Abstract
Introduction
The ADNI (Alzheimer's Disease Neuroimaging Initiative) is a large longitudinal study of patients with probable Alzheimer's disease (AD), patients with mild cognitive impairment (MCI) and healthy elderly controls followed for at least 2–3 years. Many participants in the ADNI are being treated with medications, and these may have beneficial or deleterious effects.
Objective
The goal of the study was to characterize baseline medication use in the ADNI.
Methods
Diagnosis, demographics, medication status, psychometric data and MRI measures of hippocampal volume and entorhinal cortex thickness were obtained for 818 participants from the ADNI cohort. Total number of medications, Beers list (potentially dangerous) medications and AD treatments were also tabulated. ANOVA and logistic regression were used to assess associations between baseline pharmacotherapy and diagnosis, demographics, and selected clinical and MRI variables.
Results
Of the 818 enrolled ADNI participants, 809 were available for analysis in the present study, including 184 patients with AD, 399 patients with MCI and 226 healthy elderly controls. Significant gender differences in recruitment were observed in the MCI group. The average number of medications per participant was 8 (SD 4) and 22% reported treatment with one or more Beers list medications. For symptomatic treatment of MCI or AD, donepezil and memantine were the most commonly reported drugs. As expected, MCI and AD patients with more severe impairment were more likely to be treated. Men received treatment more frequently than women. Older subjects and those with less education were less likely to receive treatment.
Conclusions
AD and MCI participants from the ADNI cohort were being treated with polypharmacy and many were also taking one or more medications with the potential for adverse effects. Off-label use of cholinesterase inhibitors and/or memantine for MCI was common, with more severely affected patients most likely to receive treatment. Differences in the frequency of symptomatic treatment were also observed as a function of age, years of education, gender and disease severity.
Introduction
The ADNI (Alzheimer's Disease Neuroimaging Initiative) is a large longitudinal study of patients with probable Alzheimer's disease (AD), patients with mild cognitive impairment (MCI) and healthy elderly controls followed for at least 2–3 years. In addition to neuroimaging and psychometric assessments, medication usage is ascertained at each study visit. As of yet, a detailed description of medication use in the ADNI at baseline has not been reported in the literature. There is considerable controversy over whether the current AD treatments have persisting symptomatic effects and/or detectable disease-modifying effects on brain structure and function. With frequent longitudinal multimodal imaging and rich clinical data, the ADNI presents an opportunity to investigate the effects of medications in general as well as those of AD-specific treatments.
The long-term effects of using multiple medications have not been well studied. Previous studies have used a variety of definitions for polypharmacy. Some have defined polypharmacy as participants taking more than five medications[1-4] or more than three medications.[5] Another study stratified polypharmacy by number of medications, including two to three medications (minor), four to five medications (moderate), and more than five medications (major).[6] Accounts of medication use in elderly individuals have also been mixed, with between 36% and 61% of elderly individuals reported to take four or more medications.[5,7] Additionally, elderly individuals receiving home care have been reported to take an average of 5.5 medications.[2]
The probability of an adverse drug event increases with the number of medications taken. For example, the probability of an adverse drug interaction is only 13% when an individual is taking two medications but rises to 82% for more than seven medications[8] and nears 100% when ten or more medications are taken.[9] Polypharmacy could be a marker for more severe illness rather than a reliable predictor of health outcomes, but a previous study has suggested that neither primary diagnosis nor number of diagnoses on hospital admission are as good at predicting health outcomes as polypharmacy.[1] Furthermore, some medications are particularly dangerous when used in elderly populations. The Beers list, which was generated by a US panel of experts in pharmacology, represents a subset of medications that may be potentially inappropriate for use in adults aged ≥65 years for various reasons such as sedation, cardiovascular risk and mechanism of elimination.[10] Medications on this list are known to cause various adverse effects of different severities and frequencies. Since the majority of subjects in the ADNI are within the target age range defined by the Beers list, these medications may have potential adverse effects for this population.
