Abstract
A small molecule inhibitor of α4 integrin-dependent cell migration was identified through a cell–based screen of small molecule libraries. Biochemical and cellular experiments suggest that this molecule functions by interacting with γ-parvin. This molecule should serve as a useful tool to study α4 integrin signaling and may lead to new therapeutics for the treatment of autoimmune diseases.
1. Introduction
Integrins are a family of cell adhesion receptors composed of an α and β subunit which mediate cell adhesion and cell migration by interacting with the extracellular matrix. The α4 integrins (α4β1 and α4β7) are essential for embryogenesis, hematopoiesis and immune responses and are of particular interest with respect to leukocyte trafficking and activation.1 The importance of α4 integrin signaling in various chronic inflammatory diseases2 and tumor metastases3 has led to the development of several α4 antagonists.4 These include antibodies that bind α4 integrins,5,6 peptidomimetics that block integrin binding,7–9 and small molecules that disrupt the α4 integrin-paxillin complex.10 Inhibition of α4 integrin signaling by targeting proteins downstream of α4 integrin may offer an alternative approach for modulating α4 integrin-mediated leukocyte trafficking, as well as provide new insights into integrin dependent signaling pathways. To this end we carried out an unbiased phenotypic screen for molecules that block α4-integrin dependent cell migration.
2. Results and Discussion
2.1. Cell–based screen of small molecule libraries
Cell migration was assayed using an automated 384-well plate wound healing assay11,12 with Chinese hamster ovary cells that were engineered to express α4 integrin (CHO–α4).13 CHO–α4 cells were plated into 384 well plates coated with a segment of fibronectin (CS–1), a ligand for α4β1 integrin. Cells were grown to high density and each well was mechanically scratched. The cells were then treated with compounds from a library of approximately 50,000 heterocycles which included known drugs, pyrrolopyrimidines, thiazoles, oxazoles, trisubstituted purines, disubstituted pyrimidines and thiazines14 at 37 °C for 12 h (5 μM final concentration). Cells were fixed and the nuclei were stained followed by automated high throughput fluorescence microscopy. Approximately 250 compounds were found to inhibit migration of CHO–α4 cells. To distinguish selective inhibitors of α4 integrin–dependent cell migration (specifically α4 versus α5 integrin-dependent cell migration in the case of CHO cells), the effects of these primary hits on migration of CHO–α4 cells in 384 well plates coated with a 9–11 fragment of fibronectin (a ligand for α5β1 integrin) were assayed. Among the primary hits that did not inhibit α5 integrin-dependent cell migration (see Supplementary Figures 1 and 2), JK273 (Figure 1)15 was the most potent (and showed dose dependent behavior). To verify JK273 activity on α4 integrin signaling in a lymphocyte derived cell line, the effect of JK273 on the motility of Jurkat T cells in a modified Boyden chamber assay was determined and significant inhibitory activity was found (IC50 0.5 μM). A preliminary structure-activity relationship (SAR) analysis revealed that the 3–chlorophenylamino group is essential for activity, while the 4-aminophenyl group can be replaced with other substituted phenyls without significant loss in activity (see Supplementary Figure 2).
Figure 1.
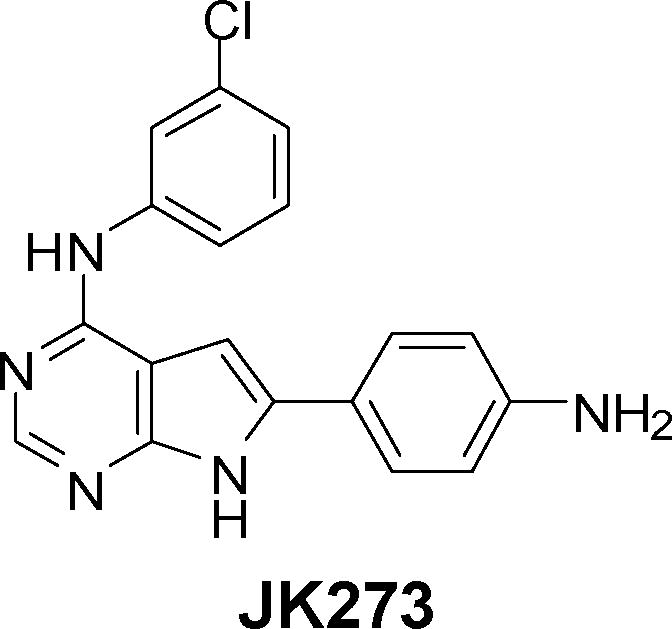
Structure of JK273.
