Abstract
Transcription factor LSF is essential for cell cycle progression, being required for activating expression of the thymidylate synthase (Tyms) gene at the G1/S transition. We previously established that phosphorylation of LSF in early G1 at Ser-291 and Ser-309 inhibits its transcriptional activity and that dephosphorylation later in G1 is required for its reactivation. Here we reveal the role of prolyl cis-trans isomerase Pin1 in activating LSF, by facilitating dephosphorylation at both Ser-291 and Ser-309. We demonstrate that Pin1 binds LSF both in vitro and in vivo. Using coimmunoprecipitation assays, we identify three SP/TP motifs in LSF (at residues Ser-291, Ser-309, and Thr-329) that are required and sufficient for association with Pin1. Co-expression of Pin1 enhances LSF transactivation potential in reporter assays. The Pin1-dependent enhancement of LSF activity requires residue Thr-329 in LSF, requires both the WW and PPiase domains of Pin1, and correlates with hypophosphorylation of LSF at Ser-291 and Ser-309. These findings support a model in which the binding of Pin1 at the Thr-329—Pro-330 motif in LSF permits isomerization by Pin1 of the peptide bonds at the nearby phosphorylated SP motifs (Ser-291 and Ser-309) to the trans configuration, thereby facilitating their dephosphorylation.
Keywords: Cell/Cycle, Phosphorylation, Phosphorylation/Transcription Factors, Protein/Prolyl Isomerase, Signal Transduction, Transcription, Pin1, Transcription/LSF
Introduction
The transcription factor LSF (also named CP2) plays a critical role in progression from G1 to S phase of the cell cycle, at least in part through specific induction of the thymidylate synthase (Tyms)3 gene prior to the G1/S transition (1). Inhibition of LSF results in either apoptosis during S phase or cell cycle arrest at the G1/S transition (1, 2). Both of these effects are abrogated by provision of exogenous thymidine and/or expression of exogenous Tyms, indicating that Tyms is a critical target for LSF during cell cycle progression (1, 2). Recent studies also indicate that regulation of Tyms by LSF plays a role in determining the sensitivity of human hepatocellular carcinomas to 5-fluorouracil, with elevated levels of LSF facilitating chemoresistance (3).
Previous work in our laboratory has demonstrated that the activity of LSF is regulated during G1 as a result of phosphorylation by both Erk and cyclin C/Cdk2 (4). LSF is rapidly and quantitatively phosphorylated by Erk on Ser-291 upon mitogenic stimulation of multiple cell types (5, 6). LSF is then phosphorylated by cyclin C/Cdk2 on Ser-309 in mouse fibroblasts, with maximal phosphorylation occurring in early G1, 1–2 h following mitogenic stimulation (4). Phosphorylation at both Ser-291 and Ser-309 inhibits the transcriptional activity of LSF, and both sites are dephosphorylated as cells progress into late G1, prior to activation of Tyms at the G1/S transition. These results suggest a novel time-delay mechanism of LSF regulation, in which phosphorylation in early G1 serves to inhibit LSF and prevent premature induction of LSF target genes. LSF is then activated by dephosphorylation of Ser-291 and Ser-309 in late G1, resulting in Tyms induction (4). Notably, although dephosphorylation of LSF is required for induction of Tyms, it does not affect induction of the E2F target genes encoding cyclin E1 and MCM3 (4), indicating that LSF may function in parallel to the well-studied Rb/E2F pathway in controlling gene expression during G1 to S progression (7).
Both Erk and Cdk2 are proline-directed kinases that target Ser/Thr residues immediately N-terminal to Pro residues, as is the case for the LSF phosphorylation sites Ser-291 and Ser-309. In considering the regulation of LSF as a result of phosphorylation at these residues, it is noteworthy that one consequence of phosphorylation at a subset of proline-directed sites is association with the prolyl isomerase Pin1. Pin1 binds its substrates and in some instances reversibly catalyzes cis-trans isomerization of the phospho-Ser/Thr-Pro peptide bond. Pin1 can thus couple phosphorylation at specific residues to conformational changes (8, 9), resulting in alterations in activity or modification of the target protein.
A number of Pin1-regulated proteins are transcription factors, suggesting that Pin1 might also play a role in regulation of LSF. Pin1 modulates the activities of its target proteins in multiple ways, including influencing the dephosphorylation of key residues (reviewed in Ref. 10–12). Many cellular phosphatases (e.g. PP2A) have a strict isomeric requirement, dephosphorylating a phospho-Ser/Thr-Pro site only when the Pro residue is in the trans configuration (13). Therefore, Pin1 can either protect against or expedite dephosphorylation by isomerizing phospho-Ser/Thr-Pro peptide bonds (10, 11). Furthermore, because Pin1 is made up of two separate domains, one of which binds phospho-Ser/Thr-Pro motifs (the WW domain) and the other of which isomerizes phospho-Ser/Thr-Pro bonds (the PPiase domain), Pin1 can associate with one site and alter the phosphorylation state of a separate site (10, 11). Pin1 regulates the dephosphorylation of c-Myc in precisely this manner, with the binding of Pin1 to phospho-Ser-58 leading to isomerization of the phospho-Ser-62-Pro-63 bond from cis to trans, which promotes the dephosphorylation of Ser-62 (14).
In this report, we describe Pin1-mediated regulation of LSF. Pin1 binds LSF by interaction with three SP/TP motifs: Ser-291 and Ser-309, which inhibit LSF transactivation when phosphorylated, and Thr-329. Interaction with Pin1 stimulates the inherent transcriptional activity of LSF and leads to its dephosphorylation at Ser-291 and Ser-309; both the WW and PPiase domains of Pin1 are required to mediate these effects. Interestingly, LSF Thr-329 is also required for Pin1 to both stimulate dephosphorylation and enhance LSF transactivation potential. In distinction to the influence of Pin1 on some other transcription factors, LSF protein stability is unaltered by its ability to interact with Pin1. The involvement of Thr-329 suggests a model in which Pin1 binding to phosphorylated Thr-329-Pro-330 is required for Pin1 to isomerize the phospho-Ser-291-Pro-292 and phospho-Ser-309-Pro-310 bonds to the trans conformation, which would facilitate dephosphorylation at Ser-291 and Ser-309 and enhancement of LSF transcription activity.
EXPERIMENTAL PROCEDURES
Cell Culture
NIH 3T3 cells were grown in Dulbecco's modification of Eagle's medium (DMEM) supplemented with 10% calf serum (JRH Biosciences), 2 mm l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C in 10% CO2.
293T cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS) (Hyclone), 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. The retroviral packaging cell line BOSC-23 (15) was propagated in DMEM supplemented with 10% heat-inactivated FBS, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Plasmid Constructs
The expression plasmids pCMV-QZ, pCMV-LSF (wild-type and S291A, S309A substitution mutants) (4), and pEF1α-LSF-HA (16) have been described previously. The retroviral vector LZRSpBMN-linker-IRES-EGFP, a derivative of the pBMN-I-GFP vector containing the EBNA-1 gene and the puromycin resistance cassette from pLZRS-LacZ(A) (17), was a gift from Gary Nolan (Stanford University). LZRSpBMN-linker-IRES-EGFP-LSF was constructed by inserting the LSF cDNA sequence from pCMV-LSF in between the BamHI and XhoI sites of the vector4; the S291A, S309A, and the S291A/S309A LSF mutants in the LZRSpBMN-linker-IRES-EGFP background were constructed by a fragment exchange from the appropriate pCMV-LSF construct. All other LSF substitution mutant cDNAs were cloned into the retroviral background by amplifying mutant LSF cDNA from the respective pCMV-LSF, digesting the amplicon with BamHI and XhoI and ligating it into BamHI- and XhoI-digested pLZRSpBMN-linker-IRES-EGFP. Inserts were confirmed by restriction digest and sequencing. The reporter plasmid pGL3B-WT4E1b has been previously described (4). The Renilla luciferase expression plasmid, phRLTK was purchased from Promega.
