Abstract
Neurogenin 3 (NGN3) commits pancreatic progenitors to an islet cell fate. We have induced NGN3 expression and identified upregulation of the gene encoding the Ras-associated small molecular mass GTP-binding protein, RAB3B. RAB3B localised to the cytoplasm of human β-cells, both during the foetal period and post natally. Genes encoding alternative RAB3 proteins and RAB27A were unaltered by NGN3 expression and in human adult islets their transcripts were many fold less prevalent than those of RAB3B. The regulation of insulin exocytosis in rodent β-cells and responsiveness to incretins are reliant on Rab family members, notably Rab3a and Rab27a, but not Rab3b. Our results support an important inter-species difference in regulating insulin exocytosis where RAB3B is the most expressed isoform in human islets.
Introduction
Understanding normal β-cell development and function underpins various efforts aimed to restore β-cells in patients with type 1 and type 2 diabetes. During the foetal development, the pancreas contains epithelial progenitor cells, which give rise to the adult cell lineages, including β-cells (Murtaugh 2007). Experiments manipulating genes in mice have discovered a multitude of transcription factors that regulate this transition (Wilson et al. 2003). Within a subset of progenitors, positive for the transcription factors Sry box 9 (Sox9) and pancreas-duodenal homeobox 1 (Pdx1), the basic helix-loop-helix (bHLH) transcription factor Neurogenin-3 (Ngn3, also known as Neurog3) becomes transiently expressed to commit cells to an endocrine fate (Schwitzgebel et al. 2000, Lynn et al. 2007, Seymour et al. 2007). Without Ngn3, islet differentiation fails (Gradwohl et al. 2000). The transcription factor has also been shown necessary for β-cell regeneration from adult precursors (Xu et al. 2008). Strategies, such as expression microarray following retroviral ectopic Ngn3 expression, have identified direct genetic targets of Ngn3 encoding transcription factors, such as NeuroD1, paired homeobox factor 4 (Pax4), Nirenberg and Kim (NK) homeobox family member Nkx2.2 and insulinoma-associated 1, all of which when inactivated in mice impair β-cell differentiation (Sosa-Pineda et al. 1997, Sussel et al. 1998, Huang et al. 2000, Heremans et al. 2002, Gasa et al. 2004, Smith et al. 2004, Mellitzer et al. 2006). Pdx1 is also increased following Ngn3 expression (Gasa et al. 2004). Several of these transcription factors downstream of Ngn3 are then required for mature β-cell function. For instance, Pdx1 regulates GLUT2, glucokinase and insulin amyloid polypeptide (IAPP) expression (McKinnon & Docherty 2001) and, in association with NeuroD1, it transactivates the insulin gene (Babu et al. 2008).
Many aspects of the β-cell phenotype are conserved across species. Nevertheless, there are subtle differences in mature β-cell function between mice and humans: the relative roles of glucose transport and phosphorylation as a part of glucose sensing (Schuit 1997); responsiveness to glucokinase activators (Johnson et al. 2007); glucose-induced desensitisation (Zawalich et al. 1998); responses to galanin (McDonald et al. 1994) and melatonin (Ramracheya et al. 2008); the roles of Pax4 (Brun et al. 2008) and p57Kip2 (Potikha et al. 2005) in β-cell proliferation; and, central to β-cell function, regulation of the insulin promoter (Hay & Docherty 2006). This makes direct study of human pancreas development and β-cells worthwhile. Studying foetuses from first trimester termination of pregnancy has provided a framework for understanding early human pancreas development with β-cells increasing rapidly after 8 weeks post conception (wpc), first as cell clusters and then within islets, where they express other markers of maturity such as prohormone convertase 1/3, IAPP, chromogranin A and some components of the glucose-sensing apparatus (Piper et al. 2004, Richardson et al. 2007). Similar studies first identified SOX9 as important for pancreatic development and β-cell differentiation; the pancreata of patients with campomelic dysplasia being hypoplastic and composed of poorly formed islets (Piper et al. 2002). Human pancreatic progenitor cells expressing PDX1 (Piper et al. 2004) and NGN3 transcripts have been identified at 8 wpc (Castaing et al. 2005).
To further address the potential inter-species differences downstream of endocrine commitment, we induced human NGN3 expression in a cell line with similarities to human foetal pancreatic progenitors, leading to increased expression of the Ras-associated small molecular mass GTP-binding protein, RAB3B. RAB3 proteins regulate intracellular trafficking and exocytosis in a range of cell types (Gonzalez & Scheller 1999) with RAB3B recently implicated in protecting and enhancing the function of dopaminergic nerve terminals (Chung et al. 2009). Inactivation of either Rab3a or Rab27, but not Rab3b, in mice causes glucose intolerance (Yaekura et al. 2003, Aizawa & Komatsu 2005, Kasai et al. 2005). In this study, we have identified RAB3B, rather than RAB27A or other RAB3 isoforms, as the predominant isoform in human islets implying an inter-species difference and providing a new candidate for mutation or abnormal function as a cause of diabetes and as a potential therapeutic target for enhancing insulin secretion in humans.
Materials and Methods
Human tissue collection
The collection of human foetal material under guidelines issued by the Polkinghorne committee has been described previously (Piper et al. 2004, Ostrer et al. 2006). Ethical approval was granted by the Southampton and South West Hampshire Local Regional Ethics committee. In these experiments, material from at least two foetuses per stage was examined. Human islets were obtained with appropriate ethical approval from the King's College Hospital Islet Transplantation Unit (King's College Hospital, London, UK). Pancreata were removed from non-diabetic cadaver organ donors and islets were isolated under aseptic conditions as described previously (Huang et al. 2004).