2.2. The molecular mechanism of JK273
To investigate the molecular mechanism by which JK273 blocks cell migration, we attempted to identify the cellular target(s) of this compound by affinity chromatography (Figure 2). An affinity matrix 2 was prepared by coupling JK273 derivative 1 to Affi-Gel 10 through the amino substituent by a polyethylene glycol linker based on the above SAR analysis (see Supplementary material). The matrix 2 was treated with Jurkat cell extracts, and retained proteins were separated by SDS-PAGE. To identify those proteins that bind specifically to JK273, cell extracts were pre-incubated with JK273 (100 μM). JK273 effectively blocked the binding of 37 kDa and 41 kDa proteins to the affinity matrix, which were identified by mass spectral analysis as γ-parvin and adenosine kinase (ADK), respectively (see Supplementary Tables 1 & 2). These results were verified by Western blotting with anti-γ-parvin and anti-ADK antibodies (Figure 2c). To confirm the involvement of γ-parvin and ADK in Jurkat cell migration, expression of these proteins was independently knocked down with interfering short-hairpin RNAs (shRNAs)16. Jurkat cells were infected with viruses producing shRNAs specific for γ-parvin or ADK and their migration was again assayed using a modified Boyden chamber assay. Downregulation of ADK with multiple shRNAs had only a small effect on cell migration, suggesting that binding of JK273 to ADK is not a major functional contributor to the cell migration phenotype (data not shown).
Figure 2.
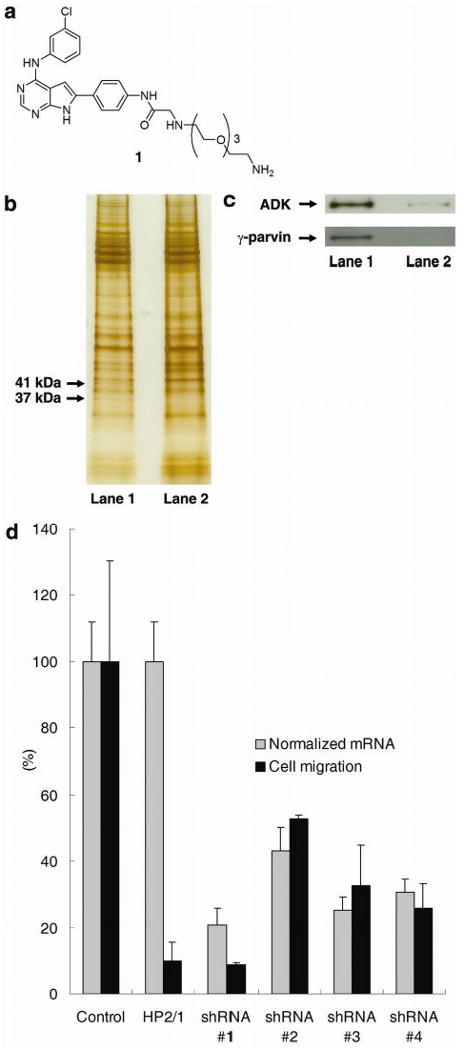
(a) Structure of ligand 1 for affinity matrix. (b) Silver-stained SDS-PAGE gel showing γ-parvin and ADK proteins pulled down by affinity matrix. Lane 1: cell extracts were treated with DMSO (1%); Lane 2: cell extracts were treated with JK273 (100 μM). (c) Confirmation of γ-parvin and ADK identity by Western blot analysis. Lane 1: cell extracts were treated with DMSO (1%); Lane 2: cell extracts were treated with JK273 (100 μM). d) Boyden chamber assay with Jurkat cells and quantitative RT-PCR analysis of γ-parvin expression after infection with lentiviruses producing shRNAs targeting γ-parvin for 84 h. An anti-α4 integrin antibody (clone HP2/1) was used as a positive control for inhibition of cell migration in the Boyden chamber assay. GAPDH was used as an internal control for quantitative RT-PCR analysis. Data are mean ± S.D.