The bacterial expression plasmid encoding glutathione S-transferase (GST), pGEX-2T was from Amersham Biosciences. Bacterial expression plasmids encoding GST-tagged Pin1, pGEX-KG-Pin1 (wild-type, Y23A, and R68A/R69A mutants) (18, 19) and wild-type, Y23A, and R68A/R69A Pin1 in p3xFLAG-14 (19) were gifts from James Manley (Columbia University). The retroviral expression vector LZRSpBMN-linker-IRES-EGFP-FLAG-Pin1 (expressing wild-type, Y23A, or R68A/R69A Pin1) was constructed from the respective 3× FLAG Pin1 cDNAs by PCR amplification using primers that introduced a 5′ BglII site and a 3′ XhoI site, followed by ligation of the amplicon into BamHI- and XhoI-digested vector. All inserts were confirmed by restriction digestion or sequencing.
Site-directed Mutagenesis
Plasmids encoding amino acid substitution mutants of LSF were generated by PCR-mediated site-directed mutagenesis in the pCMV background, using the QuikChange site-directed mutagenesis kit (Stratagene) as per the manufacturer's instructions. Generation of expression constructs for multiple amino acid mutants was accomplished using the QuikChange Multi-site directed mutagenesis kit (Stratagene). LSF codon changes for T258A, S289A, S291A, S309A were as described previously (4). Codon changes for additional Ser/Thr to Ala mutants were: S76A, TCT to GCA; S278A, TCT to GCC; T329A, ACA to GCA; S451A, TCC to GCC. For the eight amino acid substitution mutant construct, pCMV-LSF-SP/TP(8)AP, the residues Ser-76, Thr-258, Thr-329, and Ser-451 were mutated to Ala in the pCMV-LSF-SquadA background (6). For plasmids encoding LSF add-back mutants, where all but selected Ser/Thr-Pro encoded motifs were mutated to encode Ala, we mutated selected Ala-Pro codons back to the wild-type Ser/Thr-Pro codons. LSF mutant cDNAs in other plasmid backgrounds were generated by DNA fragment exchange. All codon changes and DNA fragment exchanges were confirmed by sequencing.
Cellular Extracts and Protein Stability Measurements
Total cellular lysates were prepared in lysis buffer (50 mm HEPES, pH 7.5; 250 mm NaCl; 50 mm NaF; 5 mm EDTA; 1% Triton X-100; with protease inhibitor mixture (Roche) and phosphatase inhibitor mixture II (5 μm cantharadin, 5 nm microcystin-LR, 25 μm bromotetramisole oxalate) (Calbiochem)). The lysates were rocked for 30 min at 4 °C and clarified by centrifugation at 20,000 × g at 4 °C for 10 min. Protein concentration was determined using the Bradford method (Bio-Rad).
For protein stability assays, exponentially growing NIH 3T3 cells were transfected with plasmid LZRSpBMN-linker-IRES-EGFP DNA expressing wild-type, S291A, S309A, T329A, or S291A/S309A/T329A LSF, using Lipofectamine (Invitrogen). Cells were treated with 50 μg/ml cycloheximide (Sigma) 48 h post-transfection, and harvested at 0 (untreated), 8 and 24 h post-cycloheximide treatment. Cell extracts were prepared as described above, followed by immunoblotting.
GST Fusion Protein Purification and GST-Pin1 in Vitro Protein-Protein Interaction Studies
GST fusion proteins were purified as described (20, 21) with modifications as indicated below. Protein expression was induced in BL21 Escherichia coli, by incubation with 2 mm IPTG (Promega) for 12 h at 20 °C. Cells were pelleted, resuspended in LKB buffer (25 mm HEPES, pH 7.5; 100 mm NaCl; 1 mm EDTA; 1 mm EGTA; 1% IGEPAL CA-630; 1 mm PMSF; 10 μg/ml each of aprotinin, pepstatin, and leupeptin), and sonicated. Following clarification by centrifugation, the supernatant was incubated with glutathione-Sepharose 4B resin (GE Healthcare) by rocking at 25 °C for 30 min. The resin was washed four times with LKB buffer. Bound proteins were eluted by incubating the glutathione resin in three successive changes of 25 mm HEPES, pH 7.5; 10 mm reduced glutathione at 25 °C for 10 min. Eluates were pooled and dialyzed into 20 mm HEPES, pH 7.5; 100 mm KCl; 20% glycerol; 0.2 mm EDTA; 0.2 mm EGTA; 1 mm dithiothreitol (DTT).
GST-Pin1 protein-protein interaction experiments were performed as described in Ref. 16, 18 with some modifications. Equimolar amounts of GST or GST-Pin1 were bound to glutathione-Sepharose 4B resin at 25 °C for 30 min with rocking. The beads were washed twice with LKB buffer and once with lysis buffer prior to incubation by rocking with 200 μg of total cellular extracts for 2 h at 4 °C. The beads were washed five times with lysis buffer; bound proteins were eluted by boiling in 2× SDS-PAGE buffer (125 mm Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 0.01% bromphenol blue, and 200 mm DTT). The eluted proteins were resolved by SDS-PAGE and detected by immunoblotting. To demonstrate the requirement of LSF phosphorylation for interaction with GST-Pin1, the extracts were first dephosphorylated by incubating with 50 units calf intestinal phosphatase (CIP) (New England Biolabs) for 30 min at 37 °C, prior to incubation with the GST-containing resins.
Retrovirus Production and Infection
To generate retroviruses, 7 × 105 BOSC-23 (15) cells were co-transfected with the retroviral vector LZRSpBMN-linker-IRES-EGFP (either the parental empty vector or LZRSpBMN-linker-IRES-EGFP containing Pin1 or Pin1 mutants) and the envelope vector pCL-Eco (22) using Fugene-6 reagent (Roche) and an overnight incubation. Following replacement with fresh medium, and a subsequent 24-hour incubation, medium was harvested by centrifugation, and the supernatant supplemented with 8 μg/ml polybrene (Sigma). Retroviruses were titered by serial dilution, assaying infection by determining the number of GFP-positive cells via fluorescence microscopy or flow cytometry after 24 h. The minimal amount of virus yielding infection of greater than 95% of the cells was used for all subsequent experiments.
