Abstract
Proteases are a ubiquitous group of enzymes that play key roles in the life cycle of parasites, in the host-parasite relationship, and in the pathogenesis of parasitic diseases. Furthermore, proteases are druggable targets for the development of new anti-parasitic therapy. The subtilisin protease (SUB; Clan SB, family S8) of Leishmania donovani was cloned and found to possess a unique catalytic triad. This gene was then deleted by gene knock-out, which resulted in reduced ability by the parasite to undergo promastigote to amastigote differentiation in vitro. Electron microscopy of SUB knock-out amastigotes revealed abnormal membrane structures, retained flagella, and increased binucleation. SUB-deficient Leishmania displayed reduced virulence in both hamster and murine infection models. Histology of spleens from SUB knock-out-infected hamsters revealed the absence of psammoma body calcifications indicative of the granulomatous lesions that occur during Leishmania infection. To delineate the specific role of SUB in parasite physiology, two-dimensional gel electrophoresis was carried out on SUB−/− versus wild-type parasites. SUB knock-out parasites showed altered regulation of the terminal peroxidases of the trypanothione reductase system. Leishmania and other trypanosomatids lack glutathione reductase, and therefore rely on the novel trypanothione reductase system to detoxify reactive oxygen intermediates and to maintain redox homeostasis. The predominant tryparedoxin peroxidases were decreased in SUB−/− parasites, and higher molecular weight isoforms were present, indicating altered processing. In addition, knock-out parasites showed increased sensitivity to hydroperoxide. These data suggest that subtilisin is the maturase for tryparedoxin peroxidases and is necessary for full virulence.
Keywords: Parasite, Peroxidase, Protease, Serine Protease, Trypanosome, Leishmania, Subtilisin, Trypanothione, Tryparedoxin, Donovani
Introduction
Protozoan parasites of the genus Leishmania cause a variety of vector-borne diseases in vertebrates, including cutaneous, mucocutaneous, and visceral leishmaniases in humans. Due to the lack of safe and effective treatments for this disease, leishmaniasis is classified by the World Health Organization as a Tropical Disease Research Category I disease, an emerging or uncontrolled disease (1). This kinetoplastid parasite has a relatively simple dimorphic life cycle consisting of promastigote and amastigote stages. Leishmania promastigotes multiply extracellularly as spindle-shaped flagellates in the midgut of the phlebotomine sandfly vector. The parasites are then transmitted to a mammalian host when an infected sandfly bites to take in a blood meal. In the naïve host the parasites infect macrophages and differentiate into amastigotes. This form of the parasite is an ovoid intracellular aflagellate. Throughout its life cycle, Leishmania is exposed to a variety of reactive oxygen species that it must detoxify to survive. Antioxidant defense is particularly important for amastigotes, because they must also survive the oxidative burst generated by the host macrophages (2).
Recent advances in parasite molecular biology and bioinformatics have enabled us to strategically identify and study Leishmania proteins as therapeutic targets. Parasite proteases are viable drug targets, because many of them are required for the pathogenic life cycle of the parasite (3, 4). We have identified an unusual Clan SB, family S8 subtilisin-like serine protease in Leishmania as one of these therapeutic targets. This family of endopeptidases is conserved across all biological kingdoms (5). Subtilisins are protein-processing enzymes and known virulence factors for both Plasmodium and Toxoplasma parasites (6). In Plasmodium falciparum a subtilisin-like serine protease is required for erythrocyte egress by infectious merozoites (7, 8) and is believed to be the convertase for the maturation of merozoite surface protein 1 and SERA proteins (9, 10). In Toxoplasma gondii a subtilisin is involved in rhoptry organelle protein processing (11, 12).
In this study we describe the identification and phenotypic characterization of Leishmania subtilisin. This protease was found to process the terminal peroxidases of the trypanothione reductase system. This system plays an important role in Leishmania survival within host macrophages and is being intensely studied as a target for antiparasitic drug development (13). This study has found that subtilisin is an important regulator of this system and is key for parasite infectivity and virulence.
EXPERIMENTAL PROCEDURES
Animals and Parasite Strains
Commercially bred, 6- to 8-week-old, female BALB/c mice (Mus musculus) were used for the murine footpad infection model (Charles River Laboratories International, Inc., Davis, CA). Commercially bred, 4- to 5-week-old, male Golden Syrian hamsters (Mesocricetus auratus) were used for the visceral infection model (Simonsen Laboratories, Inc., Gilroy, CA). Leishmania donovani donovani MHOM/ET/67/HU3 cloned stock and Leishmania major LV39 MRHO/SU/59/P were used for knock-out studies and for animal infections. Leishmania promastigotes were cultured at 27 °C in M199 (Sigma) liquid medium as previously described (14). Axenic amastigotes of L. donovani were cultured at 37 °C in 100% fetal bovine serum (Omega Scientific Inc., Tarzana, CA) as previously described (15).
Subtilisin Cloning and Sequencing
Genomic DNA from L. donovani was isolated as previously described (16). The subtilisin gene was then amplified from this genomic DNA by PCR using the Expand High Fidelity PCR System (Roche Diagnostics, Indianapolis, IN) in two overlapping pieces, termed the 5′ and 3′ halves. For each half, an external primer from the non-coding flanks of the gene and an internal primer were designed based on regions of identity between the known L. infantum and L. major sequences (Sanger Institute GeneDB: LinJ13_V3.0940 and LmjF13.1040). Both halves were cloned into the pGEM-7Zf(−) vector (Promega, Madison, WI) and then spliced together using an internal HindIII site. The open reading frame was sequenced using the following primers: 5′-CAT GCA TCA GCC GGT AC-3′, 5′-GCG GCA TGG TCA TCT AC-3′, 5′-TAC TCA CAA TCT CTA CG-3′, 5′-CAC CAG TAA GAG TGC GG-3′, 5′-GAA GAG CCG CCA CCG TG-3′, 5′-CGT GCT GGC AGG ACA GC-3′, 5′-TCC TCT TTG AGG GTG CG-3′, 5′-TAG CCA TAG CCC ACC GC-3′, 5′-CTC CTC TTT GAG GGT GC-3′, 5′-CGC TCT GTC TCG AGG CG-3′, 5′-GTG TGG GGC AGC GGC AG-3′, 5′-ACC GTT GGC TGT CAG AG-3′, 5′-CGT TAG GAG ACG CCG CA-3′, 5′-CGT CGT CAG CAC AAG AG-3′, 5′-ACC CAC CTT CCG CTT CG-3′, 5′-GTG CCA GCA GAC CAC GG-3′, 5′-TAG GCA GCG GTG CCG AC-3′, 5′-AAC GGC AGC AGG CTC TC-3′, 5′-GTC GGC ACC GCT GCC TA-3′, 5′-ATC GGC TAT AGG ATT CC-3′, 5′-CAG TAG CCC GCA GGT GC-3′, 5′-CGT TGT CTG TGC CGA CC-3′, and 5′-ACT GAT CAG CCA AGG CG-3′. The amino acid sequence of L. donovani subtilisin catalytic core was identified using Pfam (accession number PF00082, Subtilase family) and was aligned with homologous sequences from L. infantum, L. major, L. braziliensis, T. cruzi (1 and 2), T. brucei (1 and 2), P. falciparum (1, 2, and 3), T. gondii (1a, 1b, and 2), B. licheniformis, B. amyloliquefaciens, B. subtilis, H. sapiens (furin and Site-1), M. musculus, S. cerevisiae, S. pombe, C. intestinalis, A. mellifera, X. laevis, and D. rerio (respectively: LinJ13_V3.0940, LmjF13.1040, LbrM13_V2.0860, Tc00.1047053511045.40, Tc00.1047053511859.60, Tb11.02.1280, Tb927.3.4230, CAD51440, XP_001348051, CAD51437, XP_002370002, XP_002368971, XP_002364650, P00780, P00782, P04189, P09958, EAW95506, P23188, P13134, Q09175, XP_002122807, XP_395754, NP_001087381, and CAK04389) using the ClustalW algorithm from MegAlign (DNASTAR, Madison, WI).
