Abstract
Growth hormone (GH) excess results in structural and functional changes in the kidney and is implicated as a causative factor in the development of diabetic nephropathy (DN). Glomerular podocytes are the major barrier to the filtration of serum proteins, and altered podocyte function and/or reduced podocyte number is a key event in the pathogenesis of DN. We have previously shown that podocytes are a target for GH action. To elucidate the molecular basis for the effects of GH on the podocyte, we conducted microarray and RT-quantitative PCR analyses of immortalized human podocytes and identified zinc finger E-box-binding homeobox 2 (ZEB2) to be up-regulated in a GH dose- and time-dependent manner. We established that the GH-dependent increase in ZEB2 levels is associated with increased transcription of a ZEB2 natural antisense transcript required for efficient translation of the ZEB2 transcript. GH down-regulated expression of E- and P-cadherins, targets of ZEB2, and inhibited E-cadherin promoter activity. Mutation of ZEB2 binding sites on the E-cadherin promoter abolished this effect of GH on the E-cadherin promoter. Whereas GH increased podocyte permeability to albumin in a paracellular albumin influx assay, shRNA-mediated knockdown of ZEB2 expression abrogated this effect. We conclude that GH increases expression of ZEB2 in part by increasing expression of a ZEB2 natural antisense transcript. GH-dependent increase in ZEB2 expression results in loss of P- and E-cadherins in podocytes and increased podocyte permeability to albumin. Decreased expression of P- and E-cadherins is implicated in podocyte dysfunction and epithelial-mesenchymal transition observed in DN. We speculate that the actions of GH on ZEB2 and P- and E-cadherin expression play a role in the pathogenesis of microalbuminuria of DN.
Keywords: Diabetes, Hormones, Kidney, Transcription Factors, Transcription Regulation, Diabetic Nephropathy, E-cadherin, Podocytes, Proteinuria, ZEB2
Introduction
Pituitary growth hormone (GH)5 is essential for postnatal growth in mammals. In addition to growth, GH affects the metabolism of fat, protein, and carbohydrate (1). GH exerts these actions both by its direct effect on target organs and by stimulating the production of insulin-like growth factor-1 (IGF-1). At the tissue level, these pleiotropic actions of GH result from the interaction of GH with a specific cell surface receptor, the GH receptor (GHR). Whereas the GHR is ubiquitously expressed, the role and effects of GH have been most intensely investigated in organs and tissues such as liver, bone, muscle, and adipocytes in which GHR expression is substantial and are thus considered canonical targets of GH action. However, recent reports have highlighted the biological effects and physiological relevance of GH action in non-canonical targets such as the blastocyst (2), colonic epithelial cells (3), cardiomyocytes (4), and neurons (5).
GH excess in both the human (acromegaly) and in transgenic animal models is characterized by significant structural and functional changes in the kidney (6–8). An overactive GH/GHR axis is implicated as a causative factor in the development of diabetic nephropathy (9–11). Our previous study had identified the glomerular podocyte as a target of GH action with podocytes expressing functional GHR and exhibiting GH-dependent activation of canonical GH signaling pathways (12). Podocytes are visceral epithelial cells of the renal glomerulus. These cells, which have a complex cellular architecture that includes a cell body and primary, secondary, and tertiary foot processes, are generally considered to be terminally differentiated. Individual foot processes interdigitate with that of neighboring cells and also attach to the underlying glomerular basement membrane. Podocyte foot processes, glomerular basement membrane, and the fenestrated endothelial cells together form the glomerular filtration barrier that ensures filtration of water and solutes while preventing loss of albumin and other proteins. The cell-cell junctions between the podocyte foot processes are bridged by slit diaphragms, which play a significant role in glomerular filtration. The absolute number of podocytes and their functional integrity are critical for maintenance of normal renal function. Proteinuria ensues when the structure of podocytes is destroyed by disruption of the slit diaphragm and loss of foot processes (effacement). Podocyte injury and loss have been observed in human and experimental models of diabetic nephropathy (DN) and other glomerular diseases (13–16).
The cellular and molecular basis for GH action in the podocyte is unclear. In the current study, we report a molecular basis for GH action in the podocyte with the identification of zinc finger E-box-binding homeobox 2 (ZEB2), also known as zinc finger homeobox 1B (ZFHX1B), as a target of GH action in the podocyte. We demonstrate that GH-dependent increase in ZEB2 expression is due to increased ZEB2 promoter activity and the induction of a ZEB2 natural antisense transcript (ZEB2-NAT). Our studies further elucidate the functional implications of this novel GH action with demonstration of GH-dependent repression of canonical targets of ZEB2, E-and P-cadherins, with associated changes in podocyte function.
EXPERIMENTAL PROCEDURES
Cell Culture
Conditionally immortalized mouse podocyte cells (MPC-5) (17) were grown according to a protocol detailed previously (18). Briefly, cells were cultured under growth-permissive conditions on rat tail collagen type I-coated plastic dishes (BD Biosciences) at 33 °C with 100% relative humidity and 5% CO2 in RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin, 100 μg/ml streptomycin (Invitrogen), and 10 units/ml mouse recombinant γ-interferon (Sigma). To induce differentiation, podocytes were maintained in non-permissive conditions at 37 °C without γ-interferon for 14 days prior to experimentation. Under these culture conditions, cells stopped proliferating and were identified as differentiated podocytes by their arborized morphology and induction of podocyte-specific proteins such as synaptopodin (data not shown). Cells were routinely maintained for 12–16 h in serum-free medium before experimentation. Human podocytes (19) were cultured in RPMI 1640 medium with glutamine (Sigma) supplemented with 10% fetal bovine serum (Invitrogen) and insulin-transferrin-sodium selenite (1 ml/100 ml; Sigma). These cells were induced to differentiate using a protocol similar to that outlined above for the murine podocytes. HepG2 cells (ATCC) were cultured in DMEM supplemented with 10% FBS.
Microarray
Fully differentiated human podocytes were cultured overnight in serum-free medium and then exposed to human GH (500 ng/ml) for 1, 5, and 30 min. Total RNA was extracted from these cells using TRIzol reagent (Invitrogen) followed by repurification using a column (Qiagen, RNeasy minikit) according to the manufacture's protocol. In-column DNase digestion was performed for each sample to remove genomic DNA. RNA samples were analyzed for concentration and purity using a Nanodrop ND-1000 spectrophotometer, and integrity was checked using the Agilent 2100 Bioanalyzer. Affymetrix Human 2.0 Plus arrays (Affymetrix) were used in this study. Standard Affymetrix protocols were followed for sample labeling, hybridization, and image scanning at the University of Michigan microarray core facility.