Immunoblot Analysis
Proteins were separated by SDS-PAGE and electrophoretically transferred to PVDF membranes (New England Nuclear). The membranes were blocked with 5% dry milk and incubated overnight at 4 °C with primary antibody. Primary antibodies included; anti-LSF (Becton Dickinson); anti-LSF-314 (23); anti-LSF-49 [a gift from Bill Andrews (Sierra Sciences LLC), a rabbit polyclonal antibody raised against LSF sequence FKQEESSLPPDNENKIL]; anti-LSF S309p (4); anti-LSF S291p (4); anti-Pin1/Dodo (Calbiochem); anti-GFP (Santa Cruz Biotechnology, sc-9996) and anti-β-actin (Sigma). Subsequently, blots were incubated with HRP-conjugated goat anti-rabbit or goat anti-mouse antibodies (Bio-Rad) and developed using SuperSignal West Pico enhanced chemiluminescence kit (Pierce) and autoradiography film (Denville Scientific Inc). Film was developed on an X-omat processor (Kodak). Band quantification was carried out using ImageJ software (24).
Immunoprecipitation
For immunoprecipitating Pin1 or LSF, cell lysates from 1 × 107 asynchronous NIH 3T3 cells were incubated with 2 μg of anti-Pin1 antibody (Calbiochem) or 5 μg of anti-LSF-49 antibody, respectively, at 4 °C for 12 h. Immune complexes were recovered using fast-flow protein A Sepharose beads (Millipore). The beads were washed four times with lysis buffer; complexes were eluted using 2× SDS-PAGE loading buffer.
For immunoprecipitating FLAG-Pin1, 1.5 × 106 NIH 3T3 cells were transfected with 16 μg of plasmid DNA (FLAG-Pin1 and LSF derivatives) using 90 μl of Lipofectamine (Invitrogen). Cells were harvested after 36–48 h. Total cell extract (500 μg of total protein) was incubated at 4 °C for 1 h with 25 μl of anti-FLAG-M2-agarose beads (Sigma), preblocked with 5 mg/ml BSA. Immune complexes were recovered as described above.
For immunoprecipitating with the MPM-2 antibody, NIH 3T3 cell extracts were prepared in Nonidet P-40 lysis buffer (50 mm Tris-HCl, pH 7.4; 1% IGEPAL CA-630; 0.25% sodium deoxycholate; 150 mm NaCl; 1 mm EDTA; 1 mm PMSF; 1 μg/ml aprotinin; 1 μg/ml leupeptin; 1 mm sodium orthovanadate; 1 mm NaF; 10 nm okadaic acid). 5 μg of MPM-2 antibody (Millipore) was incubated with 500 μg of total cellular protein overnight at 4 °C. Immune complexes were captured by incubating with washed protein G-Sepharose beads (Millipore) for 2 h. The beads were washed four times with the Nonidet P-40 lysis buffer, and bound proteins were eluted with 2× SDS-PAGE buffer.
Transient Transfection and Reporter Assays
Exponentially growing NIH 3T3 cells were transfected using a total of 2 μg of plasmid DNA (including: 500 ng of pCMV-QZ or pCMV-LSF and its derivatives, 100 ng of pGL3B-WT4E1b and 50 ng of phRLTK) and 10 μl of Lipofectamine (Invitrogen) per 35-mm plate. Cells were harvested at 36–48 h post-transfection using passive lysis buffer (Promega). Firefly luciferase and Renilla luciferase activities were measured using the dual luciferase assay system (Promega). The relative activation of the reporter construct was determined by dividing the firefly luciferase values by the Renilla luciferase values to normalize for transfection efficiency.
RESULTS
LSF Interacts with Pin1 in Vitro and in Vivo
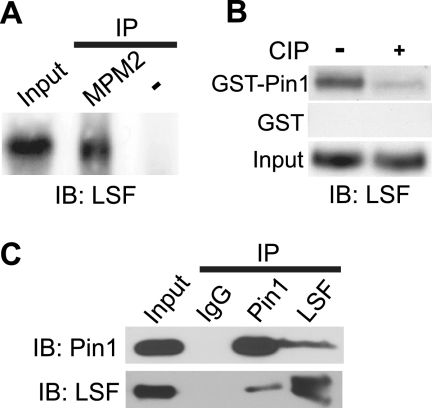
To determine whether LSF interacts with Pin1, several approaches were taken. First, we showed that endogenous LSF in NIH 3T3 cell extracts was immunoprecipitated by the MPM-2 antibody (Fig. 1A), an antibody that generally recognizes Pin1 substrates (25). To more directly assay for interaction of LSF with Pin1, we demonstrated that LSF from extracts of exponentially growing NIH 3T3 cells was retained on a GST-Pin1-bound resin, but not on the control GST-bound resin (Fig. 1B). Because the interaction between Pin1 and its substrates is strictly dependent on phosphorylated Ser/Thr-Pro motifs of the partner protein (9, 25), the NIH 3T3 extracts were also treated with calf intestinal phosphatase (CIP) prior to incubation with the GST-Pin1 resin. Dephosphorylation dramatically diminished the association of LSF with GST-Pin1, as anticipated (Fig. 1B).
FIGURE 1.
LSF associates with Pin1 both in vitro and in vivo. A, Nonidet P-40 extracts from asynchronously growing NIH 3T3 cells were immunoprecipitated with MPM-2 antibody. The precipitate and input extract (10% of amount immunoprecipitated) were resolved by SDS-PAGE on a 7.5% gel and analyzed by immunoblotting with anti-LSF-314. A control immunoprecipitate (in which the antibody was omitted) was similarly analyzed. B, mock-treated or CIP-treated NIH 3T3 whole cell extracts (200 μg each) were incubated with purified GST-Pin1 or GST preloaded onto glutathione-Sepharose beads. Retained proteins and input extracts were analyzed by immunoblotting with anti-LSF (BD). This result is representative of two independent experiments. C, either Pin1 or LSF was immunoprecipitated from NIH 3T3 whole cell extracts using anti-Pin1, anti-LSF-49, or IgG control antibodies. Immunoprecipitated proteins were resolved by SDS-PAGE on a 12.5% gel (for analysis of Pin1) or a 7.5% gel (for analysis of LSF) and immunoblotted using anti-Pin1 (Calbiochem) and anti-LSF (BD) antibodies, respectively. Results are representative of 2–6 independent experiments.
Whether endogenous LSF and Pin1 co-exist in a complex in vivo was determined by coimmunoprecipitation experiments. When Pin1 was precipitated from extracts of NIH 3T3 cells, LSF was reproducibly detected in the Pin1 immunoprecipitate, but not in the control immunoprecipitate (Fig. 1C). The complementary coimmunoprecipitation experiment also demonstrated the presence of Pin1 specifically in the LSF immunoprecipitates (Fig. 1C). Thus, cellular association of LSF and Pin1 does occur under normal physiological conditions.
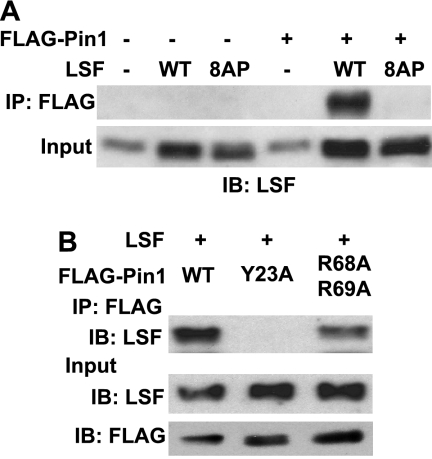
To test whether association with Pin1 in vivo requires phosphorylation of LSF, as would be required for a direct Pin1 interaction, FLAG-tagged Pin1 was expressed with either wild-type LSF or a derivative in which the Ser/Thr residues in all eight of the Ser/Thr-Pro motifs present in LSF were mutated to Ala (LSF 8AP). Whereas wild-type LSF robustly coimmunoprecipitated with FLAG-Pin1, LSF 8AP did not interact at all (Fig. 2A, top panel), even though the two LSF proteins were expressed at comparable levels (Fig. 2A, bottom panel). This requirement for SP and/or TP motifs in LSF for interaction with Pin1 in cells strongly suggests that the interaction is direct.