Expression and Purification of Recombinant SUB from Pichia pastoris
Transformation constructs were generated by PCR amplification of L. donovani and L. major SUB2 cores, adding a 5′ SalI site (bold) followed by a Kex2 cleavage site (underlined) and a 3′ SpeI site (underlined bold) using forward (L.d.: 5′-CTC GTC GAC AAA AGA GCA CAC CGT TCC ACA GAT GCG-3′; L.m.: CTC GTC GAC AAA AGA GCA CGC CGT TCC ACC GAT GCG) and reverse (L.d.: 5′-CTC ACT AGT TCA ACA CGG GCA AGT CGA TTC TGA C-3′; L.m.: 5′-CTC ACT AGT TCA ACA CGA GAG AGT CGA TTC TGA CG-3′) primers, and then cloned into pPICZa A (Invitrogen). The pPICZα-SUB constructs were electroporated into X-33 P. pastoris, and expression clones were isolated and induced for 96 h as per the manufacturer's protocol. For each L. donovani and L. major SUB, three clones were independently evaluated for protease activity. Supernatant from induced cultures was harvested by centrifugation at 3,000 × g for 10 min, followed by 0.2-μm filtration (Nalge Nunc). Expressed SUB protein was then buffer-exchanged with 50 mm Tris-HCl, pH 7.5, and concentrated on an Amicon Ultra-4 10,000 NMWL filter device (Millipore, Billerica, MA). This concentrate was then fractionated by hydrophobic interaction. The sample was diluted 1:1 to a final buffer concentration 30 mm Tris-HCl, pH 8.0, with 1 m ammonium sulfate and loaded onto a HiTrap Octyl-Sepharose 4FF hydrophobic interaction column (Amersham Biosciences). A 10 mm Tris-HCl, pH 8.0, buffer with 1 m ammonium sulfate was used for column equilibration, sample loading, and washing at a 1 ml/min flow rate. The ammonium sulfate concentration was decreased from 1–0 m over 40 column volumes to elute the SUB protein. The eluate was desalted by buffer exchanging with 50 mm Tris-HCl, pH 7.5, and concentrated by Amicon. SUB protein concentration was measured by inhibitor titration with PPACK (H-d-Phe-Pro-Arg-CMK) and NanoDrop 1000.
Protease activity was measured using peptide substrates containing C-terminal 7-amino-4-carbamoylmethylcoumarin (AMC) as the fluorogenic leaving group. The synthetic substrates Z-VFRSLK-AMC, Z-RVRR-AMC, and Z-RR-AMC were used. Initial test reactions contained 20 μm substrate. For Km determination VFRSLK was serially diluted from 100–0.05 μm, and RVRR was serially diluted from 20 to 0.025 μm. Enzyme samples were mixed with substrate in 50 mm Tris-HCl, pH 7.5, with 0.2% DMSO in 150 μl of total volume. Hydrolysis of the substrates was measured at 25 °C using a FlexStation microplate spectrofluorometer (Molecular Devices, Sunnyvale, CA). Excitation/emission for AMC and 7-amino-4-carbamoyl-methylcoumarin were 355/460 nm and 380/460 nm, respectively. Vmax values were calculated using the accompanying SoftMax Pro v4.8 software.
Southern and Northern Blot Analyses
For Southern blot analysis, genomic DNA was digested with indicated restriction endonucleases (New England Biolabs, Ipswich, MA and Roche Diagnostics, Indianapolis, IN), and fragments were separated by electrophoresis on a 0.6% agarose gel (17). These were then transferred to Hybond-N+ (Amersham Biosciences) nylon filters by the manufacturer's instructions for alkali transfer. Southern blot probes were 32P-labeled using a Rediprime II Random Prime Labeling System (Amersham Biosciences) as per the manufacturer's instructions. Hybridization and washing conditions were performed as previously described (18). RNA was isolated from L. donovani promastigotes and axenic amastigotes (19). RNA (5 μg/lane) was size-fractionated, and Northern blot hybridization was performed as previously described.
Constructs for Targeted Gene Deletion of SUB
Two knock-out cassettes (one for each allele) were created to delete the L. donovani and L. major SUB genes. These cassettes each contained an antibiotic resistance gene, hygr (conferring hygromycin B resistance) (20), pacr (conferring puromycin resistance, used for L. donovani only) (21), or satr (conferring nourseothricin resistance, L. major only) (22), followed by 1.5 kb of the 3′-untranslated region of the L. major dhfr-ts gene (23). This untranslated region ensures high level expression during the life cycle of Leishmania. To target these knock-out cassettes to the SUB locus, 5′ and 3′ targeting flanks were created and ligated into their respective sides of the cassettes. These targeting flanks were generated by PCR amplification of the untranslated regions directly 5′ of the SUB ORF and 3′ of the SUB catalytic core (L. donovani) or 3′ of the ORF (L. major). PCR primers were designed based on the published L. infantum and L. major sequences (GeneDB): L. donovani 5′ flank forward (5′-CTC ACT AGT CGC CTC CTC GTC GTC GCA CTC-3′) and reverse (5′-CTC TCT AGA CAC CAC TAC CTC AAT CGG AGC G-3′) (1.3-kb fragment), 3′ flank forward (5′-CTC ACT AGT TGG TCA TCT ACG TCC GCT GTA GC-3′) and reverse (5′-CTC TCT AGA CGT GCC CTG ATC TGC GGC AGC-3′) (0.7-kb fragment); L. major 5′ flank forward (5′-CTC ACT AGT TGC GCA ACC ACA GCG GTC ATC-3′) and reverse (5′-CTC TCT AGA TAC CTC AAT GGG AGC GTG CTT G-3′) (1.5-kb fragment), 3′ flank forward (5′-CTC ACT AGT TCG TTG GAG AGG CCA ACG CGC-3′) and reverse (5′-CTC TCT AGA CGA GTA GGA AGA GGT GAC CGT C-3′) (0.8-kb fragment) primers (SpeI sites, in bold, and XbaI sites, underlined, were included for cloning). These constructs were maintained and amplified in the pGEM-9Zf(−) vector (Promega). For targeted gene deletion, 50 μg of the targeting constructs was excised from their vectors using the flanking restriction endonucleases SpeI and XbaI (New England Biolabs) and purified by electrophoresis on 0.8% agarose gels then purified using the QIAEX II Gel Extraction Kit (Qiagen Inc., Valencia, CA).