PCR and Real Time Quantitative PCR
Total RNA was isolated and purified from differentiated human podocytes as described above. ZEB2-NAT primer sequences were as published (20). Total RNA was reverse transcribed using avian myeloblastosis virus reverse transcriptase, and PCR was performed with NAT primers using Extend Long PCR DNA polymerase (Roche Diagnostics). The reaction conditions were as follows: initial RT for 10 min at 25 °C, stringent RT for 45 min at 48 °C followed by inactivation of reverse transcriptase at 94 °C for 5 min, and PCR cycles of initial denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 68 °C for 3 min. A final extension was carried out at 68 °C for 7 min. The following primers were used: NAT primer 1F, 5′-CCT CCC GAC ACT CTT GGC GA-3′; NAT primer 2F, 5′-GGG CGA GTG GGC TTC CT-3′; NAT primer 2R, 5′-CGA TAA GAG CGG ATC AGA TGG C-3′; β-globulin 1, 5′-CCA ATC TGC TCA CAC AGG ATA GAG AGG GCA GG-3′; and β-globulin 2, 5′-CCT TGA GGC TGT CCA AGT GAT TCA GGC CAT CG-3′. Primer Express software (PE Biosystems) was used to design primers and probes for ZEB2 (encompassing exons 7 and 8) and E-cadherin (encompassing exons 5 and 6). The sequences of primers and probes used in the study are as follows: ZEB2 primer 1, 5′-CAA GGA GCA GGT AAT CGC AAG T-3′; ZEB2 primer 2, 5′-GAA CGT CAA ACC CGT GAG CAT-3′; ZEB2 probe, 5′-CAA ATG CAC AGA GTG TGG CAA GGC C-3′; E-cadherin primer 1, 5′-CGC ATT GCC ACA TAC ACT CTC T-3′; E-cadherin primer 2, 5′-CCA TTG GAT CCT CAA CTG CAT-3′; E-cadherin probe, 5′-CTC TCA CGC TGT GTC ATC CAA CGG-3′; P-cadherin primer 1, 5′-ACC GAC CAG AAT GAC CAC AAG-3′; and P-cadherin primer 2, 5′-AGA AGT ACC TGG TAG GAC TCC CTC TAA-3′.
The E-cadherin and ZEB2 probes were labeled with fluorescent reporter dye VIC (Applied Biosystems), and the GAPDH probe was labeled with FAM (6-carboxyfluorescein). Normalization and validation of data were carried out using GAPDH as an internal control, and data were compared between GH-treated and untreated samples according to the comparative CT method (21). Using a separate tube RT-PCR protocol (PE Biosystems), 4-ng aliquots of total RNA were analyzed. After RT at 48 °C for 30 min, samples were subjected to PCR analysis using the following cycling parameters: 95 °C × 10 min; 95 °C × 15 s → 60 °C × 1 min for 40 cycles. Each sample was analyzed in triplicate in individual assays performed on three or more occasions.
Plasmids
E-cadherin-luciferase reporter construct was a gift from Dr. Eric Fearon (University of Michigan). ZEB2 binding sites in E-cadherin promoter (E2 box1 and E2 box3) were mutated using the QuikChange site-directed mutagenesis kit (Stratagene). Plasmid DNA was prepared using the EndoFree plasmid maxikit (Qiagen), and all constructs were verified by DNA sequencing. ZEB2- and ZEB2-NAT-luciferase reporter constructs (20) were kindly provided by Dr. Antonio García de Herreros (Institut Municipal d'Investigació Mèdica-Hospital del Mar, Universitat Pompeu Fabra, Barcelona, Spain).
Transient Transfection and Luciferase Assay
HepG2 cells (2 × 105 cells/well in 6-well plates) were cotransfected with luciferase reporter plasmids (2 μg/well) and internal control, pRL-TK (Promega; 0.2 μg/well), expressing the Renilla luciferase. Transfections were performed using FuGENE (Roche Applied Science). 48 h after transfection, the cells were starved for 12–16 h and then treated with GH (500 ng/ml) for specified time periods. To measure luciferase activity, the wells were rinsed twice with phosphate-buffered saline, and cells were harvested with 200 μl of passive lysis buffer (Promega). Following a brief freeze-thaw cycle, the insoluble debris were removed by centrifugation at 4 °C for 2 min at 14,000 × g. 20-μl aliquots of the supernatant were then immediately processed for sequential quantitation of both firefly and Renilla luciferase activity (Dual-Luciferase Assay System, Promega) using a Monolight TD 20/20 luminometer (Turner Designs). The activity of the cotransfected Renilla reporter plasmid was used for normalization of transfection efficiency. All transfections experiments were performed in triplicates.
Western Blot Analysis
Cells were lysed in protein lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 5% 2-mercaptoethanol, 1% Triton X-100, 0.25% sodium deoxycholate, and protease inhibitor mixture (Roche Diagnostics)). The suspension was centrifuged at 12,000 × g for 15 min, supernatant was collected, and protein concentration was quantified via the Bio-Rad protein assay. Equal amounts of cell lysates were resolved through 4–16% gradient polyacrylamide gels and blotted onto nitrocellulose membrane. The blot was probed with suitable primary and secondary antibodies and then developed by the ECL chemiluminescence method (Pierce). The primary antibodies against ZEB2 (Abnova) and E- and P-cadherins (BD Biosciences) were used in a 1:1000 dilution. When indicated, the membrane was then stripped and reblotted with anti-tubulin antiserum (Sigma; 1:1000) to verify equality of sample loading.
ZEB2 Knockdown Using shRNA Lentiviral Particles
A panel of five lentiviruses expressing unique ZEB2 shRNA sequences was purchased from Sigma-Aldrich. Proliferating human podocytes were plated in a 6-well plate at 40% confluence (2 × 105 cells/well) and cultured overnight for 12–16 h. The cells were then pretreated with Polybrene (6 μg/ml for 20 h), infected with lentiviral particles, and incubated at 33 °C. The following day the medium was replaced with fresh medium containing puromycin (1 μg/ml) as a selection marker. The puromycin-resistant cells were induced to differentiate using the protocol detailed above, and ZEB2 expression was measured in the differentiated cells using RT-qPCR and Western blot analysis.
Albumin Influx Assay
Human podocytes were plated on a 12-well plate with collagen-coated Transwell filters (Corning) (6–7 × 104 cells/well) and differentiated at nonpermissive conditions (37 °C, 5% CO2) for 14 days. The cells were then placed in serum-free medium overnight and treated with GH (500 ng/ml) for 48 h. Subsequently, the serum-free medium was removed, and cells were washed twice with a 1 mm CaCl2 and 1 mm MgCl2 mixture to preserve cadherin junctions. 2 ml of RMPI 1640 medium with 40 mg/ml BSA was then placed in the bottom chamber, and 0.3 ml of RPMI 1640 medium (without albumin) was placed in the top chamber. The cells were incubated at 37 °C, aliquots of medium were collected from the top chamber at various (1-, 2-, and 4-h) time points, and albumin concentration was measured in these aliquots using the bicinchoninic acid protein assay kit (Sigma-Aldrich). Absorbance was measured at 562 nm using an ELx 800 plate reader (BioTek).