Immunohistochemistry and immunoblotting
Tissue preparation, immunoblotting, immunohistochemistry and immunofluorescence were performed as described previously (Piper et al. 2004, Piper Hanley et al. 2008). Antibodies are listed in Supplementary Table 1, see section on supplementary data given at the end of this article with dilutions, catalogue numbers and sources. Exceptions to dilutions for immunoblotting were 1:1000 for NGN3 and RAB3B. For biotinylated secondary antibodies, streptavidin (SA)–HRP (1:200, Vector Laboratories Ltd, Peterborough, UK), SA–FITC (1:150, Sigma–Aldrich Ltd), or SA–Texas Red (1:200; Vector Laboratories Ltd) conjugates were used according to the manufacturers' instructions. For bright-field immunohistochemistry, the colour reaction was developed following SA–HRP with diaminobenzidine (Merck) containing 0·1% hydrogen peroxidase (Sigma–Aldrich Ltd). Negative controls were omission of primary or secondary antibody.
Cloning of the PANC-1 cell line with inducible NGN3 expression
The human PANC-1 cell line (cat. no. 87092802) was purchased from the European Cell and Animal Culture Collection (ECACC, Salisbury, UK) and cultured in DMEM containing 10% foetal bovine serum (FBS) and prokaryotic antibiotics. All vectors were from Clontech Laboratories Inc. The human NGN3 coding sequence was amplified by PCR using primers containing HindIII and XbaI restriction sites (forward, 5′-CCCAAGCTTGACTCAAACTTACCCTTCCCTCTG-3′; reverse, 5′-GCTCTAGAGCTCCGGCCGGGTAGTGCT-3′) and cloned using these restriction sites into the pTRE2 vector to create pTRE2–NGN3. PANC-1 cells were transfected sequentially with pTet-On and pTRE2–NGN3 plus pTK-Hyg using Transfast (Promega Ltd). Stable PANC-1 clones demonstrating inducible NGN3 expression (PANC-1iNGN3) were isolated in DMEM containing tetracycline-free FBS by selection with G418 and hygromycin according to the manufacturer's instructions (Clontech Laboratories). To assess the expression of functional NGN3 protein, the proximal 1613 bp of 5′ flanking region from the mouse NeuroD1 gene was amplified to create a luciferase construct, p-1613NeuroD1-Luc. This construct contains two E-box motifs regulated by Ngn3 (Huang et al. 2000). Luciferase assays (Promega Corp.), as described previously (Hanley et al. 2001), were conducted 48 h after the addition of doxycycline (Dox) to screen PANC-1iNGN3 clones for induction of functional NGN3.
Isolation of RNA, northern blotting, reverse transcription and real-time PCR
Total RNA was isolated from PANC-1iNGN3 cells, human foetal pancreas and adult islets using Tri reagent (Sigma–Aldrich Ltd) for subsequent gel electrophoresis and reverse transcription (RT) using Superscript III (Invitrogen Ltd). RNA gel electrophoresis was carried out under denaturing conditions using 5 μg of total RNA per lane followed by washing and transfer overnight to Hybond N+ membrane (Amersham Pharmacia Biotech Ltd). The membrane was cross-linked by exposure to ultraviolet light and hybridised at 68 °C overnight with radiolabelled DNA probes to the NGN3 coding sequence. Following post-hybridisation washes, the membrane was exposed to autoradiography film at −80 °C and developed.
Real-time PCR in the foetal tissue and the PANC-1 cells used pre-designed Taqman Gene Expression assays for each gene (Applied Biosystems, Warrington, UK) and an ABI PRISM 7900HT system with standard cycling conditions. TBP and HPRT1 were used as endogenous controls. Results were analysed with SDS v2.1 software (Applied Biosystems) using the relative quantification method. mRNA was isolated from human pancreatic islets using the RNeasy Mini kit (Qiagen Ltd) according to the manufacturer's instructions and was quantified using a Nanodrop spectrometer (NanoDrop, Rockland, ME, USA). cDNA was synthesised and quantitative RT-PCR standards ranging from 10 to 109 copies DNA were prepared as described previously (Persaud et al. 2002). Real-time PCR amplification was performed using a LightCycler rapid thermal cycler system. Reactions were performed in 10 μl comprising nucleotides, Taq DNA polymerase and buffer (all included in the LightCycler FastStart Reaction Mix SYBR Green I); template cDNA; 3 mM MgCl2; and 0·5 μM primers. All PCR protocols included an initial 10 min denaturation step and each cycle subsequently included a ramp at 95 °C for denaturation, annealing for 10 s at the temperatures listed in Supplementary Table 2, see section on supplementary data given at the end of this article and a 72 °C extension phase for 14 s (β-ACTIN) or 18 s (all other genes). The amplification products of both the primer pairs were subjected to melting point analyses and subsequent gel electrophoresis to ensure specificity of amplification.
Statistical analysis
Data were expressed as means±s.e.m. Statistical analysis used paired t-test or one-way ANOVA followed by Dunnett's post hoc test, as indicated. Values with P<0·05 were considered significant.