Leishmania Transfections and Clone Isolation
Purified transfection constructs described above were used to transfect log phase Leishmania promastigotes by electroporation (2.25 kV/cm, 500 microfarads) as previously described (23). After electroporation, the cells were grown and selected using hygromycin B, puromycin, or nourseothricin on both plates and in liquid media and clones were isolated as previously described (24).
Replication Rates and in Vitro Differentiation
Day 4 SUB knock-out and wild-type parasites were split in triplicate into new M199 (for promastigote replication rates) and into 37 °C fetal bovine serum (for axenic amastigote replication). Parasite culture densities were determined on days 1–4 post-split by cell counting on a Multisizer 3 Coulter Counter (Beckman Coulter, Inc., Fullerton, CA). Axenic amastigote differentiation was observed by microscopy.
Transmission Electron Microscopy
Approximately 108 day 4 L. donovani axenic amastigotes from wild-type or SUB−/− cultures were pelleted and washed 3× in PBS. The parasites were processed for conventional EM by freeze-substitution in 1% OsO4/0.1% uranyl acetate in acetone and embedded in Epon resin. Sections were cut with a Leica Ultracut UCT Ultramicrotome (Leica Microsystems, Bannockburn, IL) and viewed on a Tecnai T20 electron microscope (FEI Co., Hillsboro, OR) with a 4000 × 4000 UltraScan charge-coupled device camera (Gatan Inc., Pleasanton, CA).
Hamster and Mouse Infections
Hamsters were infected intraperitoneally with 109 day 4 SUB knock-out or wild-type L. donovani promastigotes (groups of three) (25). Animals were weighed weekly over the length of the experiment. Hamsters were culled 200 days post-infection by CO2 inhalation followed by thoracotomy. Pieces of each spleen and liver were fixed in 10% formalin in PBS and then embedded in paraffin for histology. Sections were cut at 5 μm and stained with either Wright-Giemsa or hematoxylin and eosin by the University of California at San Francisco (UCSF) Morphology Core using standard protocols. Psamomma bodies were identified by microscopy and counted. Mice were infected with metacyclic L. major promastigotes purified from day 4 SUB+/− and wild-type cultures using negative selection by binding to peanut agglutinin as has been previously described (26). BALB/c mice (groups of 5) were anesthetized by isoflurane inhalation and infected subcutaneously in the left hind footpad with 5 × 106 metacyclic promastigotes in 50 μl of Hanks' balanced salt solution. Footpad swelling was measured weekly after inoculation using a Mitutoyo caliper. Parasites were recovered from infected mice by resection of the left popliteal lymph node.
Two-dimensional Gel Electrophoresis
Three experimental replicates were prepared from separately cultured samples of both wild-type and SUB knock-out L. donovani. Approximately 109 cells were pelleted, washed 3× with PBS, and stored at −80 °C. Lysates were prepared by resuspending the cell pellets in 2 ml of native lysis buffer containing 20 mm HEPES, pH 7.5, 250 mm sucrose, 3 mm MgCl2, 0.5% Nonidet P-40, 1 mm DTT, and 1× Halt EDTA-free protease inhibitor mixture (Pierce). Cells were then broken by mechanical lysis using 70 strokes of a Dounce homogenizer. The lysates were centrifuged at 12,000 × g for 20 min at 4 °C, and the clarified supernatants were dialyzed overnight against 50 mm Tris-HCl, pH 7.5, 100 mm NaCl using 8-kDa molecular weight cut-off dialysis membranes. The following day the protein samples were concentrated and washed by precipitation using the ReadyPrep 2-D Cleanup Kit (Bio-Rad Laboratories, Inc., Hercules, CA). Approximately 300 μg of protein per gel was brought up to 300 μl in Bio-Rad Rehydration/Sample buffer and was passively loaded onto 17-cm, 3–10 pH immobilized pH gradient isoelectric focusing strips. Isoelectric focusing was performed using slow increases in voltage over multiple steps up to 10 kV for a total of 60–65 kVh focusing time. Next, the strips were reduced and alkylated using sequential 10-min incubations in 2% DTT then 2.5% iodoacetamide and dissolved in sample equilibration buffer. The isoelectric focusing strips were run in the second dimension on 17 × 17 cm, 12.5% acrylamide Tris-glycine, SDS-PAGE gels. Gels were stained with SYPRO Ruby and imaged using a Typhoon Trio Variable Mode Imager (Amersham Biosciences). These images were utilized for spot intensity analysis using Bio-Rad PDQuest software (v. 7.4). For proteomic analysis, gels were silver-stained (27), and selected protein spots were excised and in-gel-digested with trypsin (28, 29). The resulting peptides were extracted and analyzed by on-line liquid chromatography/mass spectrometry using an Eksigent nanoflow pump (Dublin, CA) coupled to a QStar Pulsar quadrupole orthogonal acceleration, time-of-flight hybrid instrument (Applied Biosystems, Foster City, CA). The reversed-phase chromatographic column was controlled with a Famos autoinjector (Sunnyvale, CA) and Eksigent software to run at a 5–50% acetonitrile gradient in 0.1% formic acid with a 350 nl/min flow rate. Data were analyzed in Analyst 2.0 software (Applied Biosystems) with the Mascot script 1.6b20 (Matrix Science, London, UK). Analyst-processing options for peak finding in spectrum were 0.5% default threshold, 400 Gaussian filter, and a Gaussian filter limit of 10; for TOF auto-centroiding: 20 ppm merge distance, 10 ppm minimum width, 50% percentage height, and 100 ppm maximum width. Default parameters were used except that “no de-isotoping” was selected and precursor mass tolerance for grouping was set to 0.2. Database searches were performed using ProteinProspector v. 5.3.0 (available on-line) using the Batch-Tag and Search Compare modules (30). Searches were performed on the SwissProt databank (December 16, 2008) to evaluate sample purity followed by searching TriTrypDB v. 1.0 beta (available on-line, January, 2009).
Hydroperoxide Sensitivity Assay
These assays were performed as was previously described (31). Stationary phase Leishmania promastigotes were split into 2-ml M199 at 2 × 106 per ml in the presence of different concentrations tert-butylhydroperoxide (Sigma). Culture densities were determined after 5–6 days by using the Coulter Counter. Relative density was calculated by normalizing to untreated controls.