Statistical Analysis
Data are presented as mean ± S.E. unless otherwise indicated. Moderated F-statistics (Limma R package) was used to analyze microarray data. p values <0.01 and <0.001 were used to distinguish differentially expressed genes in the GH-treated podocytes compared with untreated podocytes. SPSS software (SPSS, Inc.) was used to apply Mann-Whitney and Kruskal-Wallis nonparametric tests to analyze statistical differences between the distributions of two or multiple independent samples, respectively. p values equal to or less than 0.05 were considered significant.
RESULTS
GH Increases Expression of Transcription Factor ZEB2 in Glomerular Podocyte
To identify the cellular basis for GH action in the podocyte, we used microarray analysis to conduct an unbiased survey of genes regulated in a GH-dependent manner in the podocyte. Immortalized human podocytes were exposed to GH (500 ng/ml) for varying time periods, and mRNA was extracted and subjected to microarray analysis. Threshold and probabilistic filtering of the data at a high stringency of p < 0.001 identified several genes that were altered in a GH-dependent manner (Gene Expression Omnibus (GEO) accession number GSE21327). ZEB2, whose expression was increased in a GH-dependent manner, was chosen for further analysis based on potential biological significance given the established role of ZEB2 in TGF-β signaling and the importance of TGF-β in the pathogenesis of renal disorders (22–24). ZEB2 is also known as ZFHX1B and Smad interaction protein 1 (SIP1); we refer to this gene as ZEB2 throughout this study.
Results obtained from the microarray analysis were confirmed by quantitative real time RT-PCR measurement of the steady state abundance of ZEB2 mRNA. For this purpose, immortalized human podocytes were exposed to GH, and mRNA was harvested at various time periods. These experiments revealed that levels of ZEB2 mRNA, normalized to GAPDH expression, were increased 2.0- (24 h), 2.8- (48 h), and 4.8-fold (72 h) following exposure of podocytes to GH (Fig. 1A). Immunoblotting of lysates from podocytes exposed to GH also revealed a GH dose- and time-dependent increase in the levels of ZEB2 protein (Fig. 1, B and C). Thus, the effects of GH were maximal at a concentration of 500 ng/ml, and the effect plateaued at 48–72 h of exposure to GH. We next assayed the effect of GH on the ZEB2 promoter. GH stimulation of HepG2 cells transiently transfected with a ZEB2 promoter-luciferase reporter construct verified GH-dependent increase in ZEB2 promoter activity (Fig. 1D). A canonical action of GH is stimulation of production of IGF-1. To exclude the possibility that the observed effect of GH on ZEB2 expression was mediated by locally produced IGF-1, we exposed differentiated human podocytes to IGF-1 and measured ZEB2 expression in these cells. These results indicate that IGF-1 failed to stimulate expression of ZEB2 mRNA (data not shown) and protein (Fig. 1E) in these cells. These results suggest that GH-dependent increase in ZEB2 expression is independent of IGF-1 in this model system and experimental paradigm.
FIGURE 1.
GH-dependent increase in ZEB2 expression in glomerular podocyte. A, immortalized differentiated human podocytes were exposed to hGH (500 ng/ml) for the indicated time periods as detailed under “Experimental Procedures.” The steady state abundance of the ZEB2 transcript was measured by RT-qPCR and is depicted relative to ZEB2 mRNA abundance prior to exposure to GH; GAPDH was used as an internal control. The results (n = 4–5) are depicted as mean ± S.E. *, p < 0.05 (Kruskal-Wallis test) compared with expression prior to exposure to GH. B, immortalized differentiated human podocytes were exposed to hGH (500 ng/ml) for the indicated time periods and subjected to Western blot analysis for ZEB2 and tubulin as detailed under “Experimental Procedures.” Results shown are representative of three independent experiments. C, immortalized differentiated human podocytes were exposed to the indicated concentrations of hGH for 48 h and subjected to Western blot analysis for ZEB2 and tubulin as detailed under “Experimental Procedures.” Results shown are representative of three independent experiments. D, HepG2 cells were transiently transfected with ZEB2 promoter-reporter luciferase construct as detailed under “Experimental Procedures.” Cotransfection of Renilla luciferase was used to normalize transfection efficiency. The normalized luciferase activity of the cell exposed to GH (gray bar) is depicted relative to activity of the cells exposed to vehicle alone (black bar) designated as 100%. Error bars indicate mean ± S.E.; n = 4. *, p < 0.05 compared with vehicle-treated cells. E, immortalized differentiated human podocytes were exposed to IGF-1 (200 ng/ml) for the indicated time periods and subjected to Western blot analysis for ZEB2 and tubulin as detailed under “Experimental Procedures.” Results shown are representative of two independent experiments.
Increased Expression of Natural Antisense Transcript of ZEB2 as Mechanism for GH-dependent Regulation of ZEB2 in Glomerular Podocyte
A previous report had described a NAT of ZEB2 implicated in Snail1-induced expression of ZEB2 (20). In this model, Snail1 induced the expression of a ZEB2-NAT that overlaps with a donor splice site. Thus, ZEB2-NAT by binding to the complementary sense strand inhibited the splicing and processing of the cognate 5′-UTR ZEB2 intron (Fig. 2A). This ZEB2 intron also contains an internal ribosome entry site, and thus, the maintenance of the intron by the NAT facilitates ZEB2 translation and augments ZEB2 expression. We investigated this mechanism in the GH-dependent induction of ZEB2 in the glomerular podocyte. We obtained preliminary evidence of expression of the antisense transcript by demonstrating the presence of the internal ribosome entry site-containing intron in RNA from human podocyte cells exposed to GH (Fig. 2B). Hence, PCR amplification with the 1F/2R primer dyad of RNA prepared from cells naïve to GH yielded a 236-bp product predicted from splicing of the intron. In contrast, in cells exposed to GH, this PCR yielded an additional band of ∼2.7 kb that would infer the presence of the unspliced intron in this RNA. We further confirmed these results with another set of primers, 2F/2R. These results indicate that the abundance of the intron containing the 554-bp product was much greater in RNA from cells exposed to GH (Fig. 2B). Measurement of ZEB2 antisense RNA via quantitative RT-PCR confirmed the increase in levels of the transcript following stimulation of the cells with GH (Fig. 2C). We next assayed the effect of GH on the promoter of the ZEB2-NAT. Exposure of HepG2 cells transiently transfected with a NAT promoter-luciferase reporter construct to GH verified GH-dependent increase in NAT promoter activity (Fig. 2D). These results support a role for ZEB2-NAT expression in the observed GH-dependent increase in ZEB2 expression in the glomerular podocyte.