FIGURE 2.
LSF SP/TP motifs and the WW binding domain of Pin1 are required for association of LSF with Pin1. A, NIH 3T3 cells were transfected with p3X FLAG-Pin1 (WT) and cotransfected with either the parental pCMV-QZ construct (−), pCMV-LSF (WT), or pCMV expressing LSF 8AP, a mutant LSF in which all eight Ser-Pro and Thr-Pro motifs are replaced by Ala-Pro dipeptides. FLAG-Pin1 was immunoprecipitated from cellular extracts with anti-FLAG-M2 antibody and both the immunoprecipitates and input extracts were analyzed by immunoblotting with anti-LSF-314. Results are representative of six independent experiments. B, NIH 3T3 cells were cotransfected with expression constructs for wild-type LSF and either p3X FLAG-Pin1 (WT), a WW domain mutant of Pin1 (Y23A), or a catalytic domain mutant of Pin1 (R68A/R69A). Extracts were immunoprecipitated with anti-FLAG-M2 antibody, and coimmunoprecipitated proteins and input extracts were analyzed as in B. Input extracts were also analyzed for exogenous Pin1 levels by immunoblotting with anti-FLAG-M2 antibody. Results are representative of two independent experiments.
Pin1 is comprised of two domains: an N-terminal WW interaction domain and a C-terminal catalytic, PPiase domain, connected via a flexible linker (9). Both domains are capable of interacting with substrate proteins (11). We tested the Pin1 domain requirements for association with LSF by expressing wild-type LSF along with either wild-type or mutant Pin1, tagged with FLAG, followed by coimmunoprecipitation assays. Specifically, binding activity of the WW domain is eliminated by mutating Tyr-23 to Ala (26), and the catalytic activity of the PPiase domain is diminished at least 10-fold by mutating both Arg-68 and Arg-69 to Ala (18). All exogenous Pin1 proteins were expressed at similar levels (Fig. 2B, bottom panel). Immunoprecipitation of the wild-type Pin1 with anti-FLAG antibodies resulted in LSF coimmunoprecipitation, as expected. Mutation of the WW domain abolished the ability of Pin1 to interact with LSF, while mutation of the Pin1 catalytic domain decreased Pin1-LSF association. Quantitation of the immunoblot demonstrated roughly 4-fold lower association with Pin1 R68A/R69A, as compared with wild-type Pin1 (Fig. 2B). Taken together, these results demonstrate that LSF and Pin1 associate in vivo, and that this interaction requires one or more Ser/Thr-Pro motifs of LSF and the WW domain of Pin1. Although the PPiase domain of Pin1 is not sufficient on its own to interact with LSF, it also contributes to LSF binding.
Three Ser/Thr-Pro Motifs in LSF Are Responsible for Association with Pin1
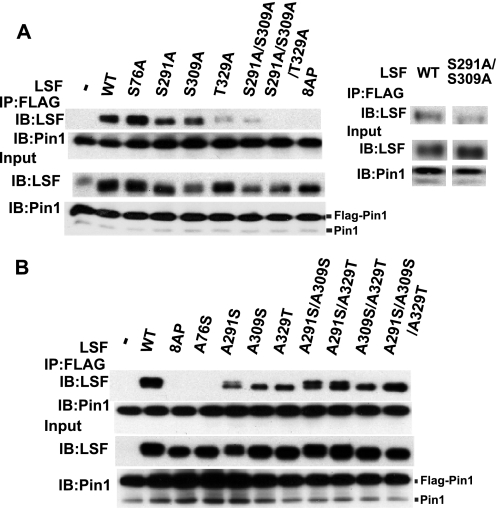
To identify the Ser/Thr-Pro motifs in LSF that are required for the interaction with Pin1, single alanine substitution mutants in all eight Ser/Thr-Pro motifs in LSF were generated. All the mutants were tested for interaction with FLAG-tagged Pin1 after simultaneous transfection of NIH 3T3 cells with plasmids expressing LSF and Pin1 constructs. Using this assay, the only amino acid substitution that reproducibly diminished the association between Pin1 and LSF was T329A (data not shown). However, the levels of Pin1 expression were variable in these transient transfection assays, making potentially more subtle effects of LSF mutants difficult to identify.
To maintain a consistent, high level of FLAG-Pin1 expression in all cells, exogenous FLAG-Pin1 was synthesized, instead, via infection with a recombinant retrovirus (Fig. 3). In this setting, we analyzed not only a select group of single Ala mutants in the LSF SP/TP motifs (Fig. 3A), but also combinations of multiple Ala substitution mutants, as well as single and multiple add-back mutants in which all but one or a small number of the Ser/Thr-Pro motifs were mutated to alanines (Fig. 3B). These types of mutations are complementary; whereas the alanine substitution mutants can identify which residues are necessary for the interaction between LSF and Pin1, the add-back mutants can identify which residues are sufficient for this interaction. Based on our previous studies, the focus was on four specific residues in LSF: Thr-329, which our initial studies pinpointed as important for the LSF-Pin1 interaction, Ser-291 and Ser-309, two sites previously demonstrated to be phosphorylated and to regulate the activity of LSF during G1 to S phase progression (4), and Ser-76, which can also impact the transcriptional activity of LSF (e.g. see Fig. 4A).
FIGURE 3.
Pin1 binds LSF through multiple Ser/Thr-Pro motifs. A, FLAG-Pin1 transduced NIH 3T3 cells were transiently transfected with retroviral expression constructs for wild-type LSF (WT), alanine substitution LSF mutants of the indicated, specific SP/TP motifs, and a LSF mutant with all eight ST/TP motifs substituted to AP (8AP). FLAG-Pin1 in the cell extracts was immunoprecipitated with anti-FLAG-M2 antibody. The immunoprecipitates and input extracts were resolved by SDS-PAGE on a 12.5% gel (for analysis of Pin1) or a 7.5% gel (for analysis of LSF) and immunoblotted using anti-Pin1 (Calbiochem) and anti-LSF-314 antibodies, respectively. FLAG-Pin1 migrates substantially more slowly than the endogenous Pin1, as indicated. Right panel, additional results from an independent experiment, in which expression levels of wild-type and S291A/S309A LSF were more comparable. B, FLAG-Pin1 transduced NIH 3T3 cells were transiently transfected with retroviral expression constructs for wild-type LSF (WT), the 8AP LSF mutant or add-back LSF mutants, where the indicated Ser or Thr residue(s) were added back into the 8AP background. Coimmunoprecipitation analysis was carried out as in A. Results are representative of 2–4 independent experiments.
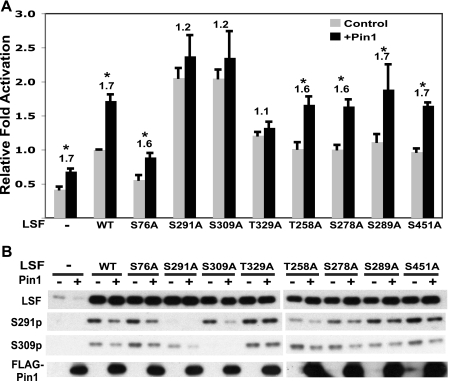
FIGURE 4.