Results
Inducible NGN3 expression by PANC-1iNGN3 cells
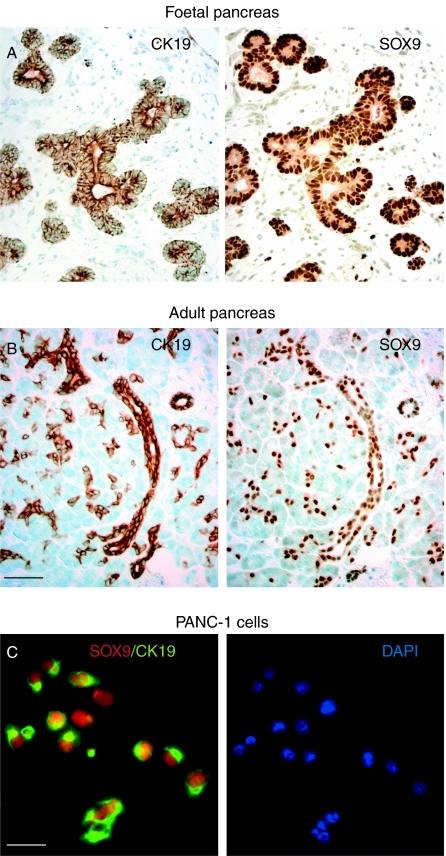
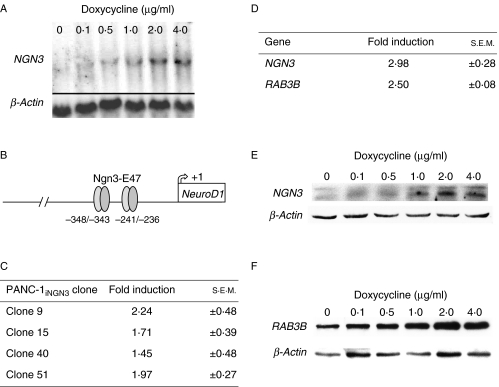
In the human pancreas, cytoplasmic CK19 and nuclear SOX9 were restricted to the foetal epithelial progenitor cells and adult ductal cells (Fig. 1A and B; Piper et al. 2004). A similar profile was identified uniformly in the human pancreatic ductal carcinoma cell line, PANC-1 (Fig. 1C), which was negative for amylase (data not shown), confirming this cell line as a suitable source in which to engineer inducible NGN3 expression. Sequential stable transfection of vectors for dox-inducible NGN3 expression resulted in the isolation of more than 60 human PANC-1iNGN3 clones for further analysis. These starting clones retained SOX9 and CK19 expression (data not shown). From selected clones, northern blotting revealed dose-responsive NGN3 expression (Fig. 2A). Four clones, 9, 15, 40 and 51, were analysed in greater detail. The ability of these clones to induce functional NGN3 protein after the addition of dox to the media was assessed using a luciferase construct containing two E-box motifs from the wild-type mouse NeuroD1 5′ flanking region (Fig. 2B; Huang et al. 2000). All the four clones increased luciferase activity on the addition of dox to the media (Fig. 2C). Clone 51 gave the most reproducible results with least background luciferase activity and, thus, was chosen for the induction of NGN3 and subsequent microarray analysis (see Supplementary Methods, see section on supplementary data given at the end of this article). As expected, NGN3 upregulation was detected by the array (Fig. 2D). The gene encoding RAB3B was also identified from candidates whose expression was induced at least twofold by 2 μg/ml dox treatment for 48 h. Reassuringly, both NGN3 and RAB3B proteins showed dose-responsive increases following dox treatment of clone 51 (Fig. 2E and F).
Figure 1.
PANC-1 cells express CK19 and SOX9. (A) Consecutive 5 μm sections of the human foetal pancreas at 8 wpc stained for CK19 and SOX9. (B) Consecutive 5 μm sections of the human adult pancreas stained for CK19 and SOX9. (C) Dual immunofluorescence of PANC-1 cells for SOX9 (red) and CK19 (green) counterstained with DAPI (blue) of the same image is shown to the right. Size bars represent 250 μm (A and B) and 50 μm (C).
Figure 2.
Inducible NGN3 expression in PANC-1 cells. (A) Northern blot analysis for NGN3 expression following the addition of doxycycline for 48 h to a clone of PANC-1iNGN3 cells. (B) Schematic of the mouse NeuroD1 promoter with two E-box motifs at −348/−343 and −241/−236 bp in its 5′ flanking region that are regulated by Ngn3-E47 dimers (Huang et al. 2000). (C) Fold induction ±s.e.m. of luciferase activity from transient transfection of p-1613NeuroD1-Luc measured 48 h after the addition of 2 μg/ml doxycycline in four PANC-1iNGN3 clones. (D) Fold increase ±s.e.m. of NGN3 and RAB3B expression from microarray analysis of PANC-1iNGN3 clone 51 by adding 2 μg/ml doxycycline for 48 h. (E and F) Western blot analysis of clone 51 PANC-1iNGN3 cells showing NGN3 (E) and RAB3B (F) protein expression following addition of increasing concentrations of doxycycline for 48 h.
Expression of RAB3 family members and RAB27A in NGN3-inducible PANC-1 cells
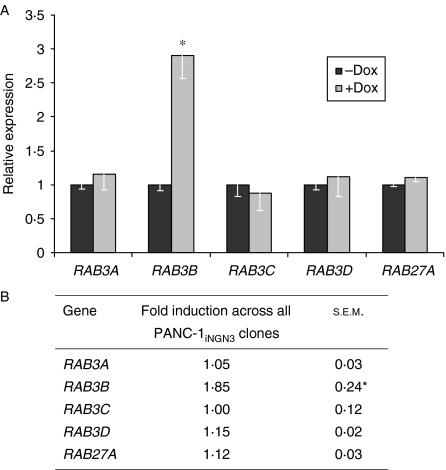
In the mouse central nervous system, Rab3 proteins functions redundantly due to other co-expressed family members (Schluter et al. 2004). In mouse β-cells, inactivation of Rab3a and Rab27a, but not Rab3b, caused glucose intolerance (Yaekura et al. 2003, Kasai et al. 2005). Therefore, we analysed whether NGN3 expression led to the expression of other RAB3 genes as well as RAB27A. Following the induction of NGN3 for 48 h, neither RAB3A, RAB3C and RAB3D family members nor RAB27A was increased in either clone 51 (Fig. 3A) or the other PANC-1iNGN3 cell clones (Fig. 3B). RAB3B was significantly increased in all the four PANC-1iNGN3 clones, albeit to a lesser extent in clones 9, 15 and 40 than in clone 51. Spurious induction of RAB3B by the antibiotic was excluded, as dox had no effect on RAB3B expression in PANC-1 cells lacking the pTRE2–NGN3 vector (data not shown).
Figure 3.