RESULTS
L. donovani SUB Uses a Non-canonical Catalytic Triad
The gene encoding L. donovani SUB was cloned as described above. Sequencing yielded a 5,235-bp gene. This sequence was submitted to GenBankTM with accession number ADA81891. The resultant 1,744-amino acid protein has an estimated molecular mass of 184.7 kDa. This protein has a predicted signal peptide (SignalP V2.0 HMM probability of 0.995) with a cleavage site between amino acids 38–39 (0.421 probability), and a probable C-terminal transmembrane helix between amino acids 1709–1731 (TMHMM v. 2.0). The published L. major SUB (GeneDB: LmjF13.1040) shares this general layout; however, SUB from the more closely related L. infantum (a subspecies from within the L. donovani complex) has a C-terminal truncation after amino acid 1192 (LinJ13_V3.0940). The Pfam predicted Subtilase family core for L. donovani SUB is between amino acids 86–414. Comparisons of L. donovani SUB to other trypanosomatid SUBs are summarized in Table 1. Interestingly, L. donovani SUB has a non-canonical catalytic triad with the catalytic Glu in place of the standard Asp due to a single C to G base pare change. L. infantum SUB also has Glu in place of the Asp, indicating that this adaptation may be specific to parasites in the L. donovani complex. The SUB catalytic core amino acid sequences are relatively conserved within the Leishmania species; however, these sequences have diverged considerably from those of the trypanosomes, with only a 40% identity between the genera.
TABLE 1.
Comparison of predicted SUB proteins from trypanosomatids
L. donovani SUB was compared to the other known trypanosomatid SUB protein sequences using ClustalW2. The catalytic cores of each protein were determined by aligning them with the Pfam Subtilase family core (PF00082). Subtilisins have the catalytic triad Asp-His-Ser. L. donovani SUB has a non-canonical catalytic triad with a Glu in place of the standard Asp due to a single C to G base pair change. L. infantum SUB also has Glu in place of the Asp, indicating that this adaptation may be specific to parasites in the L. donovani complex. The SUB catalytic core amino acid (aa) sequences are relatively conserved within the Leishmania species; however, these sequences have diverged considerably from those of the trypanosomes, with only a 40% identity between the genera. L. infantum has a truncated C terminus. All the trypanosomatid SUB proteins fall between 126 and 191 kDa.
| Species | Gene | aa identity | Core identity | Length | Molecular mass | Catalytic triad |
|---|---|---|---|---|---|---|
| % | % | aa | kDa | |||
| L. donovani | SUB | 100.0 | 100.0 | 1744 | 184.7 | Glu-97, His-130, Ser-395 |
| L. infantum | SUB | 99.6 | 99.7 | 1192 | 126.2 | Glu-97, His-130, Ser-395 |
| L. major | SUB | 89.0 | 94.5 | 1722 | 182.7 | D99, His-132, Ser-397 |
| L. braziliensis | SUB | 70.0 | 83.0 | 1785 | 190.7 | D97, His-130, Ser-405 |
| T. cruzi | SUB1 | 23.1 | 40.3 | 1430 | 160.5 | D219, His-269, Ser-502 |
| T. cruzi | SUB2 | 23.4 | 40.1 | 1408 | 158.0 | D196, His-246, Ser-480 |
| T. brucei | SUB1 | 24.4 | 39.5 | 1487 | 161.5 | D191, His-238, Ser-476 |
To determine the subfamily of Leishmania subtilisin, the catalytic core sequence was compared with the cores of other Clan SB, family S8 family members. Core sequences were aligned using ClustalW2 (EMBL-EBI), and a phylogenetic tree was generated (Fig. 1). The Leishmania SUBs group with the subfamily S8A proteases, which include the eukaryotic Site-1 peptidases and the bacterial subtilisins. This distinguishes Leishmania SUB from the Toxoplasma and Plasmodium SUBs and from the subfamily S8B kexins and furins. Site-1 peptidases are restricted to metazoan organisms and are known to process sterol regulatory element binding proteins, which are not found in trypanosomatids (32).
FIGURE 1.
The catalytic cores of the Leishmania subtilisins were compared with the cores of other Clan SB, family S8 family members, as determined by Pfam. Core sequences were aligned using ClustalW2 (EMBL-EBI). The Leishmania SUBs group with the subfamily S8A proteases, which include the eukaryotic Site- 1 peptidases and the bacterial subtilisins. This distinguishes Leishmania SUB from the Toxoplasma and Plasmodium SUBs and from the subfamily S8B kexins and furins.
L. donovani and L. major SUBs Were Recombinantly Expressed in P. pastoris
The catalytic cores of the subtilisin proteins from L. donovani and L. major were successfully recombinantly expressed. Site-1 proteases, to which Leishmania SUB is most similar, have a requirement for a lysine or arginine in the P4 position. For this reason SUB activity was evaluated using synthetic substrates with and without P4 Arg. Cleavage of the synthetic substrates RVRR and VFRSLK was detected in all of the SUB-expressing Pichia supernatants compared with the X-33 background strain. Slight activity against the RR substrate was only detected in one L. major clone. In this clone the RVRR Vmax was over six times that of RR. Poor cleavage of RR and the lack of detected protease activity in the X-33 control strain indicate that the cleavage is not due to endogenous KEX2. These results show that, like the Site-1 proteases, Leishmania SUB prefers a basic P4 residue. L. donovani and L. major SUB was isolated from the Pichia supernatants, and kcat and Km values were determined for both RVRR and VFRSLK substrates (Table 2). Both enzymes catalyzed the RVRR substrate at about a 10-fold faster rate than VFRSLK. The L. donovani SUB had a similar affinity for both substrates, whereas L. major SUB had a 10-fold higher affinity for RVRR. Interestingly, L. donovani SUB had a much lower rate of catalysis for each substrate (126- and 62-fold lower for RVRR and VFRSLK, respectively) when compared with L. major SUB. This difference could potentially be due to the non-canonical catalytic triad of L. donovani.
TABLE 2.
Kinetic analysis of L. donovani and L. major SUB
The catalytic cores of L. donovani and L. major SUBs were recombinantly expressed in P. pastoris X-33. Isolated and concentrated proteases were tested for activity against the synthetic fluorogenic substrates RVRR and VFRSLK. Km and kcat parameters were calculated for each substrate using both enzymes. Both enzymes had a higher rate of catalysis for the RVRR substrate over VSRSLK. In addition, L. major SUB had a much higher rate of catalysis compared to L. donovani SUB for both substrates.
| AMC substrate |
L. donovani SUB |
L. major SUB |
||||
|---|---|---|---|---|---|---|
| kcat | Km | kcat/Km | kcat | Km | kcat/Km | |
| S−1 | μm | S−1 μm−1 | S−1 | μm | S−1mm−1 | |
| RRVR | 2.19 × 10−3 | 1.20 | 1.83 × 10−3 | 2.78 × 10−1 | 5.64 | 4.93 × 10−2 |
| VFRSLK | 1.88 × 10−4 | 0.91 | 2.07 × 10−4 | 1.17 × 10−2 | 50.21 | 2.33 × 10−3 |
Subtilisin (SUB) −/− Parasites Have Defects in Promastigote to Amastigote Differentiation
The published L. major and L. infantum genomes (GeneDB) indicate that Leishmania sp. contain a single copy of SUB per haploid genome. This was confirmed to be true in L. donovani and L. major by Southern blot analysis of genomic DNA (data not shown). Deletion of both alleles of the single copy gene was carried out by two rounds of targeted gene replacement in L. donovani and confirmed by Southern blot (Fig. 2). Only one allele could be deleted from L. major despite multiple attempts at targeting the second allele.
FIGURE 2.