Pin1 enhances LSF transactivation potential by facilitating dephosphorylation of Ser-291 and Ser-309. A, NIH 3T3 cells were infected with either a retrovirus overexpressing FLAG-Pin1 (black bars) or the parental retrovirus as control (gray bars). These cells were cotransfected with either pCMV-QZ (−), pCMV-LSF (WT), or pCMV driving expression of the indicated alanine substitution mutants of LSF, the reporter plasmid pGL3B-WT4E1b and phRL-TK. Expression from the reporter construct in the presence of each mutant is normalized to the degree of activation by wild-type LSF in the absence of exogenous Pin1 expression. The results are a compilation of three independent experiments. Error bars represent S.E. Numbers above the bars represent the fold increase in transactivation by Pin1. Asterisks represent p < 0.05, as determined by a pair-wise t test. B, extracts prepared in passive lysis buffer from a representative experiment described in A were analyzed by immunoblotting with anti-LSF-314, anti-S291p, and anti-S309p, as indicated, following SDS-PAGE through a 7.5% gel.
As previously observed (Fig. 2A), wild-type LSF coimmunoprecipitated with FLAG-Pin1, while the mutant in all eight Ser/Thr-Pro sites (8AP) did not (Fig. 3, A and B). Consistent with the results of the transient transfection assays, substituting Thr-329 with Ala significantly decreased the extent of the LSF-Pin1 interaction, whereas substituting Ser-76, Ser-291, or Ser-309 alone did not (Fig. 3A). Interestingly however, the double alanine substitution mutant at Ser-291 and Ser-309 also reduced the interaction of LSF with Pin1 (Fig. 3A, see also additional experiment in right panel). Finally, the triple substitution mutant LSF S291A/S309A/T329A was totally incapable of associating with Pin1. These results demonstrate that Ser-291, Ser-309, and Thr-329 are required for maximal Pin1 binding to LSF.
Upon analysis of the interactions between Pin1 and single add-back mutants of LSF (containing only a single SP or TP motif), Ser-76 did not rescue the LSF-Pin1 interaction, but either Ser-291, Ser-309, or Thr-329 was sufficient to promote limited interaction between Pin1 and LSF (Fig. 3B). The double add-back mutants of Ser-291 plus Ser-309, Ser-291 plus Thr-329, or Ser-309 plus Thr-329 increased the association with Pin1, as compared with the respective single add-back mutants (Fig. 3B). Most notably, the triple add-back mutant of Ser-291, Ser-309, and Thr-329 fully restored interaction between LSF and Pin1 to a degree comparable to that obtained with wild-type LSF (Fig. 3B). These results indicate that the SP or TP motif including residues Ser-291, Ser-309, and Thr-329 is each sufficient to induce binding of Pin1 to LSF, and that all three motifs are sufficient for maximal association.
Finally, we note that LSF migrates as a doublet upon SDS-PAGE analysis, although not always well resolved. The slower migrating form reflects phosphorylation of LSF solely at Ser-291 (5, 6). Indeed, immunoblotting with the phospho-Ser-291 antibody only recognizes the single, slower migrating species, whereas pan-LSF antibodies and the phospho-Ser-309 antibody can recognize both species (4). Consistent with these previous demonstrations, only the faster migrating LSF species is observed for mutants in which Ser-291 is substituted with alanine (Fig. 3A). Similarly, for the addback mutants, only those in which Ala-291 is reverted back to Ser migrate as doublets (Fig. 3B). Both forms of LSF are present in Pin1 immunoprecipitates as well as in total cell lysates.
One complicating aspect of the regulation of LSF post-translational modifications is that the phosphorylation status of adjacent residues can affect the degree of phosphorylation at Ser-291, and vice versa (4). In the experiments shown here, this is apparent, for example, in the increase of slower migrating LSF (reflecting Ser-291 phosphorylation) in the A291S/A309S addback mutant, as compared with the amount in the A291S mutant (Fig. 3B). These synergistic modifications do not affect the general conclusions presented here with respect to the effect of Pin1 on LSF modifications and activities.
Pin1 Enhances LSF Transactivation Potential
Pin1 regulates the phosphorylation status of multiple transcription factors with which it interacts (for review see Ref. 11). Given that phosphorylation at Ser-291 and Ser-309 inhibits LSF transactivation potential in NIH 3T3 cells (4), we investigated whether Pin1 regulates the activity and/or phosphorylation status of LSF. To this end, NIH 3T3 cells were infected with either a Pin1-overexpressing retrovirus or the parental virus as a control and then transfected with a LSF luciferase reporter construct, along with constructs expressing either wild type or alanine substitution mutants of LSF. As previously observed (4), LSF S291A and LSF S309A activated transcription significantly better than wild-type LSF (Fig. 4A, gray bars), despite similar levels of expression (Fig. 4B). Additionally, substituting Ser-76 to Ala consistently decreased the transactivation ability of LSF (Fig. 4A). Addition of exogenous Pin1 reproducibly enhanced LSF-dependent reporter activity, by ∼1.7-fold, for wild-type, S76A, T258A, S278A, S289A, and S451A LSF (Fig. 4A, compare gray and black bars). In contrast, Pin1 did not significantly alter LSF-dependent luciferase activity mediated by S291A, S309A, or T329A LSF.
To investigate how Pin1 enhances LSF activity, the status of LSF phosphorylation at Ser-291 and Ser-309 was determined. Pin1 consistently led to hypophosphorylation at both Ser-291 and Ser-309 in all LSF proteins, with the exception of T329A LSF (Fig. 4B). This is despite the fact that baseline phosphorylation of Ser-291 and Ser-309 on LSF T329A, in the absence of Pin1 overexpression, was comparable to that of wild-type LSF. Thus, the T329A mutation does not affect the ability of LSF to be targeted by kinases. The correlation between effects of Pin1 on transactivation and LSF phosphorylation, coupled with our previous findings indicating that phosphorylation at Ser-291 and Ser-309 inhibits LSF transactivation (4), strongly suggests that Pin1 enhances LSF transactivation potential, at least in part, by facilitating hypophosphorylation of Ser-291 and Ser-309. In addition, Thr-329 in LSF is required in order for Pin1 to mediate hypophosphorylation at the other two residues. Notably, these same three residues are implicated in the association of Pin1 with LSF (Fig. 3).