Expression of RAB3 family members and RAB27A following induction of NGN3 expression in PANC-1iNGN3 cells. (A) Expression of RAB3 isoforms and RAB27A in PANC-1iNGN3 clone 51 after 2 μg/ml doxycycline for 48 h. Bars show mean±s.e.m. from at least two experiments. (B) Mean fold induction ±s.e.m. of expression for RAB3 isoforms and RAB27A in the other PANC-1iNGN3 clones (Fig. 2B) after 2 μg/ml doxycycline for 48 h. *P<0·05 compared to ‘−dox’ treatment.
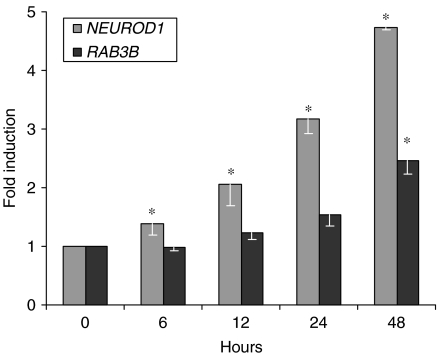
NGN3 is a transient requirement during mouse endocrine cell differentiation (Schwitzgebel et al. 2000). As exocytosis of stored hormone granules is a function of mature endocrine cells, NGN3 would not be expected to directly regulate RAB3B expression. We examined the timing of RAB3B expression in the PANC-1iNGN3 cells. By northern blotting, NGN3 transcripts were induced within 2 h of adding dox (data not shown). mRNA levels of NEUROD1, a direct target of NGN3 action (Huang et al. 2000), were increased by ∼40% at 6 h and doubled at 12 h. In contrast, RAB3B transcripts accumulated relatively slowly, levels being increased only by ∼50% at 24 h after the addition of Dox (Fig. 4). This implies that the effect of NGN3 on RAB3B transcription is indirect and mediated through other transcription factors. We used RNAi to moderate the increase in NEUROD1 expression (Supplementary Figure 1, see section on supplementary data given at the end of this article). The downstream induction of RAB3B was unaltered, indicating that it does not rely on NEUROD1 (data not shown).
Figure 4.
Timing of RAB3B expression following the induction of NGN3 in PANC-1iNGN3 cells. The relative expression of RAB3B and NEUROD1 is shown following the addition of 2 μg/ml doxycycline to PANC-1iNGN3 clone 51. Bars show mean±s.e.m. from two experiments. *P<0·05 following analysis by ANOVA and Dunnett's post hoc test.
Expression of RAB3B during human pancreas development and in islets
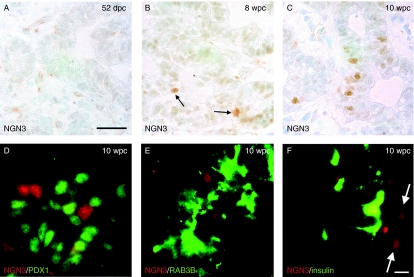
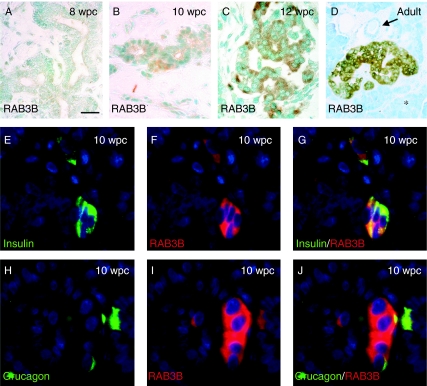
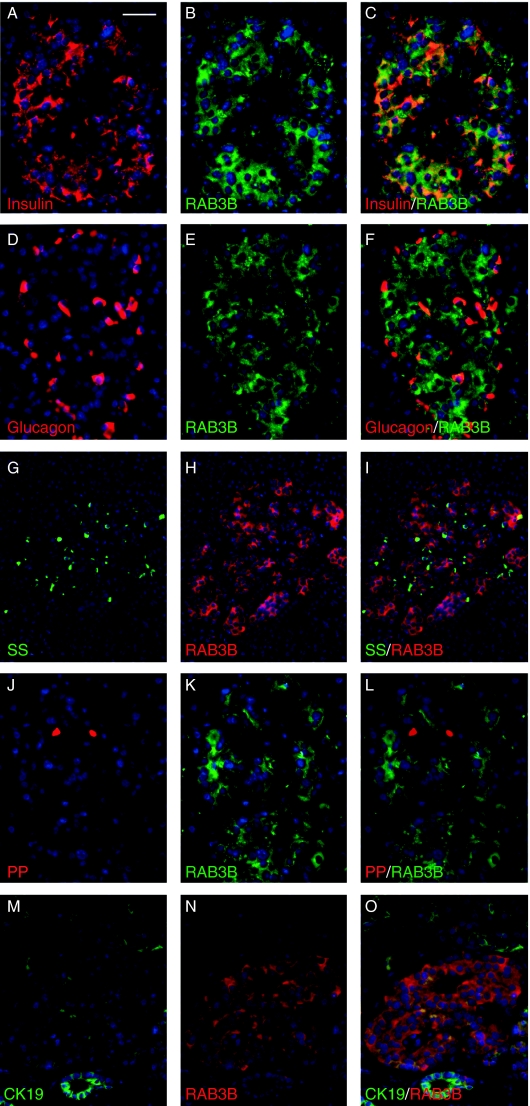
From analyses of four specimens at 50 and 52 dpc, the human embryonic pancreas was largely devoid of cells positive for NGN3 (Fig. 5A). In two specimens at 8 wpc, isolated epithelial cells with nuclei stained for NGN3 immunoreactivity were apparent centrally within the organ (arrows in Fig. 5B). At this stage, we have previously reported occasional insulin-positive cells in the same location (Piper et al. 2004). Similarly positioned NGN3-positive cells were more numerous within two specimens of larger pancreas at 10 wpc (Fig. 5C) and they did not co-localise with PDX1 (Fig. 5D). In keeping with an indirect induction by NGN3, neither RAB3B nor insulin-positive cells stained for NGN3 (Fig. 5E and F). RAB3B was first detected weakly by immunohistochemistry in the cytoplasm of clustered cells adjacent to the duct-like epithelial structures of the foetal pancreas at 10 wpc (Fig. 6A and B). It was detected more robustly in the earliest foetal islets at 12 wpc (Fig. 6C) and in the cells of adult islets (Fig. 6D). RAB3B was not found in ducts or acinar cells (Fig. 6D) of the adult pancreas. At 10 wpc, RAB3B co-localised with insulin and glucagon (Fig 6E–J). In the adult pancreas, RAB3B extensively co-localised with insulin but was somewhat variable in different β-cells (Fig. 7A–C). Some RAB3B was present in some α- and δ-cell but at relatively low level (Fig. 7D–I). In contrast, RAB3B did not co-localise with pancreatic polypeptide in the adult pancreas (Fig. 7G–L). Consistent with Fig. 6D, RAB3B was not detected in CK19-positive duct cells in the adult pancreas (Fig. 7M–O). These data are consistent with the timing of RAB3B expression following its induction by NGN3 in PANC-1iNGN3 cells (Fig. 4).