Successful deletion of both genomic copies of the SUB gene was determined by Southern blot analysis. Digested genomic DNA from wild-type, single-copy, and double-copy knockouts were analyzed at the SUB locus for the presence of either the wild-type locus or the knock-out cassette. The blot was stripped and re-probed for the core of the SUB gene itself to ensure that the gene was not relocated to another site in the genome.
Wild-type, SUB+/−, and −/− L. donovani promastigotes were cultured at 27 °C in M199. These parasites replicated at a rate comparable to wild-type parasites. To test for the ability to differentiate into axenic amastigotes, stationary phase (day 5 post-split) promastigotes were split 1:10 in FBS at 37 °C. Wild-type and SUB+/− parasites differentiated readily, however SUB−/− did not (Fig. 3). The SUB−/− parasites remained as elongated, flagellated spindles. These cells did not form aggregates of cells typically seen in axenic amastigote cultures. To test for differentiation under lower dilution conditions, stationary phase SUB−/− promastigotes were diluted 1:2 in FBS at 37 °C. After 4 days, some of the cells appeared to differentiate; however, cell aggregation did not occur.
FIGURE 3.
Wild-type, SUB+/−, and −/− L. donovani promastigotes were cultured successfully at 27 °C in M199. To test for the ability to differentiate into axenic amastigotes, stationary phase (day 5 post-split) promastigotes of each culture were split 1:10 in FBS at 37 °C. Wild-type and SUB+/− parasites differentiated readily, however SUB−/− did not. The SUB−/− parasites remained as elongated, flagellated spindles. These cells did not form aggregates of cells typically seen in axenic amastigote cultures.
Electron Microscopy of SUB−/− Amastigotes Revealed Abnormal Ultrastructures
Wild-type and SUB−/− axenic amastigotes from the low dilution differentiation were processed for transmission electron microscopy. Wild-type axenic amastigotes had typical amastigote morphology: a rounded cell body measuring ∼3 μm in diameter, no external flagellum, and a condensed electron-dense kinetoplast (Fig. 4A). SUB−/− axenic amastigotes exhibited many abnormalities (Fig. 4, B–D). Although most of these cells had rounded cell bodies, many were still elongated and spindle-shaped. Those that were rounded often had invaginations in the plasma membrane. Additionally, many of these cells were binucleated. Unlike the wild-type amastigotes, the SUB−/− cells also had multiple flagellar cross sections, including flagella appearing in the cytoplasm, outside of their expected location within the flagellar pocket. These images indicate that the SUB−/− cells were not successfully differentiating into amastigotes.
FIGURE 4.
Wild-type axenic amastigotes (A) had normal rounded cell bodies measuring ∼3 μm in diameter (scale bar = 1 μm) with no external flagellum and a condensed electron-dense kinetoplast. SUB−/− axenic amastigotes (B–D) exhibited many abnormalities. Although most of these cells had rounded cell bodies, many were still elongated and spindle-shaped (D). Those that were rounded often had invaginations in the plasma membrane (B). Additionally, many of these cells were binucleated (C). Unlike the wild-type amastigotes, the SUB−/− cells also had multiple flagellar cross-sections, including flagella appearing in the cytoplasm (B), outside of their expected location within the flagellar pocket (N = nucleus, K = kinetoplast, FP = flagellar pocket, and F = flagellum).
SUB Regulates Levels of Peroxidases from the Trypanothione Reductase System
Site-1 peptidases and the subtilisins from apicomplexan parasites such as Toxoplasma and Plasmodium are maturases that process proteins within vesicles (9, 11, 33). The presence of a signal peptide and C-terminal transmembrane domain on Leishmania SUB indicates that it may perform a similar function. We employed two-dimensional gel electrophoresis to study the main differences in protein expression and processing between wild-type and SUB−/− parasites. Gels were run in triplicate (Fig. 5). On average, gels had ∼325 well defined spots, a value typical for two-dimensional gels of this size. These protein species represent up to 4% of the predicted 8,195 protein-coding genes from the L. donovani complex (34). Spots that differed in intensity between the wild-type and SUB−/− parasites were selected for peptide sequencing by mass spectrometry. The identities of these proteins are presented in Table 3. Interestingly, nine distinct spots were found to be tryparedoxin peroxidases, the terminal peroxidases of the trypanothione reductase system. In the L. donovani complex, this family of peroxidases is comprised of three cytoplasmic tryparedoxin peroxidases encoded in a multigene array, TXNPx1–3 (TRYP1–3), and a mitochondrial peroxidoxin, Prx (35). Interestingly, L. major encodes seven cytoplasmic tryparedoxin peroxidases, TXNPx1–7, in addition to the single Prx. These enzymes are all 2-Cys peroxiredoxins and have complementary roles in parasite protection against oxidative stress (31). L. donovani TXNPx1 and -3 share 99% amino acid identity and have 94% identity to TXNPx2 (GeneDB).
FIGURE 5.
Wild-type L. donovani had high levels of TXNPx1 and -3 forming a single doublet of spots (WT spots 4 and 5, left). The level of TXNPx2 was low (spot 3) and Prx was not detectable (spot 1). In the SUB−/− parasites (right), the wild-type TXNPx1/3 doublet decreased (SUB−/− spots 4 and 5) and two new TXNPx1/3 doublets were present at a higher molecular weight (spots 6 and 7) and at a higher molecular weight with a lower pI (spots 8 and 9). Prx levels (spots 1 and 2) were elevated in these parasites.
TABLE 3.
Two-dimensional gel analysis of WT and SUB−/−Leishmania
Spots that differed in intensity between the wild-type and SUB −/− parasites were selected for peptide sequencing by mass spectrometry. Nine distinct spots were found to be tryparedoxin peroxidases, the terminal peroxidases of the trypanothione reductase system. In the L. donovani complex, this family of peroxidases is composed of three cytoplasmic tryparedoxin peroxidases, TXNPx1–3 (TRYP1–3), and a mitochondrial peroxidoxin.
| WT |
SUB −/− |
|||
|---|---|---|---|---|
| Spot | Protein | Spot | Protein | Relative to WT |
| 1 | No Prx detected | 1 | Prx | Elevated |
| 2 | Prx | Elevated | ||
| 3 | TXNPx2 | 3 | TXNPx2 | Same |
| 4 | TXNPx1/3 | 4 | TXNPx1/3 | Decreased |
| 5 | TXNPx1/3 | 5 | TXNPx1/3 | Decreased |
| 6 | TXNPx1/3 | Elevated | ||
| 7 | No TXNPx detected | 7 | TXNPx1/3 | Elevated |
| 8 | TXNPx1/3 | Elevated | ||
| 9 | TXNPx1/3 | Elevated | ||
Spot densitometry was performed on the peroxidase spots in triplicate. Wild-type L. donovani had high levels of TXNPx1 and -3 forming a single “wild-type doublet” of spots. The level of TXNPx2 was low and Prx was not detectable. In the SUB−/− parasites, the TXNPx1/3 wild-type doublet decreased (to ∼35% of the wild-type spot density), and two new TXNPx1/3 “mutant doublets” were present at a higher molecular weight and at a higher molecular weight with a lower pI. In addition, Prx levels were elevated in these parasites, which could be a compensation for the decreased level of wild-type TXNPx1/3. TXNPx2 spot density was not significantly different between the wild-type and SUB−/−. The range of mass spectrometry amino acid coverage was nearly complete for all TXNPx spots (supplemental Fig. S1), with the notable exception of the wild-type doublet spots 4 and 5 for both WT and SUB−/−. For these spots the C-terminal 20 amino acids were never detected, despite high protein abundance and the fact that these amino acids were detected in the higher molecular weight doublets. These data suggest that the lower molecular weight wild-type doublet is C-terminally cleaved. The molecular weight shift of the mutant doublets is ∼2 kDa, which is consistent with the retention of the 20-amino acid C terminus.