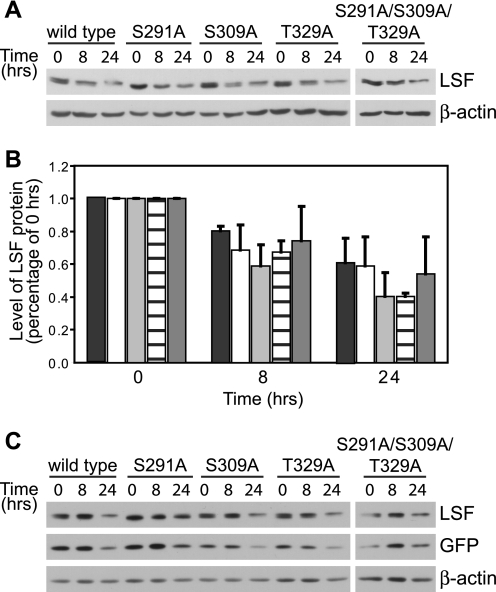
There is precedent that protein dephosphorylation, facilitated by Pin1, can alter protein stability (14). Thus, we tested whether LSF protein half-life is altered, dependent on its ability to interact with Pin1. Because of the relative stability of wild-type LSF (7), levels of wild-type and mutant proteins were compared at 0, 8, and 24 h after cycloheximide treatment. The rate at which levels of transiently overexpressed wild-type LSF decreased with time of cycloheximide treatment was comparable to the rate of decrease of levels of endogenous wild-type LSF protein (7), validating this approach. There were no significant differences in the rates of degradation of wild-type LSF, as compared with LSF mutants S291A, S309A, T329A, and the triple mutant S291A/S309A/T329A (Fig. 5). Quantitation from independent transfection experiments indicated that the levels of both wild-type and mutant proteins decreased ∼2-fold after 24 h of cycloheximide treatment (Fig. 5B). As a control for transfection efficiency, the levels of LSF were compared with those of co-expressed GFP, which is a stable protein reported to have a half-life of ∼26 h (27). Relative levels of both wild-type and mutant LSF proteins were similar to co-expressed GFP, and statistically indistinguishable from each other, throughout the cycloheximide time course (Fig. 5C). These results indicate that the ability of LSF to bind Pin1 does not significantly alter its stability.
FIGURE 5.
The ability of LSF to bind Pin1 does not alter its protein stability. A, wild-type or each indicated LSF mutant was expressed in exponentially growing NIH 3T3 cells, and cell extracts were harvested before (0 h) or after (8 and 24 h) cycloheximide addition. A representative analysis of the cell extracts from one experiment is shown, immunoblotted using anti-LSF-314 antibody (upper panel). Subsequent immunoblotting using β-actin antibodies validated equal sample loading (lower panel). B, immunoblots as in panel A were quantified for two independent experiments and normalized to levels of each LSF protein at the 0 h time point. The indicated bars represent quantification of data for wild-type LSF (black), LSF S291A (white), LSF S309A (light gray), T329A LSF (horizontal lines), and S291A/S309A/T329A LSF (dark gray). Error bars represent S.E. C, NIH 3T3 cells were transfected as above with plasmids co-expressing GFP and wild-type or mutant LSF and treated with cycloheximide for 0, 8, and 24 h. Cell extracts (20 μg of each) were immunoblotted for LSF (anti-LSF-314 antibody), GFP, and β-actin. A representative experiment is shown; relative levels of LSF compared with GFP levels, normalizing for wild-type LSF at 0 h, ranged from 0.93 to 1.46. This indicates similar decay rates of all LSF proteins and GFP. Furthermore, combining quantification of two independent experiments, there were no statistical differences in decay rates of wild-type versus mutant LSF proteins, using GFP to normalize the data.
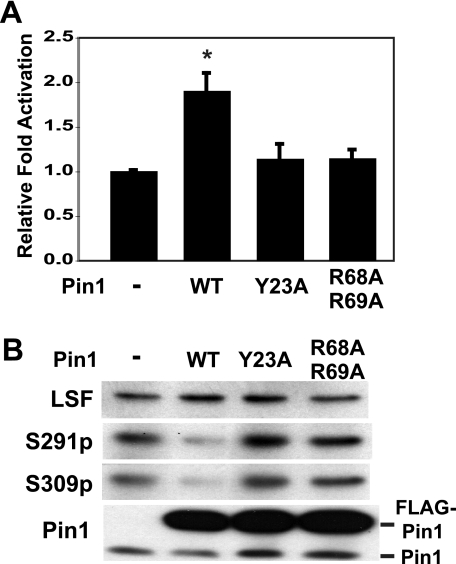
The effects of Pin1 on LSF transactivation and phosphorylation were further investigated by determining the consequences of mutations in the Pin1 WW and PPiase domains. NIH 3T3 cells were infected with different recombinant retroviruses, expressing either wild-type, Y23A, or R68A/R69A Pin1. These cells were subsequently used to assay LSF transactivation activity (Fig. 6A). As observed previously, wild-type Pin1 enhanced LSF activity by ∼1.8-fold. In contrast, neither the WW domain (Y23A) nor catalytic domain (R68A/R69A) mutants of Pin1 affected LSF activity, even though wild-type and mutant Pin1 proteins were expressed at comparable levels (Fig. 6B). Correlating with effects on LSF transactivation potential, only wild-type Pin1, but neither mutant, decreased LSF phosphorylation at Ser-291 and Ser-309 (Fig. 6B). Thus, neither the binding nor the catalytic domain of Pin1 is sufficient to mediate hypophosphorylation of Ser-291 and Ser-309. Instead, both are required to facilitate LSF dephosphorylation, as well as to enhance LSF transactivation.
FIGURE 6.
Pin1 catalytic and binding activities are required for enhancing LSF transactivation by facilitating dephosphorylation of Ser-291 and Ser-309. A, NIH 3T3 cells were infected with a parental control retrovirus (−), or retrovirus-overexpressing FLAG-tagged wild-type (WT) Pin1, the Y23A (WW domain) mutant of Pin1, or the R68A/R69A (PPiase domain) mutant of Pin1. These cells were cotransfected with pCMV or pCMV-LSF, the luciferase reporter plasmid pGL3B-WT4E1b and phRL-TK, as described in the legend to Fig. 4A. Activation by LSF of the reporter construct in the presence of each Pin1 mutant is normalized to the degree of activation by LSF in cells infected with the control parental retrovirus. The results are a compilation of two independent experiments, error bars represent S.E., and asterisks represent p < 0.05 by pair-wise t test. B, immunoblot analysis of extracts prepared in passive lysis buffer from a representative experiment described in A. The extracts were resolved by SDS-PAGE on a 12.5% gel and immunoblotted with anti-LSF-314, anti-S291p, anti-S309p, and anti-Pin1 (Calbiochem).
DISCUSSION
The transcription factor LSF, whose activity is required for cell cycle progression from G1 to S phase (1, 2), is regulated by phosphorylation on two proline-directed serine residues during G1 progression. LSF is phosphorylated by Erk on Ser-291 immediately after mitogenic stimulation of quiescent cells and by cyclin C/Cdk2 on Ser-309 shortly after re-entry into the cell cycle (4). Both of these modifications inhibit LSF transactivation potential until LSF is dephosphorylated in late G1, permitting activation of Tyms at the G1/S transition (1, 4). In this report we identify the prolyl isomerase Pin1 as an additional regulator of LSF. Pin1 facilitates dephosphorylation of Ser-291 and Ser-309, thereby boosting LSF transcriptional activity.
We demonstrated the association of LSF with Pin1 both in vitro and in vivo. In cellular extracts, coimmunoprecipitation assays revealed association between endogenous LSF and Pin1, as well as interaction between overexpressed, tagged proteins. Mutagenesis of all eight SP and TP motifs of LSF indicated that three of these motifs were involved in the interaction of LSF and Pin1: at Ser-291, Ser-309, and Thr-329. Although each of these three motifs is sufficient to promote some interaction with Pin1 in vivo, all three are required for maximal binding of LSF to Pin1. We note that Pin1 may also associate with LSF through additional Ser/Thr-Pro motifs under other cellular conditions, possibly including phosphorylation sites for JNK and p38 MAP kinases (28). Although other sites were not identified as Pin1 binding sites in this study, this could simply be due to lack of phosphorylation in proliferating cells.