Figure 5.
Expression of NGN3 during human pancreas development. (A–F) NGN3 immunohistochemistry in sections of developing human pancreas at 52 dpc (A), 8 wpc (B, arrows point to positive cells) and 10 wpc (C–F). Dual immunofluorescence of NGN3 (red) and PDX1 (green) is shown in (C). (E and F) Dual immunofluorescence of NGN3 with RAB3B (E) and insulin (F). Arrows in (F) point to NGN3-positive nuclei. Size bars represent 150 μm (A–C) and 40 μm (D–F).
Figure 6.
Expression of RAB3B during human pancreas development. (A–D) RAB3B immunohistochemistry in sections of developing human pancreas at 8 wpc (A), 10 wpc (B, arrow points to faintly positive cell cluster) and 12 wpc (C), and in the adult pancreas (D, arrow and star indicate duct and acinar tissue respectively). (E–J) Individual and dual immunofluorescence at 10 wpc counterstained with DAPI (blue). Size bar represents 150 μm (A–C), 250 μm (D) and 40 μm (E–J).
Figure 7.
Expression of RAB3B in the adult pancreas. (A–O) Immunofluorescence counterstained with DAPI (blue) in sections of the adult pancreas. Size bar represents 40 μm (A–F and J–O) and 150 μm (G–H).
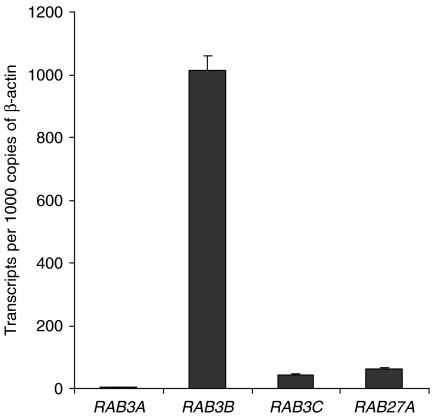
By real-time PCR, RAB3B was detected robustly in adult islets. Its transcripts were ∼500-fold increased compared to RAB3A, 25-fold increased compared to RAB3C and 17-fold increased compared to RAB27A (Fig. 8). RAB3D did not amplify despite 40 cycles of PCR, implying very low or absent expression compared to the other isoforms under investigation.
Figure 8.
Expression of RAB3 family members and RAB27A in isolated human adult islets. Real-time PCR analysis of RAB3 isoforms and RAB27A in adult islets expressed relative to β-ACTIN transcript levels. RAB3D did not amplify despite the use of more than 40 cycles of PCR.
Discussion
Studies in mice have proven that Ngn3 is required for pancreatic endocrine cell commitment. Without the bHLH transcription factor, the islet differentiation programme fails (Gradwohl et al. 2000). Conversely, driving ectopic Ngn3 expression in all pancreatic progenitor cells leads to premature over-commitment to an endocrine cell fate (Apelqvist et al. 1999). Lineage tracing of Ngn3-positive cells marks all mature pancreatic endocrine cell types (Gu et al. 2002). The transcription factor is extinguished before terminal differentiation and hormone expression (Schwitzgebel et al. 2000). Thus, it can be concluded that, in rodents, transient Ngn3 expression in an appropriate number of pancreatic epithelial progenitor cells is the normal mechanism for islet development and also appears important for potential β-cell regeneration in adult mice (Xu et al. 2008). In this study, consistent with previous data describing NGN3 transcripts in the human foetal pancreas at 8 wpc (Castaing et al. 2005), we found the transcription factor present at 8 and 10 wpc within central cells of the pancreas where endocrine differentiation is known to predominate away from the pro-proliferative, exocrine-inducing effects of peri-pancreatic mesenchyme (Miralles et al. 1998, Polak et al. 2000, Elghazi et al. 2002, Piper et al. 2004). At this stage of development, large numbers of insulin-positive cells are apparent before organisation into islets at the end of the first trimester (Piper et al. 2004). Coupled with the identification of hypomorphic NGN3 mutations in patients with juvenile-onset diabetes (Wang et al. 2006, Jensen et al. 2007), these findings make in vitro models useful in the search for downstream target genes of NGN3 expression in human (Heremans et al. 2002, Mellitzer et al. 2006) and in mouse cell types (Gasa et al. 2004).