FIGURE 2.
GH-dependent increase in expression of natural antisense transcript of ZEB2. A, schematic depicting genomic organization of ZEB2 and origin of the ZEB2-NAT. The ZEB2 open reading frame (ORF) is depicted in black, the 5′-UTR intron is in white, the 5′-UTR and 3′-UTR exons are in gray, and the ZEB2 promoter is marked by downward diagonal stripes. The relative positions of the NAT, putative internal ribosome entry site (IRES), and the oligonucleotides used in the PCR analysis are also indicated. The numbering of the base pairs of the sequence depicted corresponds to NCBI Reference Sequence accession number NM014795.2. B, GH-dependent retention of the ZEB2 intron. Immortalized differentiated human podocytes were exposed to vehicle (CON) or hGH (500 ng/ml) for 36 h, and total RNA was isolated and reverse transcribed to cDNA as detailed under “Experimental Procedures.” The cDNA or mouse genomic DNA (as control) was used in PCR (left panel, 40 cycles; right panel, 25 and 35 cycles) using either 1F/2R (left panel) or 2F/2R (right panel) primers, and the amplified product was size-fractionated via agarose electrophoresis. The sizes of the DNA bands are indicated. Equality of sample loading was verified by quantification of an unrelated gene, β-globulin, in these samples. C, GH-dependent increase in expression of ZEB2-NAT. Immortalized differentiated human podocytes were exposed to hGH (500 ng/ml) for the indicated time periods as detailed under “Experimental Procedures.” The steady state abundance of the ZEB2 transcript was measured by RT-qPCR and is depicted relative to ZEB2 mRNA abundance prior to exposure to GH; GAPDH was used as an internal control. Error bars indicate mean ± S.E.; n = 4–5. *, p < 0.05 (Kruskal-Wallis test) compared with expression prior to exposure to GH. D, HepG2 cells were transiently transfected with ZEB2-NAT promoter-reporter luciferase construct and then exposed to either vehicle or hGH (500 ng/ml) for 24 h as detailed under “Experimental Procedures.” Cotransfection of Renilla luciferase was used to normalize transfection efficiency. The normalized luciferase activity of the cell exposed to GH (gray bar) is depicted relative to activity of the cells exposed to vehicle alone (black bar) designated as 100%. Error bars indicate mean ± S.E.; n = 4. *, p < 0.05 compared with vehicle-treated cells.
GH-dependent Inhibition of E-cadherin and P-cadherin Expression in Glomerular Podocyte
ZEB2 regulates several genes that are involved with cell junctions and cell-cell adhesion, and previous studies have established that ZEB2 is a transcriptional repressor of E-cadherin and P-cadherin gene expression. E-cadherin, a hemophilic Ca2+-dependent transmembrane adhesion protein, is also expressed in the glomerular podocyte (25). Having established that GH increases ZEB2 expression, we next analyzed the expression of E-cadherin in human podocytes exposed to GH. These experiments revealed a GH-dependent down-regulation of E-cadherin mRNA (Fig. 3A) and protein expression (Fig. 3, B and C). The decrement in E-cadherin expression was dose-dependent and could be demonstrated up to 72 h following exposure to GH (Fig. 3B). These effects of GH on ZEB2 and E-cadherin expression could also be demonstrated in murine podocytes (Fig. 3, D and E).
FIGURE 3.
Reciprocal decrease in E-cadherin expression with GH-dependent increase in ZEB2 expression in glomerular podocyte. A, immortalized differentiated human podocytes were exposed to hGH (500 ng/ml) as detailed under “Experimental Procedures.” E-cadherin mRNA abundance was measured by RT-qPCR and is depicted relative to E-cadherin mRNA abundance prior to exposure to GH; GAPDH was used as an internal control. The results (n = 4–5) are depicted as mean ± S.E. *, p < 0.05 (Kruskal-Wallis test) compared with expression prior to exposure to GH. B, immortalized differentiated human podocytes were exposed to hGH (500 ng/ml) for the indicated time periods and subjected to Western blot analysis for E-cadherin and tubulin as detailed under “Experimental Procedures.” Results shown are representative of three independent experiments. C, immortalized differentiated human podocytes were exposed to the indicated concentrations of hGH for 48 h and subjected to Western blot analysis sequentially for ZEB2, E-cadherin, and tubulin as detailed under “Experimental Procedures.” Results shown are representative of three independent experiments. D, immortalized differentiated murine podocytes (MPC-5) were exposed to ovine GH (oGH) (500 ng/ml) for the indicated time periods as detailed under “Experimental Procedures.” ZEB2 mRNA abundance was measured by RT-qPCR and is depicted relative to ZEB2 mRNA abundance prior to exposure to GH; GAPDH was used as an internal control. Error bars indicate mean ± S.E.; n = 4–5. *, p < 0.05 (Kruskal-Wallis test) compared with expression prior to exposure to ovine GH. E, immortalized differentiated murine podocytes (MPC-5) were exposed to ovine GH (500 ng/ml) for the indicated time periods and then subjected to Western blot analysis sequentially for ZEB2, E-cadherin, and tubulin as detailed under “Experimental Procedures.” Results shown are representative of three independent experiments.
P-cadherin is a component of the slit diaphragm apparatus of the glomerular podocyte. Our results reveal that, similar to E-cadherin expression, expression of P-cadherin mRNA (Fig. 4A) and protein (Fig. 4B) was decreased in human podocytes exposed to GH. The time and dose dependence of the effect of GH on P-cadherin expression was similar to that observed for E-cadherin.
FIGURE 4.

GH-dependent decrease in P-cadherin expression in glomerular podocyte. A, immortalized differentiated human podocytes were exposed to hGH (500 ng/ml) for the indicated time periods as detailed under “Experimental Procedures.” P-cadherin mRNA abundance was measured by RT-qPCR and is depicted relative to P-cadherin mRNA abundance prior to exposure to GH; GAPDH was used as an internal control. Error bars indicate mean ± S.E.; n = 4–5. *, p < 0.05 (Kruskal-Wallis test) compared with expression prior to exposure to GH. B, immortalized differentiated human podocytes were exposed to hGH (500 ng/ml) for the indicated time periods and subjected to Western blot analysis for P-cadherin and tubulin as detailed under “Experimental Procedures.” HepG2 lysates were also similarly analyzed as a positive control. Results shown are representative of three independent experiments.