SUB Knock-out Parasites Have Increased Sensitivity to Oxidative Damage
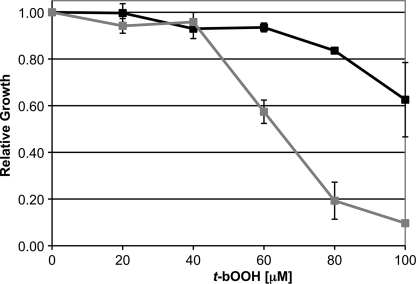
Proteomic analysis of SUB-deficient Leishmania indicated that SUB is required for normal regulation of the trypanothione reductase system. Alteration of this system would hinder the parasite's ability to detoxify hydroperoxides and thus render it more sensitive to oxidative damage (13). To evaluate sensitivity of wild-type and SUB-deficient Leishmania to oxidative damage, a hydroperoxide sensitivity assay was performed (Fig. 6). As expected, SUB knock-out Leishmania was significantly more sensitive to hydroperoxide compared with wild type. In 100 μm tert-butylhydroperoxide, wild-type parasites were over 60% viable while SUB knock-out parasites cultures had less than 10% viability.
FIGURE 6.
Leishmania major promastigote replication was measured in the presence of varying concentrations of tert-butylhydroperoxide. Wild-type (black squares) and SUB-deficient (dark gray squares) were grown to stationary phase and counted on a Multisizer 3 Coulter Counter. Values are expressed as the percent culture density relative to the untreated controls.
Loss of SUB Results in Delayed Lesion Formation in Mice and the Absence of Psammoma Body Lesions in Hamsters
Within the host, Leishmania is exposed to a variety of oxidative stresses particularly within host macrophages. It was predicted that SUB-deficient parasites would have reduced virulence in animal infection models due to the altered regulation of the trypanothione reductase system. Indeed, SUB-deficient Leishmania were found to be less virulent in both the mouse and hamster systems.
BALB/c mice were infected subcutaneously into their left hind footpads with either wild-type or SUB-deficient parasites. Footpad swelling was measured weekly (Fig. 7A). Swelling was evident in the wild-type-infected mice after 7 weeks; however, significant swelling (compared with the contralateral footpad) was not observed in mice infected with SUB-deficient parasites until after 14 weeks. The SUB-deficient infections were not self-limiting and continued to increase footpad swilling, however the lesion size was consistently 7–8 weeks delayed compared with the wild-type infections.
FIGURE 7.
BALB/c mice (n = 5) were infected subcutaneously in the left hind footpads with wild-type (solid circle) or SUB+/− (empty circle) parasites. Footpad swelling was measured weekly and the footpad thickness was plotted over time. Footpad size was significantly larger (p = 0.001) in WT infections after 40 days PI. Error bars indicate the ± S.D. between the 5 mice in each group. The mice were sacrificed at 180 days post infection. No swelling was observed in the right hind (uninfected) footpads. Male Golden Syrian hamsters (n = 3) were infected intraperitoneally with wild-type or SUB−/− L. donovani. At 200 days post-infection, the hamsters were sacrificed, and their spleens were sectioned, H&E-stained, and histologically examined. All wild-type-infected hamsters' spleens (B and C) contained psammoma body calcifications (arrows). No psammoma bodies were observed in the spleens of hamsters infected with SUB-deficient Leishmania (D and E).
For the visceral leishmaniasis infection model, male Golden Syrian hamsters were infected intraperitoneally with wild-type or SUB-deficient parasites. At 200 days post-infection, the hamsters were sacrificed, and their spleens were sectioned and histologically examined. Spleens were enlarged in both wild-type- and knock-out-infected animals. All wild-type-infected hamsters' spleens contained psammoma body calcifications (Fig. 7, B and C), indicative of granulomatous lesions that occur in visceral leishmaniasis (36, 37). Strikingly, no psammoma bodies were observed in the spleens of hamsters infected with SUB-deficient Leishmania (Fig. 7, D and E).
DISCUSSION
Leishmania is a dimorphic parasite that must survive and replicate in two vastly different environments: the gut of a phlebotomine sandfly and within the parasitophorous vacuole in phagocytic cells of vertebrates. Throughout this life cycle, the parasite is exposed to a variety of oxidative insults, including the reactive oxygen species produced by host macrophages. Antioxidant defense is therefore extremely important for parasite survival. Leishmania, along with the other kinetoplastids, uses an unusual hydroperoxide metabolic pathway, the trypanothione reductase system, which employs trypanothione as the main transporter of electrons (38). This system has been identified as an important target for antiparasitic drug development. Our research has shown that Leishmania parasites contain an unusual subtilisin-like enzyme that governs the levels of key peroxidases in the trypanothione reductase system. This serine protease, therefore, represents a potential target for rational drug design (39, 40).
We have identified and cloned a novel subtilisin-like protease from the parasite L. donovani. Phylogenetic sorting of known subtilisin catalytic cores showed that the Leishmania SUBs fall within the subfamily S8A and are most closely related to site-1 proteases. Interestingly, L. donovani (and the related L. infantum) SUB has a non-canonical catalytic triad. Clan SB serine proteases use an Asp-His-Ser triad, whereas L. donovani complex SUBs use a Glu in place of the Asp. Glu and Asp are identical save for one additional carbon in the side chain of Glu. Subtilisins are known to be pliable enzymes; however, a search of the MEROPS database has shown that there are currently no other known cases of Glu in the catalytic triad of a Clan SB protease (33). To verify that L. donovani subtilisin is an active enzyme, the catalytic cores of both L. donovani and L. major were recombinantly expressed in Pichia pastoris. Activity was recovered for both SUB cores indicating that the Glu-His-Ser catalytic triad is functional; however, kcat values for this non-canonical triad were around 100-fold lower than for the canonical triad. Proteolytic cleavage preferentially occurred when substrates had a basic residue in the P4 position, much like the site-1 proteases (41, 42). This strengthens the placement of Leishmania subtilisin in subfamily S8A.