Our in vitro cell line model to study the downstream consequences of NGN3 expression is an imperfect replica of human foetal pancreatic progenitors. In our experience, PANC-1 cells lack PDX1 protein. On the microarray, hybridisation signal was weak for PDX1 and unaltered by the dox treatment. However, similar in vitro models have been used by others (Gasa et al. 2004) and PDX1 was absent in the NGN3-positive foetal pancreatic cells both in this study and in mouse (Schwitzgebel et al. 2000). Our cloned cells did uniformly express SOX9 and CK19 mimicking foetal pancreatic progenitor cells and an adult ductal phenotype. Nevertheless, by the ectopic expression of a single gene in a tumour cell line, it is inconceivable to generate bona fide β-cell precursors with complete, faithful gene expression profiles. Hence, our model of inducible NGN3 expression was used as a tool to identify downstream candidate markers of human β-cells for validation in the native cell type. NGN3 expression promptly activated NEUROD1, which encodes a transcription factor that has been linked causally with various forms of non-autoimmune diabetes (Frayling et al. 2001). Our model also allowed detection of RAB3B, which was subsequently shown to localise robustly to the human foetal and adult β-cells.
There are four RAB3 family members. Regazzi et al. (1996) previously showed RAB3B and RAB3C, but not RAB3A, by immunoblotting of whole human islets, comprising multiple hormone-secreting cell types. At that time, RAB3D was not assessed. In rat and mouse, Rab3a and Rab27a are very important regulators of exocytosis, both in vitro and in vivo, in β-cells (Regazzi et al. 1996, Yi et al. 2002, Waselle et al. 2003, Yaekura et al. 2003, Kasai et al. 2005, Abderrahmani et al. 2006). Through the interaction with a network of interacting proteins, they coordinate the intracellular trafficking of insulin granules that culminate in docking at the cell membrane and insulin release (Yi et al. 2002, Waselle et al. 2003). Rab3a and Rab27a knockout mice show defects in glucose-stimulated insulin secretion (GSIS) similar to those observed in patients with type 2 diabetes and in mice lacking the glucagon-like peptide-1 (GLP-1) receptor (Scrocchi et al. 1996, Yaekura et al. 2003, Aizawa & Komatsu 2005, Kasai et al. 2005). Furthermore, hyperglycaemia drastically reduced levels of Rab3a and Rab27a protein in rat β-cells as a consequence of expression of the transcriptional repressor, inducible cAMP early repressor (Abderrahmani et al. 2006). It transpires that GLP-1 potentiation of insulin secretion requires a complex between the cAMP sensor protein cAMP–GEFII, bound to the sulphonylurea receptor 1, a protein called Piccolo and Rab-interacting molecule 2 (RIM2; Ozaki et al. 2000, Kashima et al. 2001, Fujimoto et al. 2002, Shibasaki et al. 2004). RIM2 then interacts with the RAB protein at the insulin granule, thus linking GLP-1 signalling and events at the ATP-sensitive potassium channel to insulin secretion. This emerging understanding of an important mechanism underlying GSIS makes the identification of the prevalent RAB isoform(s) in human β-cells important.
In this study, RAB3B was the only family member induced as a consequence of NGN3 expression. Its induction by NGN3 occurred later than that of NEUROD1, which, along with the non-overlapping expression profiles in developmental material, implies indirect regulation of RAB3B by NGN3. We show that RAB3B was extensively localised to β-cells both during human foetal development and in adult islets with some expression in some adult α- and δ-cells. Analysis of human adult islets demonstrated RAB3B expression to be approximately equal to the transcript numbers of β-ACTIN, present in all islet cell types, and greatly in excess of those for RAB3A, RAB3C, RAB3D and RAB27A. The level of RAB3B transcripts detected equates to ∼20–50% of those which we have previously found for insulin (data not shown). In the mouse central nervous system, Rab3 family members regulate the exocytosis of neurotransmitters in a redundant manner, all isoforms needing inactivation to generate an epileptic phenotype (Schluter et al. 2004). This raises the question of whether redundant function could also affect pancreatic β-cell function. Although feasible, this seems unlikely: RAB3A and RAB3C transcripts were only weakly detected and RAB3D was not detected in islets whereas in the central nervous system, under normal conditions, all Rab3 isoforms are expressed (Schluter et al. 2004); in in vitro analysis of rat melanotrophs, Rab3b could not substitute for the function of Rab3a (Rupnik et al. 2007); inactivation of either Rab3a or Rab27a in isolation produced glucose intolerance in mice (implying non-redundant function; Yaekura et al. 2003, Kasai et al. 2005); over-expression of Rab3a and Rab27a protein in MIN6 cells generated different effects on insulin secretion (Yi et al. 2002); and in dopaminergic nerves, over-expressing RAB3B, but not RAB3A, was protective in the models of Parkinson's disease (Chung et al. 2009). Interestingly, on searching the Unigene database, Rab3b is absent from cDNA libraries generated from mouse or rat insulinoma cell lines. Twelve of the 13 pancreatic clones for human RAB3B arise from the islet or insulinoma sources. Conversely, RAB3A, inactivation of which causes glucose intolerance in mice (Yaekura et al. 2003), has not been identified from Unigene human islet or insulinoma cDNA libraries.
There is a clear involvement of mutations in the pathway between the ATP-sensitive potassium channel and insulin secretion as causes of permanent neonatal diabetes (Gloyn et al. 2004). RAB3B localises to 1p31–p32, a locus previously linked to glucose intolerance and diabetes (Hsueh et al. 2003). We have conducted preliminary screens in three cases of permanent neonatal diabetes with linkage to this locus without identifying causative mutations in the RAB3B coding region. Irrespective of this, the potential for agents targeted at RAB function as novel potentiators of GSIS has already been proposed (Aizawa & Komatsu 2005). It will be important to ensure such efforts target the appropriate protein. In this study, we identified RAB3B as an indirect target of NGN3 expression. Under normal circumstances, RAB3B is clearly the predominant family member in human islets representing a significant difference in gene expression across species. Specifically, neither RAB3A nor RAB27A appears as likely to play the important role in human β-cells that has been described for the equivalent rodent cells (Regazzi et al. 1996, Yi et al. 2002, Waselle et al. 2003, Kasai et al. 2005, Abderrahmani et al. 2006).