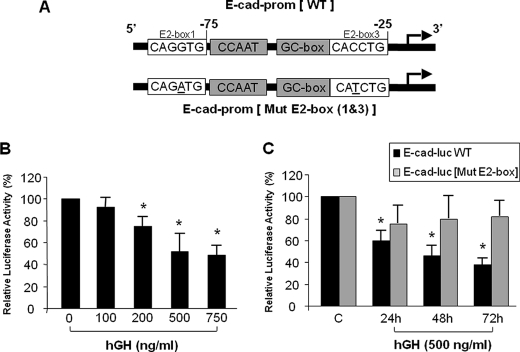
GH-dependent Inhibition of E-cadherin Promoter Activity
ZEB2 protein is characterized by a homeodomain flanked by two clusters of zinc fingers. Whereas each zinc finger cluster binds independently to CACCT sequences, a full-length monomeric ZEB2 can bind with high affinity to bipartite elements in the target gene composed of one CAGGTG target site and one CACCTG target site (26). The mouse and human E-cadherin promoters have a conserved modular structure with positive regulatory elements including two E2 boxes (CACCTG) that bind ZEB2 and transduce the suppressive action of ZEB2 on E-cadherin expression (Fig. 5A) (27–29). The results described above indicate that GH increases expression of ZEB2 in the glomerular podocyte and also decreases E-cadherin expression in these cells. Based on the temporal relationship between these two effects of GH in the podocyte, we hypothesized that the effect of GH on E-cadherin expression was transduced by the increase in ZEB2 expression. To test this hypothesis, we analyzed the effect of GH on the E-cadherin promoter. For this purpose, an E-cadherin promoter-luciferase reporter construct was transiently transfected into HepG2 cells, and luciferase activity was measured. These results indicate that GH induced a dose- and time-dependent decrease in the activity of the E-cadherin promoter (Fig. 5, B and C). To verify that the observed effect of GH on the E-cadherin promoter was due to alteration in interaction of ZEB2 with the E-cadherin promoter, we engineered an E-cadherin-promoter luciferase construct with mutation in the E2 boxes that bind ZEB2 and tested the effect of GH on the activity of this mutant luciferase reporter construct (Fig. 5A). These experiments reveal that, in contrast to the wild type E-cadherin promoter-reporter construct, GH failed to alter the activity of the mutant E-cadherin promoter-reporter construct (Fig. 5C). Hence, these results support a model wherein GH decreases E-cadherin expression via an increase in ZEB2 expression in the glomerular podocyte.
FIGURE 5.
GH-dependent decrease in E-cadherin promoter activity is mediated via ZEB2 binding sites on E-cadherin promoter. A, spatial organization of conserved E2 boxes (putative ZEB2 binding sites) on the E-cadherin promoter (E-cad-prom) (top schematic). The bottom schematic depicts the point mutations (underlined) introduced in the two E-boxes (Mut E2-box) designed to abrogate binding of ZEB2 to the E2 box ZEB2 binding sites on the E-cadherin promoter. B, HepG2 cells were transiently transfected with E-cadherin promoter-luciferase construct and then exposed to the indicated concentration of hGH for 24 h as detailed under “Experimental Procedures.” Cotransfection of Renilla luciferase was used to normalize transfection efficiency. The normalized luciferase activity of the cell exposed to GH is depicted relative to activity of the cells exposed to vehicle alone designated as 100%. Error bars indicate mean ± S.E.; n = 4. *, p < 0.05 compared with vehicle-treated cells. C, HepG2 cells were transiently transfected with either E-cadherin promoter-luciferase (E-cad-luc) (black bar) or E-cadherin promoter-luciferase construct with point mutations in the two E2 boxes (gray bar) and exposed to hGH (500 ng/ml) for the indicated time periods as detailed under “Experimental Procedures.” Cotransfection of Renilla luciferase was used to normalize transfection efficiency. The normalized luciferase activity for each individual construct exposed to GH is depicted relative to activity of the respective construct exposed to vehicle alone designated as 100%. Error bars indicate mean ± S.E.; n = 5–7. *, p < 0.01 compared with vehicle-treated cells.
ZEB2 Knockdown Prevents GH-dependent Decrease in E-cadherin in Human Podocytes
We next ascertained whether ZEB2 played an essential role in the GH-dependent decrease in E-cadherin expression. For this purpose, we used shRNA lentivirus to engineer human podocytes with specific knockdown of ZEB2 expression. The decrement in ZEB2 expression was confirmed by both RT-qPCR of mRNA and immunoblotting of cell lysates (Fig. 6, A and B). After having confirmed the efficacy and specificity of the shRNA lentivirus-mediated knockdown, these cells were exposed to GH, and the levels of E-cadherin were measured by immunoblotting. In podocytes infected with lentivirus expressing ZEB2 shRNA, GH failed to elicit a decrease in E-cadherin expression (Fig. 6C). Hence, these results indicate that ZEB2 is essential for transducing the effect of GH on E-cadherin expression in the glomerular podocyte.
FIGURE 6.
Knockdown of ZEB2 expression in glomerular podocytes abrogates GH-dependent decrease in E-cadherin expression. A, immortalized human podocytes cultured under growth-permissive conditions were transduced with lentiviral constructs expressing either ZEB2 shRNA (shRNAs 1 and 2) or scrambled shRNA. Following transduction, cells were expanded, and aliquots were induced to differentiate for 14 days prior to harvesting of RNA for analysis. Steady state ZEB2 mRNA abundance was measured by RT-qPCR; GAPDH was used as an internal control. The results (n = 4–5) are depicted as mean ± S.E. The steady state abundance of the ZEB2 transcript is depicted relative to ZEB2 mRNA abundance in non-transduced naïve cells. B, immortalized human podocytes transduced with lentiviral constructs expressing either ZEB2 shRNA (shRNAs 1 and 2) or scrambled shRNA were differentiated for 14 days and then subjected to Western blot analysis for ZEB2 and tubulin as detailed under “Experimental Procedures.” Results shown are representative of two independent experiments. C, immortalized human podocytes transduced with lentiviral construct expressing either scrambled shRNA (left panel) or ZEB2 shRNA 2 (right panel) were differentiated, exposed to hGH (500 ng/ml), and subjected to Western blot analysis for E-cadherin and tubulin as detailed under “Experimental Procedures.” Densitometric analysis of the E-cadherin band, normalized for respective tubulin expression, is depicted relative to expression prior to exposure to GH. Error bars indicate mean ± S.E.; n = 4. *, p < 0.05 (Kruskal-Wallis test) compared with expression prior to exposure to GH.