Current US FDA-approved therapies for AD include cholinesterase inhibitors and the NMDA-partial receptor antagonist memantine. Cholinesterase inhibitors that are indicated for mild-to-moderate stage AD include donepezil,[11] galantamine[12] and rivastigmine.[13] Cholinesterase inhibitors, as a group, have similar efficacy and adverse effect profiles in patients with AD. The most significant differences between cholinesterase inhibitors are found in their pharmacokinetics, including different routes of administration[14] and metabolic pathways.[15] Memantine is prescribed for the treatment of AD either as monotherapy or in combination with one of the cholinesterase inhibitors. The combination of donepezil and memantine is indicated for moderate-to-severe stage AD.[16] In fact, this combination treatment was found to be more effective than donepezil alone in moderate-to-severe stage AD.[17]
MCI is defined as a pre-dementia condition with objective evidence of progressive impairment of memory or other cognitive domains without substantial impairment in activities of daily living. To date, the FDA has declined to accept MCI as an entity that is sufficiently well defined and widely accepted to merit being an approved indication for drug therapy. Recent trends suggest that many patients currently labelled as MCI may be captured by criteria such as those proposed by Dubois et al.[18] for defining an earlier stage of AD.
Many physicians recommend non-regulatory authority-approved use of cholinesterase inhibitors for symptomatic treatment of cognitive dysfunction in MCI. However, a meta-analysis of placebo-controlled trials of cholinesterase inhibitors in MCI showed that none of these medications delayed the onset of AD and their use resulted in frequent adverse effects.[19] In fact, the FDA issued a healthcare provider alert in relation to use of galantamine as a symptomatic therapy for MCI in light of the higher frequency of deaths with galantamine treatment relative to placebo in two placebo-controlled trials.[20]
The goal of the present study was to characterize baseline medication use in the ADNI cohort and to investigate the association between specific drug treatments and a range of independent demographic, clinical and neuroimaging variables. Specifically, we assessed the frequency of medication use in the ADNI cohort as a whole and between baseline diagnosis groups, focusing primarily on usage of medications found on the Beers list and medications indicated for the treatment of AD. Additionally, we examined the ability of demographic variables and disease severity to predict AD-indicated medication usage within the AD and MCI diagnostic groups.
Methods
The ADNI
The ADNI is a consortium study designed to test whether neuroimaging, biological, genetic, clinical and neuropsychological markers can predict the progression of MCI and early AD. The ADNI was launched in 2004 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the FDA, private pharmaceutical companies and nonprofit organizations. A total of 818 participants aged 55–90 years have been recruited from 59 sites across the US and Canada and followed for 2–3 years with neuroimaging and clinical assessments. For additional information about the ADNI, see www.adni-info.org and Mueller et al.[21,22]
Data
Baseline 1.5T MRI T1-weighted magnetization-prepared rapid acquisition with gradient echo (MP-RAGE) scans from all available participants were downloaded from the ADNI database hosted by the University of California, Los Angeles (UCLA) Laboratory of Neuroimaging (LONI) website (http://www.loni.ucla.edu/ADNI) between January and April 2008. Data on baseline diagnosis, demographic information, psychometric scores and medication usage were obtained from the ADNI database (download version 24 October 2008). Information on medications was extracted and the number of medications for each ADNI participant at the baseline visit was counted. Vitamins, over-the-counter and as-needed medications were included, while inhaled and topical medications were excluded, yielding a conservative estimate of total baseline medication usage. One patient taking 41 different medications was excluded. Eight additional participants were also excluded for failed MRI scan processing (n = 6) or missing baseline MRI data (n = 2), leaving 809 evaluable patients. The number of Beers list medications at baseline was determined for each ADNI participant. All over-the-counter and as-needed Beers list medications were included, as was digoxin at any dose and for any indication. Finally, the presence or absence of donepezil, rivastigmine, galantamine or memantine, as well as any concurrent usage, was tabulated for each participant at baseline.
Since memory and global functioning may be sensitive to medication adverse effects, we extracted baseline scores for the Rey Auditory Verbal Learning Test (RAVLT) and the Clinical Dementia Rating (CDR). The RAVLT is a test of episodic memory that assesses the ability to acquire and recall 15 words across five learning trials, after an intervening interference list, and to recall and recognize the words after a 30-minute delay. The CDR is a clinician's assessment, following interviews of both the patient and an informant, of each of six domains: memory, orientation, judgement and problem solving, community affairs, home and hobbies, and personal care. The CDR sum of boxes (CDR-SB) is the summation of the scores for each of the six domains that make up the CDR. Further information on these assessment tools is available in the ADNI procedure manuals and website.