We thus turned our attention to the role of the JK273-γ-parvin interaction in the inhibition of α4-dependent cell migration. γ-parvin is a CH domain containing protein that is highly expressed in leukocytes, localized to focal adhesions, and a binding partner with several integrin-signaling related proteins including paxillin, integrin-linked kinase (ILK), α-actinin, and ARHGEF6.16 Reduction of transcript levels of γ-parvin with RNA interference caused a corresponding reduction of migration of Jurkat cells (Figure 2d); when γ-parvin mRNA levels were reduced by >75%, the degree of inhibition of cell migration was similar to that from blocking α4-integrin function with an inhibitory α4 integrin antibody. These results are in agreement with recent studies by Yoshimi et al. who further suggest that γ-parvin plays an essential role in cell spreading in leukocytes.17 To determine if JK273 affects α4 integrin dependent cell attachment, U937 or Jurkat cells were stimulated with phorbol 12-myristate 13-acetate (PMA) or stromal cell derived factor 1-α (SDF1-α) to induce adhesion to fibronectin-coated plastic.18 Treatment of a suspension of U937 or Jurkat cells with JK273 reduced attachment to fibronectin coated surfaces by 40–80% (supplementary Figure 3), confirming that JK273 activity can phenocopy γ-parvin loss-of-function via RNAi. Taken together, these data suggest that JK273 acts through alteration of γ-parvin function. The detailed mechanism by which JK273 binds and inhibits γ-parvin activity and the JK273 specificity profile for other parvins (not detected in Jurkat cells19) are currently under investigation.
3. Conclusion
We have identified a selective small molecule inhibitor of α4 integrin-dependent cell migration using a pathway specific cellular screen. Biochemical experiments suggest JK273 binds to γ-parvin, a component of focal adhesions and previously untargeted class or proteins. JK273 should serve as a useful chemical probe of α4 integrin signaling and may ultimately lead to the synthesis of new therapeutics for the treatment of autoimmune diseases.
4. Experimental
4.1. Cell culture
All culture media and supplements were purchased from Invitrogen/Gibco unless otherwise specified. All cells were purchased from American Type Culture Collection (ATCC), and incubated at 37 °C, in a humidified atmosphere with 5 % CO2. Chinese hamster ovary cells expressing α4 integrin (CHO-α4)13 were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with fetal bovine serum (FBS, 10%) and non-essential amino acids (Sigma, 1%). Human T-cell leukemia Jurkat (Clone E6-1) and human histiocytic lymphoma U937 cells were maintained in RPMI 1640 medium with HEPES (10 mM), L-glutamine (2 mM) and FBS (10%). Human embryonic kidney (HEK) 293T cells were cultured in DMEM medium supplemented with L-glutamine (2 mM) and FBS (10%). Additionally, all media were supplemented with penicillin (50 unit/mL) and streptomycin (50 μg/mL).
4.2. Automated cell migration assay
CHO–α4 cells were plated into 384-well assay plates (12,000 cells/well) coated with CS-1 and allowed to form monolayers at 37 °C and 5 % CO2 for 5 h. After wounds were generated by an automated wound-healing assay device,11 cells were treated with a library of heterocycles (5 μM final concentration). The cells were allowed to migrate for 12 h, fixed with paraformaldehyde (4%), and stained with DAPI. Each well of the 384–well plates was photographed with an IC100 (Beckman Coulter) to automate image capture. A ×4 objective lens was used to visualize a majority of the area of each well within one field of view.