Essential Role of ZEB2 in Effect of GH on Filtration Barrier Function of Podocytes
Results described above indicated that GH decreased E- and P-cadherin expression in the glomerular podocyte. These cadherins are expressed in the podocyte and the podocyte slit diaphragm, and the slit diaphragm is a key component of the glomerular filtration barrier. Hence, we hypothesized that the effect of GH on E- and P-cadherin expression would manifest as changes in the functioning of the filtration barrier of the podocyte. To assess the functional consequence of podocyte exposure to GH, we examined the filtration barrier function of podocyte via a paracellular permeability assay that measures the rate of albumin flux across the monolayer of differentiated podocytes (30). For these experiments, human podocytes were grown and differentiated on a Transwell filter and exposed to GH (500 ng/ml) for 48 h, and the influx of albumin across the Transwell membrane was measured. These results reveal a GH-dependent increase in albumin influx across the podocyte monolayer (Fig. 7A). The magnitude of the GH-dependent increase in albumin efflux was similar to that observed for TGF-β (Fig. 7A and Ref. 30). To delineate the role of ZEB2 in this effect of GH on the permeability of the podocyte monolayer, we next investigated this phenomenon in podocytes engineered with shRNA lentivirus-mediated knockdown of ZEB2 expression. These results indicate that knockdown of ZEB2 expression resulted in blunting of the ability of GH to elicit an increase in permeability of the podocyte monolayer to albumin (Fig. 7B). Hence, these results indicate that ZEB2 is essential for transducing the effect of GH on the permeability of the podocyte monolayer.
FIGURE 7.
ZEB2 is essential for GH-dependent increase in permeability of podocyte monolayer. Immortalized human podocytes transduced with lentiviral construct expressing ZEB2 shRNAs 1 and 2 (right), untransduced (left), or transduced with lentiviral construct expressing scrambled shRNA (left) were grown as a monolayer on collagen-coated Transwell filters and induced to differentiate for 14 days prior to incubation with vehicle (black bar), hGH (500 ng/ml) (gray bar), or TGF-β (white bar) for 48 h, and albumin permeability across the podocyte monolayer was determined at 1, 2, and 4 h following the 48-h exposure to hGH. Error bars indicate mean ± S.E.; n = 4. The data for the TGF-β experiments are depicted as the average of two experiments. *, p < 0.05 versus control (CON).
DISCUSSION
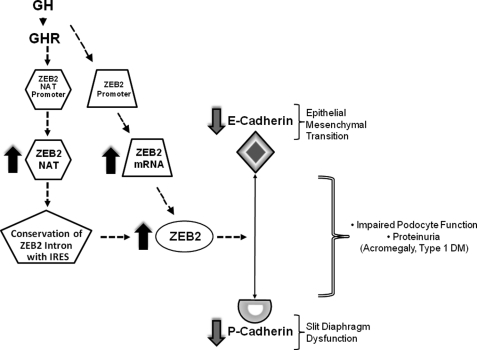
The glomerular podocyte plays an essential role in glomerular filtration, and previous studies have indicated that the podocyte is a target of GH action. We undertook the current investigation to understand the cellular and molecular basis for the effects of GH on the podocyte. Our results indicate a novel action of GH with induction of the zinc finger E-box-binding transcription factor ZEB2. This increase in ZEB2 protein expression is due to the GH-dependent up-regulation of ZEB2 mRNA expression and the induction of a NAT of the ZEB2 gene. We demonstrate that GH-dependent induction of ZEB2 in the podocyte results in decreased expression of P- and E-cadherins. P-cadherin is a component of the podocyte slit diaphragm, and E-cadherin participates in epithelial-mesenchymal transition (EMT). Our studies also establish that GH increases permeability of a podocyte monolayer, and this effect is consequent to GH-dependent induction of ZEB2. An overactive GH/GHR axis is implicated in the early changes of DN, and our results provide a cellular and molecular basis for the role of GH in the pathogenesis of DN and renal changes associated with acromegaly (Fig. 8).
FIGURE 8.
Proposed model for role of ZEB2 in GH action on glomerular podocyte. Engagement of the GHR by GH results in increased activity of promoters for ZEB2-NAT and ZEB2 mRNA. These changes in promoter activities result in increased expression of ZEB2-NAT and ZEB2 mRNA. ZEB2-NAT impairs splicing of the internal ribosome entry site (IRES)-containing intron, thus facilitating translation of ZEB2 protein. ZEB2 protein interacts with promoters of P- and E-cadherins to inhibit expression of the cognate gene. P-cadherin is an essential component of the slit diaphragm apparatus, which is central to the size selectivity of the glomerular filtration barrier. Deceased E-cadherin expression is implicated in epithelial-mesenchymal transition that results in podocyte dehiscence and loss in disease states such as diabetes mellitus. These changes in P- and E-cadherin expression will manifest as impaired podocyte function and proteinuria in pathological states of GH/GHR axis overactivity in the kidney (e.g. acromegaly and type 1 diabetes mellitus (DM)).
Our results establish that GH stimulates expression of ZEB2. ZEB2, also known as SIP1 and ZFHX1B, belongs to the δEF1 or ZEB protein family. This family of proteins is characterized by the presence of a homeodomain flanked by two conserved zinc finger clusters comprising a four-zinc finger N-terminal cluster and three-zinc finger C-terminal cluster (31). ZEB2 acts as a transcriptional repressor and contains consensus binding sites for the corepressor C-terminal binding protein. Gene repression by ZEB2 can be either C-terminal binding protein-dependent or -independent (32). Because ZEB2 has many potential targets including P- and E-cadherins, the increase in ZEB2 in a GH-dependent manner could have pleiotropic effects on the functioning of the glomerular podocyte. P-cadherin, a canonical target of ZEB2, is a member of the cadherin superfamily and is a component of the slit diaphragm apparatus (25). P-cadherin mediates homophilic interactions between cells, and one of the roles attributed to P-cadherin is mediating cell-cell adhesion between adjacent podocyte foot processes. It is hypothesized that P-cadherin serves as the basic scaffold for the slit diaphragm and is physically linked to the actin cytoskeleton of the foot process through cadherin adapter proteins α-, β-, and γ-catenin and ensuing interactions with α-actinin and MAGI (membrane-associated guanylate kinases with inverted domain structure) proteins (33). Decreased expression of P-cadherin is implicated in the pathogenesis of proteinuria in type 1 diabetes mellitus (T1DM) (34). E-cadherin is also a bona fide target for transcriptional repression by ZEB2. E-cadherin has been demonstrated to exist in cytoplasmic vesicles in podocytes in culture (25). The precise role of E-cadherin in the podocyte is unclear, although a role in the recently described EMT phenomenon in podocytes is a possibility (see below). It is noteworthy that the key findings of the current study, GH-dependent increase in ZEB2 and resultant decrease in E- and P-cadherins, were observed in both human and murine podocytes.