A previous assessment[21] indicated that hippocampal volume and medial temporal lobe thickness are sensitive markers of disease progression. Therefore, baseline hippocampal volume and entorhinal cortex thickness values were extracted using FreeSurfer V4 (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, USA)[23-26] as previously described.[21] More information about ADNI MRI methods is available on the ADNI website (http://www.adni-info.org) and in the article by Jack et al.[27]
Statistical Analyses
Age, gender, years of education, RAVLT score, CDR-SB, hippocampal volume and entorhinal cortex thickness were compared between diagnostic groups (AD, MCI, healthy controls) using ANOVAs for continuous variables and chi-squared (χ2) tests for categorical variables. These demographic, psychometric and imaging variables were also compared between MCI and AD patients treated with a cholinesterase inhibitor and untreated participants, as well as between patients treated with memantine and untreated participants, using two-sample t-tests for continuous variables and χ2 tests for categorical variables. Logistic regression models were used to identify factors associated with either cholinesterase inhibitor or memantine treatment within the MCI and AD groups independently. The dependent variable in the logistic models was a binary variable indicating the presence or absence of cholinesterase inhibitor and/or memantine treatment at baseline. Independent variables assessed in the logistic regression models included baseline age, years of education, gender, RAVLT, CDR-SB, left hippocampal volume and left entorhinal cortex thickness. The sample size for the AD logistic regression was 178. Two AD participants were excluded because they lacked RAVLT scores, and four AD participants were excluded because they lacked hippocampal volumes. The sample size for the MCI logistic regression was 394. Six participants were excluded because they lacked hippocampal volumes. The SAS® statistical package (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
The mean age of the ADNI cohort participants was 75 years, with no significant difference in age between diagnostic groups with AD (mean 75.4, range 55.2–91.0 years), MCI (mean 74.8, range 54.6–89.4 years) and healthy controls (mean 75.9, range 60.0–89.7 years). However, a significant difference in gender distribution was found across diagnostic groups, with females comprising only 36% of the MCI group (257 males, 142 females), compared with 48% of both the AD (96 males, 88 females) and healthy control (118 males, 108 females) groups (p < 0.006).
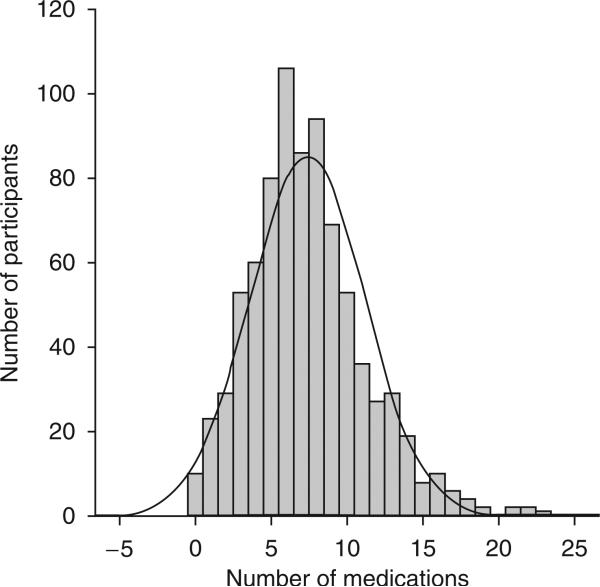
The total number of medications per participant in the ADNI cohort (n = 809) was approximately normally distributed (figure 1) with a mean of 8 (SD 4). Twenty-two percent (n = 179) of participants reported treatment with one or more Beers list medications. The ten most frequent Beers list medications reported in the ADNI cohort are listed in table I. Neither the total number of medications nor the presence of treatment with one or more Beers list medications was associated with diagnosis.
Fig. 1.
Baseline polypharmacy in the ADNI cohort (n = 809). The mean number of medications in the ADNI cohort was 8 with a standard deviation of 4.
Table I.