4.3. Transwell cell migration assay (modified Boyden chamber assay)
Transwell plate inserts (Corning Costar, 6.5 mm diameter, 3 μm pore size) were coated with CS-1 (10 μg/mL) in 0.1 M of NaHCO3 solution at 4 °C for 16 h and blocked with 1.5% of heat inactivated BSA in PBS at room temperature for 0.5 h. 500 μL of serum free medium and 200 μL of a cell suspension (2 × 105 cells/well) were added to the lower chamber and upper chamber, respectively. SDF1-α (Sigma, 15 ng/mL) was added to the lower chamber and the chambers were incubated in a CO2 incubator at 37 °C for 4.5 h. Migrated cells (both in medium and adherent on the bottom of the inserts) were collected and counted using a hemacytometer. Cell dissociation buffer (Invitrogen/Gibco, 500 μL/well) was used to detach adherent cells on the bottom of the inserts.
4.4. Cell adhesion assay
Jurkat or U937 cells (2 × 104 cells/well, 1 mL) were plated into 12 well plates coated with fibronectin (BD BioCoat™) and treated with phorbol 12-myristate 13-acetate (PMA, Sigma,100 nM) or SDF1-α (Sigma, 150 ng/mL) in the presence or absence of JK273 (1 or 5 μM) for 4.5 h. The wells were gently washed with PBS twice and the adherent cells were fixed with formaldehyde (4%) in PBS. 3 areas/well were selected randomly and the adherent cells were counted under a microscope.
4.5. Production of lentivirus
All recombinant lentiviruses were produced by transient transfection of 293T cells using Fugene 6 (Roche) according to manufacturer's protocol. Briefly, subconfluent 293T cells (30–40%) in 10 cm dish were co-transfected with 10 μg of transfer plasmid containing the shRNA, packaging plasmids producing RRE (6.5 μg) and REV (2.5 μg), and 3.5 μg of envelope vector producing VSVG. After 24 h, the medium was replaced with fresh medium, and 24 h later the supernatants were harvested, filtered through 0.45 μm pore-sized membranes.
4.6. RNA interference (RNAi) experiments
Jurkat cells (2 × 106 cells/dish) were plated into 10 cm dishes and polybrene (Sigma, 4 μg/mL) was added. The cells were infected with lentiviruses producing shRNAs targeting γ-parvin or adenosine kinase, incubated for 84 h and harvested for a transwell cell migration assay. Transcript levels of γ-parvin and adenosine kinase were measured by TaqMan quantitative RT-PCR.
Sequences of shRNAs targeting γ–parvin
shRNA#1: GGACGTCTTTGATGAATTA (sense sequence)
shRNA#2: GGGCCTGTCTGTGCAGAAT (sense sequence)
shRNA#3: GCTTCTTCCTGCACTTAAA (sense sequence)
shRNA#4: GAAGGCTTCTTCCTGCACT (sense sequence)
Sequences of shRNAs targeting ADK
shRNA#1: GCCACACAAAGCAGCAACA (sense sequence)
shRNA#2: GGCTTTGAGACTAAAGACA (sense sequence)
shRNA#3: GGTCCCTCATAGCTAATCT (sense sequence)
4.7. TaqMan quantitative RT-PCR
Jurkat cells were harvested and total RNA was extracted using Trizol (Invitrogen) and an RNeasy purification kit (Qiagen). cDNAs of the total mRNAs were synthesized using the cDNA Archive kit (Applied Biosystems), and transcript levels of γ-parvin were measured using TaqMan Universal PCR Master Mix (Applied Biosystems) and TaqMan Gene Expression Assays (primers & probe sets, Applied Biosystems) following the manufacturer's protocol. GAPDH was used as an internal control.
Supplementary Material
Acknowledgments
This work was supported by the Novartis Research Foundation and The Skaggs Institute of Chemical Biology (P.G.S.), and partially by the Postdoctoral Fellowship of Korea Research Foundation (J.L., 04–12–07–1). We thank Ms. Myleen Medina (Genomics Institute of the Novartis Research Foundation) for providing us with both γ-parvin and ADK shRNA clones.
Footnotes
Supplementary data associated with this article can be found, in the online version, at….