To phenotypically characterize the function of subtilisin in Leishmania, the genes were disrupted in L. donovani and L. major by homologous recombination. Both alleles of the L. donovani gene were knocked out; however, only one allele could be deleted in L. major despite multiple attempts at gene targeting. This could be due to either a greater requirement for subtilisin in L. major or a compensatory change in L. donovani that allowed for the full knock-out to be generated. Knock-out parasites of both species grew well in vitro as promastigotes; however, attempts to grow the L. donovani SUB−/− parasites as amastigotes revealed a defect in their ability to differentiate. This indicates that subtilisin may be beneficial for survival in the amastigote stage. Electron microscopy of amastigote-like cells from the SUB−/− differentiation experiments revealed that these cells were either extremely abnormal or had not fully differentiated. Commonly seen ultrastructural abnormalities included elongated cell bodies, severe membrane invaginations, binucleation, and multiple flagellar cross sections. These abnormalities are likely due to parasite distress in response to the lack of subtilisin enzyme. This research suggested that the biological role of subtilisin within Leishmania may be as a maturase for a protein or pathway that promotes amastigote survival.
To test this hypothesis and to uncover the pathway catalyzed by subtilisin, proteomic analysis was performed on Leishmania wild-type and SUB knockouts. We uncovered five sets of protein spots that differed considerably between wild-type and SUB−/− parasites. All five of these sets were identified as members of the tryparedoxin peroxidase family, the terminal peroxidases of the trypanothione reductase system. Both the cytoplasmic tryparedoxin peroxidases (TXNPx1, -2, and -3) and the mitochondrial peroxidoxin (Prx) were identified. Wild-type Leishmania had high amounts of TXNPx1 and -3 forming a single doublet of spots. Knocking out SUB resulted in a reduction of this wild-type doublet and the appearance of higher molecular weight mutant doublets. TXNPx2 was unchanged following SUB knock-out. Peptide analysis of these spots revealed that all the wild-type TXNPx1 and -3 spots did not contain the C termini of the proteins, whereas the mutant spots retained these termini (supplemental Fig. S1). Subtilisin is therefore putatively responsible for C-terminal processing of the wild-type tryparedoxin peroxidases 1 and 3. This conclusion is further supported by the fact that the mutant doublet had a mass shift of 2 kDa, which is the calculated mass of the removed C termini. Interestingly, TXNPx2, which was not found to be processed, already has an abbreviated C terminus.
Although the tryparedoxin peroxidases are primarily cytoplasmic they, along with other members of the trypanothione reductase system, can be targeted to the trypanosomatid peroxisomes, known as glycosomes (43). The targeting of these enzymes to the glycosomes requires a canonical type-1 peroxisome/glycosome-targeting signal, PTS1 (44). The PST1 is comprised of a short C-terminal extension with a terminal tripeptide SKL, or a conserved variant of SKL (45, 46). Both TXNPx1 and -3 C termini encode a PTS1 (SKL and SKQ, supplemental Fig. S1); however, TXNPx2 lacks this tripeptide targeting sequence. In addition, TXNPx1 and -3, but not TXNPx2, contain a potential subtilisin cleavage motif at the site where processing is believed to occur. This Lys-Lys-Gly-Ala motif is nearly identical to a potential autocatalytic cleavage site of Leishmania subtilisin, Lys-Tyr-Gly-Ala. C-terminal proteolytic processing of TXNPx1 and -3 by subtilisin can therefore have a role in balancing levels of the peroxidases in the glycosomes and in the cytoplasm. Trypanothione reductase, which itself can be targeted to the glycosome using a PTS1, also contains a potential subtilisin cleavage motif (Lys-Met-Gly-Ala) (GeneDB), indicating that subtilisin may control targeting of multiple enzymes in the tryparedoxin peroxidase pathway.
Proteomic analysis of SUB−/− parasites also showed increased levels of Prx compared with wild-type. The function of mitochondrial Prx is believed to be complementary to that of cytosolic TXNPx (31), thus the increase in Prx may be a compensatory change due to the reduction of functional TXNPx. This hypothesis is supported by the fact that both alleles of the SUB gene could not be deleted in L. major. Although L. donovani complex parasites encode three cytosolic tryparedoxin peroxidase genes in the TXNPx gene array, L. major encodes seven cytosolic tryparedoxin peroxidases (GeneDB). L. major therefore relies more heavily on these cytosolic enzymes, thereby making a sufficient compensatory increase in Prx more difficult.
SUB-deficient Leishmania was found to have increased sensitivity to hydroperoxides compared with wild-type parasites in vitro. Reduced viability of SUB-deficient L. major and L. donovani amastigotes was also exhibited in vivo using the murine and hamster infection models, respectively. In both systems, the SUB knock-out parasites had clearly reduced virulence. Our research has shown that subtilisin promotes survival of Leishmania amastigotes by serving as a maturase for the trypanothione reductase system, thus aiding in redox homeostasis and protecting the parasite from oxidative stresses in the host macrophage. Because parasite proteases are known to be viable chemotherapeutic targets (3, 4), Leishmania subtilisin represents a new potential target for rational drug design.
Supplementary Material
Acknowledgments
We graciously thank Margaret Mays at the UCSF Morphology Core for tissue histology, K. C. Lim in the UCSF Dept. of Pathology for his help with the hamster infections, and Rick Fetter for the electron microscopy. Mass spectrometry analysis was provided by the Bio-Organic Biomedical Mass Spectrometry Resource at UCSF (A. L. Burlingame, Director) supported by the Biomedical Research Technology Program of the National Institutes of Health National Center for Research Resources, NIH NCRR P41RR001614, and National Institutes of Health NCRR RR012961.
This work was supported by a National Science Foundation Graduate Research Fellowship and the Sandler Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- SUB
- subtilisin
- AMC
- 7-amino-4-methylcoumarin
- Site-1
- membrane-bound transcription factor peptidase
- TR
- trypanothione reductase
- TS2 and T(SH)2
- oxidized and reduced trypanothione
- TXN
- tryparedoxin
- TXNPx
- tryparedoxin peroxidase
- Prx
- peroxidoxin
- Z
- benzyloxycarbonyl.