Analysis of Pin1 mutants further demonstrated that both the WW domain and the PPiase domain contribute to the binding of Pin1 to LSF. The association of Pin1 with LSF thus appears to involve the interaction of both the WW and PPiase domains of Pin1 with multiple phosphorylated Ser/Thr-Pro sites on LSF. The interaction of Pin1 with several substrates, including Tau, Cdc25, and c-Myc, similarly involves Pin1 binding to multiple phosphorylated Ser/Thr-Pro motifs (11, 13). The Pin1 WW domain is generally required for stable interaction with its substrates (29). The catalytic PPiase domain also contributes to binding, such that binding by both domains appears to select for a subset of proline-directed phosphorylation sites (11). Thus the characteristics of the association of Pin1 with LSF fit with the binding mode of Pin1 to other target proteins.
Functionally, Pin1 overexpression enhances the transcriptional activity of LSF and leads to dephosphorylation of both of the regulatory sites that inhibit LSF during early G1: Ser-291 and Ser-309. Notably, Thr-329 is required for Pin1 to both enhance LSF activity and to facilitate dephosphorylation of Ser-291 and Ser-309, as are both the WW and PPiase domains of Pin1. These results suggest that binding of the WW domain to Thr-329 mediates association of Pin1 with LSF, allowing the PPiase domain to interact with and facilitate dephosphorylation of Ser-291 and Ser-309. For several proteins, Pin1 has been shown to enhance dephosphorylation catalyzed by PP2A, presumably by facilitating the isomerization of SP bonds. In the context of phosphorylated SP or TP motifs, only the trans, and not the cis conformation of proline is recognized as a substrate by PP2A and other phosphatases (10, 11, 30). As one example, binding of Pin1 to c-Myc at Ser-58 promotes dephosphorylation of Ser-62 by PP2A in late G1 (14). However, unlike the situation with c-Myc, neither the ability of LSF to interact with Pin1 nor its phosphorylation state significantly alters its stability. The half-lives of all the LSF mutants were comparable to that of wild-type LSF; all are relatively stable proteins.
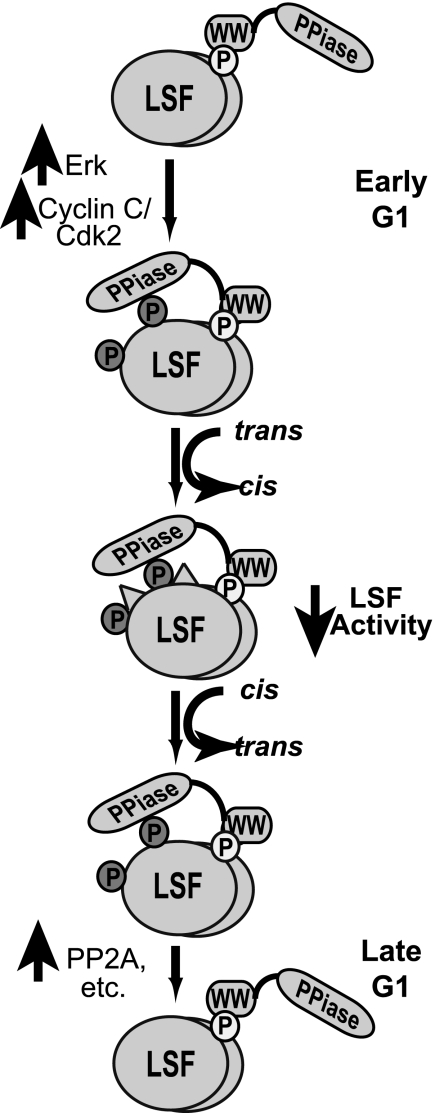
Because Pin1 isomerizes proline bonds both from cis to trans and from trans to cis, the directionality of the isomerization depends on a combination of structural constraints within the individual protein (32, 33) and on other drivers in the cellular environment that push the reaction in one direction or the other. In the case of LSF, Pin1 coimmunoprecipitates with LSF throughout the G1 phase (data not shown). Given constitutive interaction between LSF and Pin1 in G1, there must be other aspects of the cellular environment that impact the drive to dephosphorylation in late G1. We hypothesize that such changes are related both to inherent structural constraints of the phosphorylated SP motifs at Ser-291 and Ser-309 (favoring the cis configuration) and to the activities of the LSF kinases and phosphatases in the cell that alter the status of Ser-291 and Ser-309 (Fig. 7). At the peak activities of Erk and cyclin C/Cdk2 in early G1, LSF is efficiently phosphorylated on Ser-291 and Ser-309. Through association with phosphor-Thr-329-Pro-330, Pin1 then facilitates isomerization of Ser-291-Pro-292 and Ser-309-Pro-310 bonds to the cis conformation, which is presumed to be the energetically favored configuration of these phosphorylated residues due to structural constraints. This predominant cis conformation would also limit dephosphorylation of Ser-291 and Ser-309 by phosphatases such as PP2A. As cells progress through the G1 phase, Erk, and cyclin C/Cdk2 kinase activities decrease. Furthermore, LSF specifically associates with PP2A in late G1, but not early G1 (31). This switch in the balance of kinases and phosphatases would lead to removal of the phosphates on LSF. In late G1, phosphatase activities would then become the prevailing driving force for the isomerization reaction, as they deplete only the trans conformation of phosphorylated Ser-291-Pro-292 and Ser-309-Pro-310 dipeptides, present in limiting amounts. Upon depletion of this conformation, Pin1 would mediate additional isomerization of phosphorylated Ser-Pro dipeptides to the trans conformation, which would further facilitate LSF dephosphorylation at these sites. This concerted dephosphorylation of LSF on Ser-291 and Ser-309 in late G1 would serve to enhance LSF transactivation of G1/S regulated gene(s).
FIGURE 7.
Model of Pin1 regulation of LSF. A detailed discussion of the model is provided in the text. Only the LSF modifications discussed in this study are indicated, with their relationship to Pin1. Other protein-protein interactions and modifications that may differentially alter LSF activities are not shown or discussed here; see Ref. 7 for some additional insights. LSF is indicated as a dimer of ovals, with phosphorylation sites shown only on a single subunit, for clarity of presentation. Phosphorylated residues are indicated as small circles: Thr-329, light circle; Ser-291 and Ser-309, dark circles. The cis configuration of the Ser-Pro bonds in LSF is indicated as raised triangles on the surface of the oval. Pin1 is indicated as two domains: PPiase is the catalytic domain and WW is the binding domain; a flexible linker in the form of a line connects the two domains.
These results provide further support for the novel time-delay mechanism for LSF activation, where rapid inhibition of LSF activity in early G1 prevents premature induction of its target genes. We propose that, by promoting LSF dephosphorylation in late G1, Pin1 is a molecular facilitator by which LSF integrates signals from different signaling pathways. This mechanistically ensures the appropriate induction of LSF target genes at the G1/S transition. It is also noteworthy that LSF has recently been shown to function as an oncogene in hepatocellular carcinoma (34). Because Pin1 is also overexpressed in a significant fraction of hepatocellular carcinomas (35, 36), aberrant LSF-Pin1 interactions could also contribute to oncogenesis.