References and notes
- 1.Shimizu Y, Rose DM, Ginsberg MH. Advan Immunol. 1992;72:325–380. doi: 10.1016/s0065-2776(08)60024-3. [DOI] [PubMed] [Google Scholar]
- 2.von Andrian UH, Mackay CR. N Engl J Med. 2000;343:1020–1033. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 3.Holzmann B, Gosslar U, Bittner M. Curr Top Microbiol Immunol. 1998;231:125–141. doi: 10.1007/978-3-642-71987-5_8. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DY. Curr Pharm Design. 2002;8:1229–1253. doi: 10.2174/1381612023394737. [DOI] [PubMed] [Google Scholar]
- 5.Podolsky DK, Lobb RR, King N, Benjamin CD, Pepinsky B, Sehgal P, deBeaumont M. J Clin Invest. 1993;92:372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hesterberg PE, Winsor-Hines D, Briskin MJ, Soler-Ferran D, Merrill C, Mackay CR, Newman W, Ringler DJ. Gastroenterology. 1996;111:1373–1380. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- 7.Fotouhi N, Joshi P, Fry D, Cook C, Tilley JW, Kaplan G, Hanglow A, Rowan K, Schwinge V, Wolitzky B. Bioorg Med Chem Lett. 2000;10:1171–1173. doi: 10.1016/s0960-894x(00)00174-8. [DOI] [PubMed] [Google Scholar]
- 8.Jackson DY, Quan C, Artis DR, Rawson T, Blackburn B, Struble M, Fitzgerald G, Chan K, Mullins S, Burnier JP, Fairbrother WJ, Clark K, Bersini M, Chui H, Renz M, Jones S, Fong S. J Med Chem. 1997;40:3359–3368. doi: 10.1021/jm970175s. [DOI] [PubMed] [Google Scholar]
- 9.Souers AJ, Virgilio AA, Schürer SS, Ellman JA, Kogan TP, West HE, Ankener W, Vanderslice P. Bioorg Med Chem Lett. 1998;8:2297–2302. doi: 10.1016/s0960-894x(98)00416-8. [DOI] [PubMed] [Google Scholar]
- 10.Ambroise Y, Yaspan B, Ginsberg MH, Boger DL. Chem Biol. 2002;9:1219–1226. doi: 10.1016/s1074-5521(02)00246-6. [DOI] [PubMed] [Google Scholar]
- 11.Collins CS, Hong J, Sapinoso L, Zhou Y, Liu Z, Micklash K, Schultz PG, Hampton GM. Proc Natl Acad Sci USA. 2006;103:3775–3780. doi: 10.1073/pnas.0600040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ. Bmc Biotechnol. 2004;4:21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, Ginsberg MH. Nature. 1999;402:676–681. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- 14.Ding S, Gray NS, Wu X, Ding Q, Schultz PG. J Am Chem Soc. 2002;124:1594–1596. doi: 10.1021/ja0170302. [DOI] [PubMed] [Google Scholar]
- 15.Trexler P, Bold G, Lang M. WO 9807726 Frei J PCT Int Appl.
- 16.Sandy P, Ventura A, Jacks T. Biotechniques. 2005;39:215–224. doi: 10.2144/05392RV01. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimi R, Yamaji S, Suzuki A, Mishima W, Okamura M, Obana T, Matsuda C, Miwa Y, Ohno S, Ishigatsubo Y. J Immunol. 2006;176:3611–3624. doi: 10.4049/jimmunol.176.6.3611. [DOI] [PubMed] [Google Scholar]
- 18.Maqueda A, Moyano JV, Gutiérrez-López MD, Ovalle S, Rodríeguez-Frade JM, Cabañas C, Garcia-Pardo A. J Cell Physiol. 2006;207:746–756. doi: 10.1002/jcp.20624. [DOI] [PubMed] [Google Scholar]
- 19.Chu H, Thieessen I, Sixt M, Lämmermann T, Waisman A, Braun A, Noegel AA, Fässler R. Mol Cell Biol. 2006;26:1817–1825. doi: 10.1128/MCB.26.5.1817-1825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