REFERENCES
- 1.Scientific Working Group on Leishmaniasis and UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (2004) Report of the Scientific Working Group Meeting on Leishmaniasis, Geneva, 2–4 February, 2004, World Health Organization, Geneva [Google Scholar]
- 2.Levick M. P., Tetaud E., Fairlamb A. H., Blackwell J. M. (1998) Mol. Biochem. Parasitol. 96, 125–137 [DOI] [PubMed] [Google Scholar]
- 3.Sajid M., McKerrow J. H. (2002) Mol. Biochem. Parasitol. 120, 1–21 [DOI] [PubMed] [Google Scholar]
- 4.McKerrow J. H., Caffrey C., Kelly B., Loke P., Sajid M. (2006) Annu. Rev. Pathol. 1, 497–536 [DOI] [PubMed] [Google Scholar]
- 5.Siezen R. J., de Vos W. M., Leunissen J. A., Dijkstra B. W. (1991) Protein Eng. 4, 719–737 [DOI] [PubMed] [Google Scholar]
- 6.Miller S. A., Binder E. M., Blackman M. J., Carruthers V. B., Kim K. (2001) J. Biol. Chem. 276, 45341–45348 [DOI] [PubMed] [Google Scholar]
- 7.Blackman M. J. (2008) Cell. Microbiol. 10, 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arastu-Kapur S., Ponder E. L., Fonović U. P., Yeoh S., Yuan F., Fonović M., Grainger M., Phillips C. I., Powers J. C., Bogyo M. (2008) Nat. Chem. Biol. 4, 203–213 [DOI] [PubMed] [Google Scholar]
- 9.Barale J. C., Blisnick T., Fujioka H., Alzari P. M., Aikawa M., Braun-Breton C., Langsley G. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 6445–6450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeoh S., O'Donnell R. A., Koussis K., Dluzewski A. R., Ansell K. H., Osborne S. A., Hackett F., Withers-Martinez C., Mitchell G. H., Bannister L. H., Bryans J. S., Kettleborough C. A., Blackman M. J. (2007) Cell 131, 1072–1083 [DOI] [PubMed] [Google Scholar]
- 11.Miller S. A., Thathy V., Ajioka J. W., Blackman M. J., Kim K. (2003) Mol. Microbiol. 49, 883–894 [DOI] [PubMed] [Google Scholar]
- 12.Kim K. (2004) Acta Trop. 91, 69–81 [DOI] [PubMed] [Google Scholar]
- 13.Krauth-Siegel R. L., Comini M. A. (2008) Biochim. Biophys. Acta 1780, 1236–1248 [DOI] [PubMed] [Google Scholar]
- 14.Wallis A. E., McMaster W. R. (1987) J. Exp. Med. 166, 1814–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle P. S., Engel J. C., Pimenta P. F., da Silva P. P., Dwyer D. M. (1991) Exp. Parasitol. 73, 326–334 [DOI] [PubMed] [Google Scholar]
- 16.Medina-Acosta E., Cross G. A. (1993) Mol. Biochem. Parasitol. 59, 327–329 [DOI] [PubMed] [Google Scholar]
- 17.Maniatis T., Fritsch E. F., Sambrook J. (1982) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 18.Button L. L., Russell D. G., Klein H. L., Medina-Acosta E., Karess R. E., McMaster W. R. (1989) Mol. Biochem. Parasitol. 32, 271–283 [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P., Sacchi N. (1987) Anal. Biochem. 162, 156–159 [DOI] [PubMed] [Google Scholar]
- 20.Gritz L., Davies J. (1983) Gene 25, 179–188 [DOI] [PubMed] [Google Scholar]
- 21.Lacalle R. A., Pulido D., Vara J., Zalacaín M., Jiménez A. (1989) Gene 79, 375–380 [DOI] [PubMed] [Google Scholar]
- 22.Joshi P. B., Webb J. R., Davies J. E., McMaster W. R. (1995) Gene 156, 145–149 [DOI] [PubMed] [Google Scholar]
- 23.Joshi P. B., Sacks D. L., Modi G., McMaster W. R. (1998) Mol. Microbiol. 27, 519–530 [DOI] [PubMed] [Google Scholar]
- 24.Joshi P. B., Kelly B. L., Kamhawi S., Sacks D. L., McMaster W. R. (2002) Mol. Biochem. Parasitol. 120, 33–40 [DOI] [PubMed] [Google Scholar]
- 25.Wyllie S., Fairlamb A. H. (2006) Acta Trop. 97, 364–369 [DOI] [PubMed] [Google Scholar]
- 26.Sacks D. L., Hieny S., Sher A. (1985) J. Immunol. 135, 564–569 [PubMed] [Google Scholar]
- 27.Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 28.Hellman U., Wernstedt C., Góñez J., Heldin C. H. (1995) Anal. Biochem. 224, 451–455 [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld J., Capdevielle J., Guillemot J. C., Ferrara P. (1992) Anal. Biochem. 203, 173–179 [DOI] [PubMed] [Google Scholar]
- 30.Chalkley R. J., Baker P. R., Medzihradszky K. F., Lynn A. J., Burlingame A. L. (2008) Mol. Cell. Proteomics 7, 2386–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro H., Sousa C., Santos M., Cordeiro-da-Silva A., Flohé L., Tomas A. M. (2002) Free Radic. Biol. Med. 33, 1552–1562 [DOI] [PubMed] [Google Scholar]
- 32.Barrett A. J., Rawlings N. D., Woessner J. F. (2004) Handbook of Proteolytic Enzymes, 2nd Ed., Elsevier Academic Press, Amsterdam [Google Scholar]
- 33.Rawlings N. D., Morton F. R., Kok C. Y., Kong J., Barrett A. J. (2008) Nucleic Acids. Res. 36, D320–D325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peacock C. S., Seeger K., Harris D., Murphy L., Ruiz J. C., Quail M. A., Peters N., Adlem E., Tivey A., Aslett M., Kerhornou A., Ivens A., Fraser A., Rajandream M. A., Carver T., Norbertczak H., Chillingworth T., Hance Z., Jagels K., Moule S., Ormond D., Rutter S., Squares R., Whitehead S., Rabbinowitsch E., Arrowsmith C., White B., Thurston S., Bringaud F., Baldauf S. L., Faulconbridge A., Jeffares D., Depledge D. P., Oyola S. O., Hilley J. D., Brito L. O., Tosi L. R., Barrell B., Cruz A. K., Mottram J. C., Smith D. F., Berriman M. (2007) Nat. Genet. 39, 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flohé L., Harris J. R. (2007) in Subcellular Biochemistry, Vol. 44, pp. 231–251, Springer, New York [Google Scholar]
- 36.Kahl L. P., Byram J. E., David J. R., Comerford S. A., Von Lichtenberg F. (1991) Am. J. Trop. Med. Hyg. 44, 218–232 [DOI] [PubMed] [Google Scholar]
- 37.Wilson M. E., Innes D. J., Sousa A. D., Pearson R. D. (1987) J. Parasitol. 73, 55–63 [PubMed] [Google Scholar]
- 38.Flohé L., Steinert P., Hecht H., Hofmann B. (2002) Methods Enzymol. 347, 244–258 [DOI] [PubMed] [Google Scholar]
- 39.Bal G., Van der Veken P., Antonov D., Lambeir A. M., Grellier P., Croft S. L., Augustyns K., Haemers A. (2003) Bioorg. Med. Chem. Lett. 13, 2875–2878 [DOI] [PubMed] [Google Scholar]
- 40.Caler E. V., Vaena de Avalos S., Haynes P. A., Andrews N. W., Burleigh B. A. (1998) EMBO J. 17, 4975–4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenz O., ter Meulen J., Klenk H. D., Seidah N. G., Garten W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12701–12705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincent M. J., Sanchez A. J., Erickson B. R., Basak A., Chretien M., Seidah N. G., Nichol S. T. (2003) J. Virol. 77, 8640–8649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith K., Opperdoes F. R., Fairlamb A. H. (1991) Mol. Biochem. Parasitol. 48, 109–112 [DOI] [PubMed] [Google Scholar]
- 44.Parsons M. (2004) Mol. Microbiol. 53, 717–724 [DOI] [PubMed] [Google Scholar]
- 45.Emanuelsson O., Elofsson A., von Heijne G., Cristóbal S. (2003) J. Mol. Biol. 330, 443–456 [DOI] [PubMed] [Google Scholar]
- 46.Naderer T., Ellis M. A., Sernee M. F., De Souza D. P., Curtis J., Handman E., McConville M. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5502–5507 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.