The ten most commonly reported Beers list medications in the ADNI cohort at baseline
| Drug name | No. of participants reporting usage |
|---|---|
| Estrogen | 29 |
| Celecoxib | 22 |
| Doxazosin | 21 |
| Naproxen | 21 |
| Digitoxin | 21 |
| Benzodiazepines | 18 |
| alprazolam | 7 |
| temazepam | 3 |
| lorazepam | 3 |
| diazepam | 2 |
| chlordiazepoxide | 1 |
| clorazepate | 1 |
| oxazepam | 1 |
| Fluoxetine | 15 |
| Nifedipine | 8 |
| Oxybutynin | 7 |
| Amiodarone | 6 |
At baseline, 45% (n = 179) of MCI patients and 86% (n = 159) of AD patients reported treatment with a cholinesterase inhibitor (table II). Donepezil was the most frequently used cholinesterase inhibitor reported in the ADNI cohort, followed by galantamine and rivastigmine. Notably, 5% (n = 18) of the MCI patients were being treated with galantamine despite the FDA warning. Eleven percent (n = 44) of MCI patients and 46% (n = 85) of AD patients reported treatment with memantine. Finally, combination treatment was frequent, with 82% (n = 36) of memantine-treated MCI patients and 85% (n = 72) of memantine-treated AD patients reporting concurrent cholinesterase inhibitor treatment. The most common combination therapy was donepezil and memantine (table II).
Table II.
Cholinesterase inhibitor and memantine treatment in Alzheimer's disease (AD) and mild cognitive impairment (MCI) patients from the ADNI cohort
| Treatment | Total no. of patients | MCI [n = 399] (%) | AD [n = 184] (%) |
|---|---|---|---|
| Donepezil | 189 | 120 (30.0) | 69 (37.5) |
| Galantamine | 30 | 16 (4.0) | 14 (7.6) |
| Rivastigmine | 11 | 7 (1.8) | 4 (2.2) |
| Memantine | 21 | 8 (2.0) | 13 (7.1) |
| Donepezil and memantine | 88 | 33 (8.3) | 55 (29.9) |
| Galantamine and memantine | 13 | 2 (0.5) | 11 (6.0) |
| Rivastigmine and memantine | 7 | 1 (0.3) | 6 (3.3) |
A significant difference in memantine (but not cholinesterase inhibitor) therapy as a function of gender was found in the MCI group, with 13.6% (n = 35) of males compared with 6.3% (n = 9) of females reporting memantine therapy (p = 0.025). On the other hand, the AD group showed a significant difference in cholinesterase inhibitor (but not memantine) therapy by gender, with 93.8% (n = 90) of men and only 78.4% (n = 69) of women reporting treatment (p = 0.002). Complete results for analyses of demographic, psychometric and imaging variables by treatment status for AD and MCI patients are presented in tables S1 and S2, Supplemental Digital Content 1, http://links.adis online.com/DAZ/A6.
In the AD group, logistic regression demonstrated no association between cholinesterase inhibitor treatment and RAVLT score, CDR-SB, hippocampal volume or entorhinal cortex thickness. However, male gender (p = 0.011; odds ratio [OR] 3.61; 95% CI 1.35, 9.66) and years of education (p = 0.036; OR 1.17; 95% CI 1.01, 1.35) were significantly positively associated with cholinesterase inhibitor treatment in AD patients. Memantine treatment in AD patients was positively associated with CDR-SB (p = 0.016; OR 1.27; 95% CI 1.05, 1.53).
Significant associations between demographic, psychometric and imaging variables and cholinesterase inhibitor and memantine treatment in MCI patients were observed (table III). Specifically, age (p = 0.009), RAVLT score (p = 0.014) and hippocampal volume (p = 0.023) were negatively associated with cholinesterase inhibitor treatment in MCI, while CDR-SB (p = 0.003) was positively associated with cholinesterase inhibitor treatment in MCI. Age (p = 0.008), RAVLT score (p < 0.001) and hippocampal volume (p = 0.023) were also negatively associated with memantine treatment in MCI patients. Additionally, male gender (p = 0.028) and years of education (p = 0.023) were positively associated with memantine treatment in MCI.
Table III.