ZEB2 expression is controlled at multiple levels including transcriptional and post-transcriptional. Our results indicate that GH controls ZEB2 expression both by modulating ZEB2 gene transcription, as evidenced by an increase in ZEB2 promoter activity, and by regulating post-transcriptional events, as evidenced by an increase in abundance of a ZEB2-NAT. ZEB2-NAT is a recently described mechanism implicated in the control of ZEB2 expression, and to date, this mechanism has been described in Snail1-dependent regulation of ZEB2 expression (20). Our results demonstrate that GH increases the promoter activity of the putative NAT promoter. An in silico analysis (data not shown) of the ZEB2-NAT promoter revealed three putative STAT binding sites. The precise role(s) of these canonical GH response cis-elements in GH-dependent increase in ZEB2-NAT expression remains to be defined. In addition to the glomerular podocyte, our finding of GH-dependent increase in ZEB2 expression is also relevant to the role of GH in malignancies such as breast and prostate cancer wherein the GH/GHR axis has been demonstrated to play a permissive role (35). It is noteworthy that we were unable to verify expression of a closely related member of the ZEB family of protein, ZEB1 (δEF1/ZFHX1A), in the podocyte (data not shown).
The clinical hallmarks of DN include progressive albuminuria followed by a gradual decline in renal function concluding, after 5–15 years, with end stage renal disease (36). Several reports have suggested that the loss of glomerular podocytes precedes and predicts the onset of clinical nephropathy and may be an early and important pathological manifestation of DN (37–39). Epidemiology of Diabetes Interventions and Complications and related studies have revealed long term effects (“metabolic memory”) of glycemic control on glomerular function, suggesting that events early in the course of T1DM have long lasting effects on renal function (40). In this regard, it is noteworthy that podocytes are terminally differentiated cells, and thus, podocyte loss incurred early in the course of T1DM is likely to impact glomerular function later in the course of the disease. This sequence of events underscores the importance of fully understanding the myriad processes that could play a role in podocyte dysfunction and/or loss in T1DM. Our results establish that GH stimulates expression of ZEB2 and consequent ZEB2-dependent suppression of E-cadherin expression. Decreased E-cadherin expression is believed to play an essential role in the conversion of epithelial cells to a more fibroblast-like morphology, a process termed epithelial-mesenchymal transition (41). Recently EMT has been implicated in podocyte loss in DN (30, 42, 43). The original reports describing podocyte EMT alluded to the role of TGF-β in inducing EMT (43). Results presented in the current study indicate that GH can also trigger podocytes to undergo EMT. The previous report describing EMT of podocytes in DN also demonstrated increased expression of the fibroblast-specific protein 1 (FSP-1) gene in the podocyte in DN (43). FSP-1 (also termed S100A4 and metastatin) is a calcium-binding protein implicated in EMT induction (44). It is noteworthy that our studies reveal that GH also increases expression of FSP-1 in immortalized human podocytes (data not shown). Because excessive GH secretion is a hallmark of poorly controlled T1DM, we suggest that overactivity of the GH/GHR axis contributes to the dysfunction and ultimate loss of podocytes and thus plays a central role in the early pathogenesis of DN. These findings have direct translational significance because measures to blunt the effect of excess GH activity especially early in the course of T1DM could have a salutary effect on podocyte loss and thus positively influence the course of DN.
In summary, this report describes a novel action of GH on the glomerular podocyte. Our results demonstrate that GH increases ZEB2 expression in the podocyte, and this action of GH is mediated, in part, via increased expression of NAT. The GH-dependent increase in ZEB2 results in decreased expression of P- and E-cadherin in the podocyte. Furthermore, GH increases the permeability of a podocyte monolayer, and GH-induced increase in ZEB2 is essential for this effect of GH on the podocyte permeability barrier. We propose that the GH-dependent increase in ZEB2 plays a role in EMT in the podocyte and that this novel action of GH provides a cellular and molecular basis for the role of GH in the pathogenesis of DN.
Acknowledgments
We acknowledge the generous provision of reagents by Drs. Eric R. Fearon (University of Michigan), Stuart J. Frank (University of Alabama at Birmingham), Antonio García de Herreros (Institut Municipal d'Investigació Mèdica-Hospital del Mar, Universitat Pompeu Fabra, Barcelona, Spain), and Moin A. Saleem (University of Bristol).
This work was supported, in whole or in part, by National Institutes of Health Grants T32 DK071212 (to K. K.), DK49845 (to R. K. M.), and P60DK-20572 (to the Michigan Diabetes Research and Training Center) and the D.R.E.A.M. Foundation.
- GH
- growth hormone
- DN
- diabetic nephropathy
- EMT
- epithelial-mesenchymal transition
- GHR
- GH receptor
- NAT
- natural antisense transcript
- T1DM
- type 1 diabetes mellitus
- ZEB2
- zinc finger E-box-binding homeobox 2
- ZFHX1B
- zinc finger homeobox 1B
- qPCR
- quantitative PCR
- IGF-1
- insulin-like growth factor-1
- SIP1
- Smad interaction protein 1
- FSP-1
- fibroblast-specific protein 1
- hGH
- human GH.