Acknowledgments
We thank James Manley (Columbia University) for the gifts of GST-Pin1 and Flag-Pin1 constructs, Gary Nolan (Stanford University) for retroviral vectors, and Bill Andrews (Sierra Sciences) for LSF antibody. We also appreciate the technical assistance of Trevor Grant, Roxanne Cacioppo, and Sarah Oppelt in generating mutant LSF constructs.
This work was supported, in whole or in part, by Public Health Service National Institutes of Health Grants R01 CA-081157 (to U. H.) and R01 CA-18689 (to G. M. C.).
E. Drouin and U. Hansen, unpublished data.
- Tyms
- thymidylate synthase
- CIP
- calf intestinal phosphatase.
REFERENCES
- 1.Powell C. M., Rudge T. L., Zhu Q., Johnson L. F., Hansen U. (2000) EMBO J. 19, 4665–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruni P., Minopoli G., Brancaccio T., Napolitano M., Faraonio R., Zambrano N., Hansen U., Russo T. (2002) J. Biol. Chem. 277, 35481–35488 [DOI] [PubMed] [Google Scholar]
- 3.Yoo B. K., Gredler R., Vozhilla N., Su Z. Z., Chen D., Forcier T., Shah K., Saxena U., Hansen U., Fisher P. B., Sarkar D. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12938–12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxena U. H., Powell C. M., Fecko J. K., Cacioppo R., Chou H. S., Cooper G. M., Hansen U. (2009) Mol. Cell. Biol. 29, 2335–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagon Z., Volker J., Cooper G. M., Hansen U. (2003) J. Cell. Biochem. 89, 733–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volker J. L., Rameh L. E., Zhu Q., DeCaprio J., Hansen U. (1997) Genes Dev. 11, 1435–1446 [DOI] [PubMed] [Google Scholar]
- 7.Hansen U., Owens L., Saxena U. H. (2009) Cell. Cycle. 8, 2146–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu K. P., Finn G., Lee T. H., Nicholson L. K. (2007) Nat. Chem. Biol. 3, 619–629 [DOI] [PubMed] [Google Scholar]
- 9.Ranganathan R., Lu K. P., Hunter T., Noel J. P. (1997) Cell 89, 875–886 [DOI] [PubMed] [Google Scholar]
- 10.Lippens G., Landrieu I., Smet C. (2007) FEBS J. 274, 5211–5222 [DOI] [PubMed] [Google Scholar]
- 11.Lu K. P., Zhou X. Z. (2007) Nat. Rev. Mol. Cell. Biol. 8, 904–916 [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K., Uchida C., Shin R. W., Shimazaki K., Uchida T. (2008) Cell. Mol. Life. Sci. 65, 359–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X. Z., Kops O., Werner A., Lu P. J., Shen M., Stoller G., Küllertz G., Stark M., Fischer G., Lu K. P. (2000) Mol. Cell. 6, 873–883 [DOI] [PubMed] [Google Scholar]
- 14.Yeh E., Cunningham M., Arnold H., Chasse D., Monteith T., Ivaldi G., Hahn W. C., Stukenberg P. T., Shenolikar S., Uchida T., Counter C. M., Nevins J. R., Means A. R., Sears R. (2004) Nat. Cell. Biol. 6, 308–318 [DOI] [PubMed] [Google Scholar]
- 15.Pear W. S., Nolan G. P., Scott M. L., Baltimore D. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drouin E. E., Schrader C. E., Stavnezer J., Hansen U. (2002) J. Immunol. 168, 2847–2856 [DOI] [PubMed] [Google Scholar]
- 17.Kinsella T. M., Nolan G. P. (1996) Hum. Gene Ther. 7, 1405–1413 [DOI] [PubMed] [Google Scholar]
- 18.Shen M., Stukenberg P. T., Kirschner M. W., Lu K. P. (1998) Genes Dev. 12, 706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y. X., Hirose Y., Zhou X. Z., Lu K. P., Manley J. L. (2003) Genes Dev. 17, 2765–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu K. P., Osmani S. A., Means A. R. (1993) J. Biol. Chem. 268, 8769–8776 [PubMed] [Google Scholar]
- 21.Xiao S. H., Manley J. L. (1997) Genes Dev. 11, 334–344 [DOI] [PubMed] [Google Scholar]
- 22.Cherry S. R., Biniszkiewicz D., van Parijs L., Baltimore D., Jaenisch R. (2000) Mol. Cell. Biol. 20, 7419–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Repetny K. J., Zhong X., Holodick N. E., Rothstein T. L., Hansen U. (2009) Eur. J. Immunol. 39, 1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasband W. S. (1997–2009) Image J, NIH [Google Scholar]
- 25.Yaffe M. B., Schutkowski M., Shen M., Zhou X. Z., Stukenberg P. T., Rahfeld J. U., Xu J., Kuang J., Kirschner M. W., Fischer G., Cantley L. C., Lu K. P. (1997) Science 278, 1957–1960 [DOI] [PubMed] [Google Scholar]
- 26.Lu P. J., Zhou X. Z., Shen M., Lu K. P. (1999) Science 283, 1325–1328 [DOI] [PubMed] [Google Scholar]
- 27.Corish P., Tyler-Smith C. (1999) Protein Eng. 12, 1035–1040 [DOI] [PubMed] [Google Scholar]
- 28.Ylisastigui L., Kaur R., Johnson H., Volker J., He G., Hansen U., Margolis D. (2005) J. Virol. 79, 5952–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu P. J., Zhou X. Z., Liou Y. C., Noel J. P., Lu K. P. (2002) J. Biol. Chem. 277, 2381–2384 [DOI] [PubMed] [Google Scholar]
- 30.Gray C. H., Barford D. (2003) Cell. Cycle. 2, 500–502 [DOI] [PubMed] [Google Scholar]
- 31.Cacioppo R. (2007) Cell Growth Regulation of Mammalian Transcription Factor LSF by Protein Phosphatase 2A, Ph.D. Thesis, Boston University [Google Scholar]
- 32.Pastorino L., Sun A., Lu P. J., Zhou X. Z., Balastik M., Finn G., Wulf G., Lim J., Li S. H., Li X., Xia W., Nicholson L. K., Lu K. P. (2006) Nature 440, 528–534 [DOI] [PubMed] [Google Scholar]
- 33.Ramelot T. A., Nicholson L. K. (2001) J. Mol. Biol. 307, 871–884 [DOI] [PubMed] [Google Scholar]
- 34.Yoo B. K., Emdad L., Gredler R., Fuller C., Dumur C. I., Jones K. H., Jackson-Cook C., Su Z. Z., Chen D., Saxena U. H., Hansen U., Fisher P. B., Sarkar D. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 8357–8362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang R., Yuen J., Yuen M. F., Lai C. L., Lee T. K., Man K., Poon R. T., Fan S. T., Wong C. M., Ng I. O., Kwong Y. L., Tse E. (2004) Oncogene 23, 4182–4186 [DOI] [PubMed] [Google Scholar]
- 36.Bao L., Kimzey A., Sauter G., Sowadski J. M., Lu K. P., Wang D. G. (2004) Am. J. Pathol. 164, 1727–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]