Significant independent variables in logistic regression models associated with the presence of treatment in mild cognitive impairment (MCI) patients from the ADNI cohorta
| Variable | Association type | Odds ratio | 95% CI | p-Value |
|---|---|---|---|---|
| Cholinesterase inhibitor treatment | ||||
| CDR-SB | Positive | 1.453 | 1.137, 1.856 | 0.0028 |
| Baseline age | Negative | 0.959 | 0.930, 0.989 | 0.0085 |
| RAVLT total score | Negative | 0.969 | 0.945, 0.994 | 0.0141 |
| Left hippocampal volumeb | Negative | 0.604 | 0.391, 0.933 | 0.0231 |
| Memantine treatment | ||||
| Gender (male) | Positive | 2.577 | 1.106, 6.001 | 0.0282 |
| Years of education | Positive | 1.157 | 1.020, 1.312 | 0.0229 |
| Baseline age | Negative | 0.934 | 0.888, 0.982 | 0.0079 |
| RAVLT total score | Negative | 0.913 | 0.868, 0.960 | 0.0004 |
| Left hippocampal volumeb | Negative | 0.419 | 0.197, 0.891 | 0.0239 |
Independent variables were considered significant at p < 0.05.
Hippocampal volume was scaled by three orders of magnitude.
CDR-SB = Clinical Dementia Rating sum of boxes; RAVLT = Rey Auditory Verbal Learning Test.
Discussion
In this study, we assessed the prevalence of reported medication usage in the ADNI cohort at baseline, with particular focus on the use of Beers list medications and AD-indicated treatments. Additionally, we examined differences in demographic, psychometric and neuroimaging variables between patients treated with AD-indicated medications and untreated patients within the AD and MCI groups. Finally, we used logistic regression models to assess the relationship between demographic, psychometric and neuroimaging biomarkers and the presence or absence of cholinesterase inhibitor and memantine treatment in the ADNI patient groups.
The total number of medications reported by participants in the ADNI is slightly higher than that reported in previous literature reports[2,5,7] of medication use by elderly individuals in the general community, with 85% of ADNI participants taking ≥4 medications. This significant poly-pharmacy may increase the possibility of adverse events in this cohort. Additional potential adverse effects in this cohort may result from usage of Beers list medications, as 22% of ADNI participants reported treatment with one or more Beers list medications. Some of the more notable potential concerns about Beers list medications that ADNI participants reported using include the following: (i) the narrow therapeutic window of digoxin combined with the loss of muscle mass and renal clearance present in the elderly;[28] (ii) the increased risk of gastrointestinal ulcers and cardiovascular problems associated with use of NSAIDs;[29,30] (iii) the increased cerebrovascular risk associated with use of estrogen in elderly women;[31] (iv) the long half-lives of fluoxetine and some of its metabolites, which when combined with the drug's strong cytochrome P450 2D6 inhibitory effects, make it a suboptimal selective serotonin reuptake inhibitor in this population;[32] (v) use of oxybutynin[33] and benzodiazepines[34] in cognitively impaired elderly patients, who are potentially more vulnerable to drug-induced encephalopathy; (vi) use of amiodarone in elderly patients, who have increased susceptibility to the toxicities of the drug;[35] and (vii) the fact that use of doxazosin and nifedipine has been associated with hypotension in the elderly.[36]
As expected, the prevalence of cholinesterase inhibitor and/or memantine treatment was high in the AD group, with nearly the whole sample reporting treatment (86% of AD patients were receiving a cholinesterase inhibitor and 45% of AD patients were receiving memantine). In this respect, our analysis agrees with another recent report on ADNI clinical characteristics.[37] Anotable difference in memantine treatment by disease severity as defined by higher CDR-SB scores was found in the AD group. Disease severity, as reflected in lower RAVLT, higher CDR-SB and decreased left hippocampal volume and entorhinal cortex thickness, was not associated with the frequency of cholinesterase inhibitor treatment in AD patients. This result was expected, as the most commonly prescribed cholinesterase inhibitor, donepezil, is FDA approved for all stages of AD and therefore is likely to be used in the majority of patients diagnosed with AD, regardless of severity. However, memantine is FDA approved only for the moderate and severe stages of AD and is more commonly prescribed in patients with increased impairment.