REFERENCES
- 1.Møller N., Jørgensen J. O. (2009) Endocr. Rev. 30, 152–177 [DOI] [PubMed] [Google Scholar]
- 2.Pantaleon M., Whiteside E. J., Harvey M. B., Barnard R. T., Waters M. J., Kaye P. L. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 5125–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han X., Ren X., Jurickova I., Groschwitz K., Pasternak B. A., Xu H., Wilson T. A., Hogan S. P., Denson L. A. (2009) Gut 58, 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu C., Schwartzbauer G., Sperling M. A., Devaskar S. U., Thamotharan S., Robbins P. D., McTiernan C. F., Liu J. L., Jiang J., Frank S. J., Menon R. K. (2001) J. Biol. Chem. 276, 22892–22900 [DOI] [PubMed] [Google Scholar]
- 5.Baudet M. L., Rattray D., Martin B. T., Harvey S. (2009) Endocrinology 150, 2758–2766 [DOI] [PubMed] [Google Scholar]
- 6.Manelli F., Bossoni S., Burattin A., Doga M., Solerte S. B., Romanelli G., Giustina A. (2000) Metabolism 49, 634–639 [DOI] [PubMed] [Google Scholar]
- 7.Hoogenberg K., Sluiter W. J., Dullaart R. P. (1993) Acta Endocrinol. 129, 151–157 [DOI] [PubMed] [Google Scholar]
- 8.Baldelli R., De Marinis L., Bianchi A., Pivonello R., Gasco V., Auriemma R., Pasimeni G., Cimino V., Appetecchia M., Maccario M., Lombardi G., Pontecorvi A., Colao A., Grottoli S. (2008) J. Clin. Endocrinol. Metab. 93, 710–714 [DOI] [PubMed] [Google Scholar]
- 9.Cummings E. A., Sochett E. B., Dekker M. G., Lawson M. L., Daneman D. (1998) Diabetes 47, 1341–1346 [DOI] [PubMed] [Google Scholar]
- 10.Christiansen J. S., Gammelgaard J., Orskov H., Andersen A. R., Telmer S., Parving H. H. (1981) Eur. J. Clin. Invest. 11, 487–490 [DOI] [PubMed] [Google Scholar]
- 11.Blankestijn P. J., Derkx F. H., Birkenhäger J. C., Lamberts S. W., Mulder P., Verschoor L., Schalekamp M. A., Weber R. F. (1993) J. Clin. Endocrinol. Metab. 77, 498–502 [DOI] [PubMed] [Google Scholar]
- 12.Reddy G. R., Pushpanathan M. J., Ransom R. F., Holzman L. B., Brosius F. C., 3rd, Diakonova M., Mathieson P., Saleem M. A., List E. O., Kopchick J. J., Frank S. J., Menon R. K. (2007) Endocrinology 148, 2045–2055 [DOI] [PubMed] [Google Scholar]
- 13.Stitt-Cavanagh E., MacLeod L., Kennedy C. (2009) ScientificWorldJournal 9, 1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathieson P. W. (2009) Curr. Opin. Nephrol. Hypertens. 18, 206–211 [DOI] [PubMed] [Google Scholar]
- 15.Reddy G. R., Kotlyarevska K., Ransom R. F., Menon R. K. (2008) Curr. Opin. Nephrol. Hypertens. 17, 32–36 [DOI] [PubMed] [Google Scholar]
- 16.Asanuma K., Mundel P. (2003) Clin. Exp. Nephrol. 7, 255–259 [DOI] [PubMed] [Google Scholar]
- 17.Endlich N., Kress K. R., Reiser J., Uttenweiler D., Kriz W., Mundel P., Endlich K. (2001) J. Am. Soc. Nephrol. 12, 413–422 [DOI] [PubMed] [Google Scholar]
- 18.Mundel P., Reiser J., Zúñiga Mejía Borja A., Pavenstädt H., Davidson G. R., Kriz W., Zeller R. (1997) Exp. Cell Res. 236, 248–258 [DOI] [PubMed] [Google Scholar]
- 19.Saleem M. A., O'Hare M. J., Reiser J., Coward R. J., Inward C. D., Farren T., Xing C. Y., Ni L., Mathieson P. W., Mundel P. (2002) J. Am. Soc. Nephrol. 13, 630–638 [DOI] [PubMed] [Google Scholar]
- 20.Beltran M., Puig I., Peña C., García J. M., Alvarez A. B., Peña R., Bonilla F., de Herreros A. G. (2008) Genes Dev. 22, 756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmittgen T. D., Livak K. J. (2008) Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 22.Hills C. E., Squires P. E. (2010) Am. J. Nephrol. 31, 68–74 [DOI] [PubMed] [Google Scholar]
- 23.Chen S., Jim B., Ziyadeh F. N. (2003) Semin. Nephrol. 23, 532–543 [DOI] [PubMed] [Google Scholar]
- 24.Ziyadeh F. N. (2004) J. Am. Soc. Nephrol. 15, Suppl. 1, S55–S57 [DOI] [PubMed] [Google Scholar]
- 25.Reiser J., Kriz W., Kretzler M., Mundel P. (2000) J. Am. Soc. Nephrol. 11, 1–8 [DOI] [PubMed] [Google Scholar]
- 26.Remacle J. E., Kraft H., Lerchner W., Wuytens G., Collart C., Verschueren K., Smith J. C., Huylebroeck D. (1999) EMBO J. 18, 5073–5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behrens J., Löwrick O., Klein-Hitpass L., Birchmeier W. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 11495–11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giroldi L. A., Bringuier P. P., de Weijert M., Jansen C., van Bokhoven A., Schalken J. A. (1997) Biochem. Biophys. Res. Commun. 241, 453–458 [DOI] [PubMed] [Google Scholar]
- 29.Comijn J., Berx G., Vermassen P., Verschueren K., van Grunsven L., Bruyneel E., Mareel M., Huylebroeck D., van Roy F. (2001) Mol. Cell 7, 1267–1278 [DOI] [PubMed] [Google Scholar]
- 30.Li Y., Kang Y. S., Dai C., Kiss L. P., Wen X., Liu Y. (2008) Am. J. Pathol. 172, 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandewalle C., Van Roy F., Berx G. (2009) Cell. Mol. Life Sci. 66, 773–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Grunsven L. A., Michiels C., Van de Putte T., Nelles L., Wuytens G., Verschueren K., Huylebroeck D. (2003) J. Biol. Chem. 278, 26135–26145 [DOI] [PubMed] [Google Scholar]
- 33.Faul C., Asanuma K., Yanagida-Asanuma E., Kim K., Mundel P. (2007) Trends Cell Biol. 17, 428–437 [DOI] [PubMed] [Google Scholar]
- 34.Xu Z. G., Ryu D. R., Yoo T. H., Jung D. S., Kim J. J., Kim H. J., Choi H. Y., Kim J. S., Adler S. G., Natarajan R., Han D. S., Kang S. W. (2005) Nephrol. Dial. Transplant. 20, 524–531 [DOI] [PubMed] [Google Scholar]
- 35.Corrêa L. L., Lima G. A., Paiva H. B., Silva C. M., Cavallieri S. A., Miranda L. C., Gadelha M. R. (2009) Arq. Bras. Endocrinol. Metabol. 53, 963–968 [DOI] [PubMed] [Google Scholar]
- 36.Mauer S. M. (1994) Kidney Int. 45, 612–622 [DOI] [PubMed] [Google Scholar]
- 37.Pagtalunan M. E., Miller P. L., Jumping-Eagle S., Nelson R. G., Myers B. D., Rennke H. G., Coplon N. S., Sun L., Meyer T. W. (1997) J. Clin. Investig. 99, 342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer T. W., Bennett P. H., Nelson R. G. (1999) Diabetologia 42, 1341–1344 [DOI] [PubMed] [Google Scholar]
- 39.Susztak K., Böttinger E. P. (2006) J. Am. Soc. Nephrol. 17, 361–367 [DOI] [PubMed] [Google Scholar]
- 40.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2003) JAMA 290, 2159–216714570951 [Google Scholar]
- 41.Yang J., Weinberg R. A. (2008) Dev. Cell 14, 818–829 [DOI] [PubMed] [Google Scholar]
- 42.Reidy K., Susztak K. (2009) Am. J. Kidney Dis. 54, 590–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi Y., Iwano M., Suzuki D., Nakatani K., Kimura K., Harada K., Kubo A., Akai Y., Toyoda M., Kanauchi M., Neilson E. G., Saito Y. (2009) Am. J. Kidney Dis. 54, 653–664 [DOI] [PubMed] [Google Scholar]
- 44.Boye K., Maelandsmo G. M. (2010) Am. J. Pathol. 176, 528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]