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1677/JOE-10-0120.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was funded by an innovation grant from the Juvenile Diabetes Research Foundation (JDRF). NH is a Wellcome Trust Senior Fellow in Clinical Science and received support from the Wellcome Trust (in partnership with JDRF). NH and KPH received support from the Manchester NIHR Biomedical Research Centre.
Acknowledgements
The authors are grateful to Anne Chad and colleagues at the Quays Clinic and the Princess Anne Hospital for the collection of human foetal material and to Dr Guo-Cai Huang, Dr Shanta Persaud and Prof. Stephanie Amiel and Prof. Peter Jones (all King's College Hospital, London, UK) for supplying the human islets used in this study.
Footnotes
(K Piper Hanley and T Hearn contributed equally to this work)
References
- Abderrahmani A, Cheviet S, Ferdaoussi M, Coppola T, Waeber G, Regazzi R. ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis. EMBO Journal. 2006;25:977–986. doi: 10.1038/sj.emboj.7601008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa T, Komatsu M. Rab27a: a new face in beta cell metabolism-secretion coupling. Journal of Clinical Investigation. 2005;115:227–230. doi: 10.1172/JCI24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Babu DA, Chakrabarti SK, Garmey JC, Mirmira RG. Pdx1 and BETA2/NeuroD1 participate in a transcriptional complex that mediates short-range DNA looping at the insulin gene. Journal of Biological Chemistry. 2008;283:8164–8172. doi: 10.1074/jbc.M800336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun T, He KH, Lupi R, Boehm B, Wojtusciszyn A, Sauter N, Donath M, Marchetti P, Maedler K, Gauthier BR. The diabetes-linked transcription factor Pax4 is expressed in human pancreatic islets and is activated by mitogens and GLP-1. Human Molecular Genetics. 2008;17:478–489. doi: 10.1093/hmg/ddm325. [DOI] [PubMed] [Google Scholar]
- Castaing M, Duvillie B, Quemeneur E, Basmaciogullari A, Scharfmann R. Ex vivo analysis of acinar and endocrine cell development in the human embryonic pancreas. Developmental Dynamics. 2005;234:339–345. doi: 10.1002/dvdy.20547. [DOI] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Hallett PJ, Isacson O. Functional enhancement and protection of dopaminergic terminals by RAB3B overexpression. PNAS. 2009;106:22474–22479. doi: 10.1073/pnas.0912193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghazi L, Cras-Meneur C, Czernichow P, Scharfmann R. Role for FGFR2IIIb-mediated signals in controlling pancreatic endocrine progenitor cell proliferation. PNAS. 2002;99:3884–3889. doi: 10.1073/pnas.062321799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling TM, Evans JC, Bulman MP, Pearson E, Allen L, Owen K, Bingham C, Hannemann M, Shepherd M, Ellard S, et al. β-Cell genes and diabetes: molecular and clinical characterization of mutations in transcription factors. Diabetes. 2001;50(Suppl 1):S94–S100. doi: 10.2337/diabetes.50.2007.S94. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP–GEFII.Rim2.Piccolo complex in cAMP-dependent exocytosis. Journal of Biological Chemistry. 2002;277:50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- Gasa R, Mrejen C, Leachman N, Otten M, Barnes M, Wang J, Chakrabarti S, Mirmira R, German M. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. PNAS. 2004;101:13245–13250. doi: 10.1073/pnas.0405301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. New England Journal of Medicine. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Jr, Scheller RH. Regulation of membrane trafficking: structural insights from a Rab/effector complex. Cell. 1999;96:755–758. doi: 10.1016/S0092-8674(00)80585-1. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. PNAS. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Hanley NA, Rainey WE, Wilson DI, Ball SG, Parker KL. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Molecular Endocrinology. 2001;15:57–68. doi: 10.1210/me.15.1.57. [DOI] [PubMed] [Google Scholar]
- Hay CW, Docherty K. Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes. 2006;55:3201–3213. doi: 10.2337/db06-0788. [DOI] [PubMed] [Google Scholar]
- Heremans Y, Van De Casteele M, in't Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. Journal of Cell Biology. 2002;159:303–312. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh WC, St Jean PL, Mitchell BD, Pollin TI, Knowler WC, Ehm MG, Bell CJ, Sakul H, Wagner MJ, Burns DK, et al. Genome-wide and fine-mapping linkage studies of type 2 diabetes and glucose traits in the Old Order Amish: evidence for a new diabetes locus on chromosome 14q11 and confirmation of a locus on chromosome 1q21–q24. Diabetes. 2003;52:550–557. doi: 10.2337/diabetes.52.2.550. [DOI] [PubMed] [Google Scholar]
- Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Molecular and Cellular Biology. 2000;20:3292–3307. doi: 10.1128/MCB.20.9.3292-3307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GC, Zhao M, Jones P, Persaud S, Ramracheya R, Lobner K, Christie MR, Banga JP, Peakman M, Sirinivsan P, et al. The development of new density gradient media for purifying human islets and islet-quality assessments. Transplantation. 2004;77:143–145. doi: 10.1097/01.TP.0000100401.62912.B2. [DOI] [PubMed] [Google Scholar]
- Jensen JN, Rosenberg LC, Hecksher-Sorensen J, Serup P. Mutant neurogenin-3 in congenital malabsorptive diarrhea. New England Journal of Medicine. 2007;356:1781–1782. doi: 10.1056/NEJMc063247. [DOI] [PubMed] [Google Scholar]
- Johnson D, Shepherd RM, Gill D, Gorman T, Smith DM, Dunne MJ. Glucokinase activators: molecular tools for studying the physiology of insulin-secreting cells. Biochemical Society Transactions. 2007;35:1208–1210. doi: 10.1042/BST0351208. [DOI] [PubMed] [Google Scholar]
- Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. Journal of Clinical Investigation. 2005;115:388–396. doi: 10.1172/JCI22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP–GEFII-Rim2 complex in incretin-potentiated insulin secretion. Journal of Biological Chemistry. 2001;276:46046–46053. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. PNAS. 2007;104:10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald TJ, Tu E, Brenner S, Zabel P, Behme M, Panchal C, Hramiak I, Barnett WB, Miller D, Dupre J. Canine, human, and rat plasma insulin responses to galanin administration: species response differences. American Journal of Physiology. 1994;266:E612–E617. doi: 10.1152/ajpendo.1994.266.4.E612. [DOI] [PubMed] [Google Scholar]
- McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia. 2001;44:1203–1214. doi: 10.1007/s001250100628. [DOI] [PubMed] [Google Scholar]
- Mellitzer G, Bonne S, Luco RF, Van De Casteele M, Lenne-Samuel N, Collombat P, Mansouri A, Lee J, Lan M, Pipeleers D, et al. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO Journal. 2006;25:1344–1352. doi: 10.1038/sj.emboj.7601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development. 1998;125:1017–1024. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–438. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- Ostrer H, Wilson DI, Hanley NA. Human embryo and early fetus research. Clinical Genetics. 2006;70:98–107. doi: 10.1111/j.1399-0004.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, et al. cAMP–GEFII is a direct target of cAMP in regulated exocytosis. Nature Cell Biology. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- Persaud SJ, Roderigo-Milne HM, Squires PE, Sugden D, Wheeler-Jones CP, Marsh PJ, Belin VD, Luther MJ, Jones PM. A key role for beta-cell cytosolic phospholipase A(2) in the maintenance of insulin stores but not in the initiation of insulin secretion. Diabetes. 2002;51:98–104. doi: 10.2337/diabetes.51.1.98. [DOI] [PubMed] [Google Scholar]
- Piper K, Ball SG, Keeling JW, Mansoor S, Wilson DI, Hanley NA. Novel SOX9 expression during human pancreas development correlates to abnormalities in Campomelic dysplasia. Mechanisms of Development. 2002;116:223–226. doi: 10.1016/S0925-4773(02)00145-4. [DOI] [PubMed] [Google Scholar]
- Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. Journal of Endocrinology. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- Piper Hanley K, Oakley F, Sugden S, Wilson DI, Mann DA, Hanley NA. Ectopic SOX9 mediates extracellular matrix deposition characteristic of organ fibrosis. Journal of Biological Chemistry. 2008;283:14063–14071. doi: 10.1074/jbc.M707390200. [DOI] [PubMed] [Google Scholar]
- Polak M, Bouchareb-Banaei L, Scharfmann R, Czernichow P. Early pattern of differentiation in the human pancreas. Diabetes. 2000;49:225–232. doi: 10.2337/diabetes.49.2.225. [DOI] [PubMed] [Google Scholar]
- Potikha T, Kassem S, Haber EP, Ariel I, Glaser B. p57Kip2 (cdkn1c): sequence, splice variants and unique temporal and spatial expression pattern in the rat pancreas. Laboratory Investigation. 2005;85:364–375. doi: 10.1038/labinvest.3700229. [DOI] [PubMed] [Google Scholar]
- Ramracheya RD, Muller DS, Squires PE, Brereton H, Sugden D, Huang GC, Amiel SA, Jones PM, Persaud SJ. Function and expression of melatonin receptors on human pancreatic islets. Journal of Pineal Research. 2008;44:273–279. doi: 10.1111/j.1600-079X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Regazzi R, Ravazzola M, Iezzi M, Lang J, Zahraoui A, Andereggen E, Morel P, Takai Y, Wollheim CB. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. Journal of Cell Science. 1996;109:2265–2273. doi: 10.1242/jcs.109.9.2265. [DOI] [PubMed] [Google Scholar]
- Richardson CC, Hussain K, Jones PM, Persaud S, Lobner K, Boehm A, Clark A, Christie MR. Low levels of glucose transporters and K+ATP channels in human pancreatic beta cells early in development. Diabetologia. 2007;50:1000–1005. doi: 10.1007/s00125-007-0644-x. [DOI] [PubMed] [Google Scholar]
- Rupnik M, Kreft M, Nothias F, Grilc S, Bobanovic LK, Johannes L, Kiauta T, Vernier P, Darchen F, Zorec R. Distinct role of Rab3A and Rab3B in secretory activity of rat melanotrophs. American Journal of Physiology. Cell Physiology. 2007;292:C98–C105. doi: 10.1152/ajpcell.00005.2006. [DOI] [PubMed] [Google Scholar]
- Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. Journal of Neuroscience. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit FC. Is GLUT2 required for glucose sensing? Diabetologia. 1997;40:104–111. doi: 10.1007/s001250050651. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nature Medicine. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. PNAS. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. Journal of Biological Chemistry. 2004;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- Smith SB, Watada H, German MS. Neurogenin3 activates the islet differentiation program while repressing its own expression. Molecular Endocrinology. 2004;18:142–149. doi: 10.1210/me.2003-0037. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. New England Journal of Medicine. 2006;355:270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- Waselle L, Coppola T, Fukuda M, Iezzi M, El-Amraoui A, Petit C, Regazzi R. Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Molecular Biology of the Cell. 2003;14:4103–4113. doi: 10.1091/mbc.E03-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mechanisms of Development. 2003;120:65–80. doi: 10.1016/S0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, Poitout V, Baskin DG, Wang Y, Philipson LH, et al. Insulin secretory deficiency and glucose intolerance in Rab3A null mice. Journal of Biological Chemistry. 2003;278:9715–9721. doi: 10.1074/jbc.M211352200. [DOI] [PubMed] [Google Scholar]
- Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Molecular and Cellular Biology. 2002;22:1858–1867. doi: 10.1128/MCB.22.6.1858-1867.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich WS, Bonnet-Eymard M, Zawalich KC. Glucose-induced desensitization of the pancreatic beta-cell is species dependent. American Journal of Physiology. 1998;275:E917–E924. doi: 10.1152/ajpendo.1998.275.6.E917. [DOI] [PubMed] [Google Scholar]