MCI patients in the ADNI cohort also frequently reported use of cholinesterase inhibitors and memantine. The off-label use of cholinesterase inhibitors in MCI in this cohort was expected, and these therapies are supported by professional society peer-reviewed medical education[38] and suggestive but not definitive data from prospective clinical trials,[19] in which cholinesterase inhibitors were reported to potentially help with cognitive symptoms in MCI patients. However, given an increase in mortality seen in two double-blind, placebo-controlled trials of galantamine, the FDA has specifically recommended against use of this drug in MCI.[20] On the other hand, the reported use of memantine in MCI patients in the ADNI cohort is somewhat surprising since MCI memantine therapy is not supported by evidence-based clinical resources or professional medical society recommendations regarding treatment.
In the MCI group, pharmacological therapy was more commonly reported by patients with more severe impairment as defined by decreased RAVLT score, a higher CDR-SB and increased hippocampal atrophy. Previous analysis of the ADNI data[39] has shown that hippocampal atrophy is a sensitive measure of MCI to probable AD conversion within 1 year. Therefore, the negative association between left hippocampal volume and frequency of medication in MCI patients likely reflects a higher usage by more impaired patients.
In addition to increased disease severity as defined by greater impairment and brain atrophy, a number of demographic variables were found to be factors associated with or influencing treatment prevalence in MCI and AD patients. In MCI, all treatments were more frequent among younger participants and those with more education. Pharmacological treatments were also associated with gender, with cholinesterase inhibitor treatment being more commonly reported in males than females with AD, and memantine treatment being more commonly reported in males than females with MCI. There may be a number of reasons for the discrepancies in treatment frequency between genders. Given the greater life expectancy of females, males with AD may be more commonly cared for by female spouses. Spousal caregivers may have been more likely to select treatment.[40,41] Additionally, patient gender may affect the frequency with which physicians prescribe particular treatments. Finally, women may have decreased tolerance to treatment because of lower weight and/or other physiological factors. The last possibility seems less likely, as doses are frequently titrated to tolerance and the previous placebo-controlled trials have not shown significant differences in adverse effect profiles by gender. Physician prescribing patterns and whether they are influenced by physician and patient gender could be epidemiologically studied as could patient and caregiver qualitative beliefs about treatment by gender.
In addition to monotherapy with either cholinesterase inhibitors or memantine, combination therapy was relatively common in both AD and MCI participants in the ADNI cohort, with 39% of AD patients and 9% of MCI patients being treated with a cholinesterase inhibitor and memantine. Combination therapy with both a cholinesterase inhibitor and memantine has been shown to be more beneficial and better tolerated than either therapy alone in moderate-to-severe stage AD.[17] However, as previously discussed, the use of memantine either as monotherapy or in combination with other treatments may be inadvisable in MCI patients.
Clearly, the baseline ADNI data reviewed here demonstrate considerable variability in how drugs approved for treating AD are utilized by treating physicians, particularly in early stages of the illness (MCI). Several clinical and demographic factors were identified as being associated with AD treatment. Better understanding of this background pharmacotherapy and how it varies over time as the illness progresses should aid in improving methodology and analyses in future AD disease progression trials.
Conclusions
Our analysis showed that AD and MCI participants from the ADNI cohort were being treated with multiple drugs and many were also taking medications that had the potential to cause adverse effects. Off-label use of cholinesterase inhibitors and/or memantine in patients with MCI was also common, with more severely affected patients being most likely to receive treatment. Differences in the frequency of symptomatic treatment were also observed as a function of age, years of education, gender and disease severity.
Studies by our group and others are underway to investigate the role of neuroimaging and genetics in the detection and monitoring of MCI and AD, as well as in the prediction of the rate of disease progression. Future studies should consider medication status as a potential independent variable. Studies to elucidate the role of AD-indicated treatments in disease progression, as determined by changes in clinical diagnosis, cognitive functioning assessed by psychometric measures and longitudinal changes in neuroimaging biomarkers, will be important. Future studies should also assess the role of genetic factors in predicting medication usage, tolerance and efficacy.
Supplementary Material
Acknowledgements
Data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu/ADNI). As such, the investigators within the ADNI contributed to the design and implementation of the ADNI and/or provided data but did not participate in the analysis or writing of this report. For a complete list of investigators involved in the ADNI see: http://www.loni.ucla.edu/ADNI/Data/ADNI_Authorship_List.pdf.
Data collection and sharing was funded by the ADNI (Principal Investigator: Michael Weiner; National Institutes of Health [NIH] grant U01 AG024904). The ADNI is funded by the NIA, the NIBIB and through generous contributions from the following: Pfizer, Wyeth, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, AstraZeneca, Novartis, the Alzheimer's Association, Eisai, Elan, Forest and the Institute for the Study of Aging, with participation by the FDA. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the UCLA. Data analysis was supported in part by the following grants from the NIH: NIA R01 AG19771, P30 AG10133, NIBIB R03 EB008674 and by the Indiana Economic Development Corporation (IEDC #87884).
Dr Farlow has received research funds from Bristol-Myers Squibb, Danone, Elan, Eli Lilly, Forest, Medivation, Novartis, OctaPharma, Pfizer and Sonexa; has acted as a consultant and/or speaker for and has received honoraria from Accera, Adamas, Adlyfe, Astellas, AstraZeneca, CoMentis, Cortex, DS-Pharma (Dainippon Sumitomo Pharma), Eli Lilly, Eisai, Forest, GlaxoSmithKline, Medivation, Merck, Novartis, Noven, OctaPharma, Pfizer, QR Pharma, Sanofi-Aventis, Schering-Plough, Suven Life Sciences and Toyama; has given expert testimony for Forest; has a spouse with Eli Lilly stock; and receives royalties from Elan for a genetically engineered mouse model. Dr Epstein, Dr Saykin, Dr Gao and Shannon Risacher have no conflicts of interest that are directly relevant to the content of this study.
All authors (Dr Epstein, Dr Saykin, Shannon Risacher, Dr Gao and Dr Farlow) contributed to this study. Dr Gao, who is a professor of biostatistics at Indiana University, completed the statistical analysis.
References
- 1.Alarcon T, Barcena A, Gonzalez-Montalvo JI, et al. Factors predictive of outcome on admission to an acute geriatric ward. Age Ageing. 1999;28(5):429–32. doi: 10.1093/ageing/28.5.429. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty JH, Perry HM, 3rd, Lynchard GS, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55(10):M554–9. doi: 10.1093/gerona/55.10.m554. [DOI] [PubMed] [Google Scholar]
- 3.Satish S, Winograd CH, Chavez C, et al. Geriatric targeting criteria as predictors of survival and health care utilization. J Am Geriatr Soc. 1996;44(8):914–21. doi: 10.1111/j.1532-5415.1996.tb01860.x. [DOI] [PubMed] [Google Scholar]
- 4.Shorr RI, Ray WA, Daugherty JR, et al. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157(15):1681–6. [PubMed] [Google Scholar]
- 5.Jensen GL, Friedmann JM, Coleman CD, et al. Screening for hospitalization and nutritional risks among community-dwelling older persons. Am J Clin Nutr. 2001;74(2):201–5. doi: 10.1093/ajcn/74.2.201. [DOI] [PubMed] [Google Scholar]
- 6.Veehof LJ, Stewart RE, Meyboom-de Jong B, et al. Adverse drug reactions and polypharmacy in the elderly in general practice. Eur J Clin Pharmacol. 1999;55(7):533–6. doi: 10.1007/s002280050669. [DOI] [PubMed] [Google Scholar]
- 7.Cohen I, Rogers P, Burke V, et al. Predictors of medication use, compliance and symptoms of hypotension in a community-based sample of elderly men and women. J Clin Pharm Ther. 1998;23(6):423–32. doi: 10.1046/j.1365-2710.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RM, Mabee J, Chan L, et al. Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med. 1996;14(5):447–50. doi: 10.1016/S0735-6757(96)90147-3. [DOI] [PubMed] [Google Scholar]
- 9.Nolan L, O'Malley K. Prescribing for the elderly: part I. Sensitivity of the elderly to adverse drug reactions. J Am Geriatr Soc. 1988;36(2):142–9. doi: 10.1111/j.1532-5415.1988.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 10.Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–24. doi: 10.1001/archinte.163.22.2716. published erratum appears in Arch Intern Med 2004; 164 (3): 298. [DOI] [PubMed] [Google Scholar]
- 11.Doody RS, Geldmacher DS, Gordon B, et al. Donepezil Study G. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58(3):427–33. doi: 10.1001/archneur.58.3.427. [DOI] [PubMed] [Google Scholar]
- 12.Razadyne [package insert] Ortho-McNeil Neurologics, Inc.; Titusville (NJ): 2005. [Google Scholar]
- 13.Exelon [package insert] Novartis Pharmaceuticals; East Hanover (NJ): 2001. [Google Scholar]
- 14.Winblad B, Grossberg G, Frolich L, et al. IDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology. 2007;69(4 Suppl. 1):S14–22. doi: 10.1212/01.wnl.0000281847.17519.e0. [DOI] [PubMed] [Google Scholar]
- 15.Cacabelos R, Llovo R, Fraile C, et al. Pharmacogenetic aspects of therapy with cholinesterase inhibitors: the role of CYP2D6 in Alzheimer's disease pharmacogenetics. Curr Alzheimer Res. 2007;4(4):479–500. doi: 10.2174/156720507781788846. [DOI] [PubMed] [Google Scholar]
- 16.Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348(14):1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 17.Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–24. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 18.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007 Aug;6(8):734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 19.Raschetti R, Albanese E, Vanacore N, et al. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med. 2007;4(11):e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration [2009 Jun 30];Alert for healthcare professionals on galantamine hydrochloride (marketed as Reminyl) 2005 [online]. Available from URL: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085186.htm.
- 21.Mueller SG, Weiner MW, Thal LJ, et al. The Alzheimer's Disease Neuroimaging Initiative. Neuroimaging Clin N Am. 2005;15(4):869–77. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer's disease: the Alzheimer's Disease Neuroimaging Initiative (ADNI). Alzheimers Dement. 2005;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82(1):87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 29.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 30.ADAPT Group Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT). PLOS Clin Trials. 2006;1(7):E33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulley SB, Grady D. The WHI estrogen-alone trial: do things look any better? JAMA. 2004;291(14):1769–71. doi: 10.1001/jama.291.14.1769. [DOI] [PubMed] [Google Scholar]
- 32.Preskorn SM. Clinical pharmacology of SSRIs. 1st ed. Professional Communications; Wichita (KS): 1996. [Google Scholar]
- 33.Kay GG, Abou-Donia MB, Messer WS, Jr, et al. Anti-muscarinic drugs for overactive bladder and their potential effects on cognitive function in older patients. J Am Geriatr Soc. 2005;53(12):2195–201. doi: 10.1111/j.1532-5415.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 34.Paterniti S, Dufouil C, Alperovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging study. J Clin Psychopharmacol. 2002;22(3):285–93. doi: 10.1097/00004714-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Kilborn MJ, Rathore SS, Gersh BJ, et al. Amiodarone and mortality among elderly patients with acute myocardial infarction with atrial fibrillation. Am Heart J. 2002;144(6):1095–101. doi: 10.1067/mhj.2002.125836. [DOI] [PubMed] [Google Scholar]
- 36.Potentially harmful drugs in the elderly: Beers’ list and more [letter]. Prescr Lett. 2007;23(9):230907. [Google Scholar]
- 37.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI) clinical characterization. Neurology. 2010;74(3):201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Academy of Neurology Dementia. Continuum. 2007;13(2):13–58. [Google Scholar]
- 39.Risacher S, Saykin A, West J, et al. Baseline MRI predictors for conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6(4):347–61. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirschman KB, Joyce CM, James BD, et al. Would care-givers of Alzheimer disease patients involve their relative in a decision to use an AD-slowing medication? Am J Geriatr Psychiatry. 2005;13(11):1014–21. doi: 10.1176/appi.ajgp.13.11.1014. [DOI] [PubMed] [Google Scholar]
- 41.Karlawish JH, Casarett D, Klocinski J, et al. How do AD patients and their caregivers decide whether to enroll in a clinical trial? Neurology. 2001;56(6):789–92. doi: 10.1212/wnl.56.6.789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.