Abstract
AKT phosphorylates components of the intrinsic cell survival machinery and promotes survival to various stimuli. In the present study, we identified CDC-like kinase 2 (CLK2) as a new substrate of AKT activation and elucidated its role in cell survival to ionizing radiation. AKT directly binds to and phosphorylates CLK2 on serine 34 and threonine 127, in vitro and in vivo. CLK2 phosphorylation was detected in HeLa cells overexpressing active AKT. In addition, we demonstrated that ionizing radiation induces CLK2 phosphorylation via AKT activation. In contrast, the suppression of endogenous AKT expression by siRNA inhibited CLK2 phosphorylation in response to 2 gray of γ-ray or insulin. Furthermore, we examined the effect of CLK2 on the survival of irradiated CCD-18Lu cells overexpressing Myc-CLK2. CLK2 overexpression significantly increased cell growth and inhibited cell death induced by 2 gray. The role of CLK2 in cell survival to ionizing radiation was dependent on the phosphorylation of serine 34 and threonine 127. Our results suggest that AKT activation controls cell survival to ionizing radiation by phosphorylating CLK2, revealing an important regulatory mechanism required for promoting cell survival.
Keywords: Akt PKB, Cell Death, Gene Regulation, Insulin, Protein Phosphorylation, CLK2, Cell Survival, Ionizing Radiation
Introduction
AKT (protein kinase B) is a serine/threonine kinase and a key component of a pathway that controls cell survival and proliferation by inhibiting apoptosis signals, increasing glucose uptake (1), and promoting cell cycle progression (2, 3). AKT is potently activated in response to a wide variety of stimuli, including growth factors and ionizing radiation (4, 5). Full activation of AKT, in response to ionizing radiation, is mediated by ATM (6). AKT predominantly localizes to the cytoplasm and translocates to the membrane or nucleus upon cellular stimulation (7–9). To understand stimulus-dependent signal transduction through AKT, it is important to identify substrates of AKT and elucidate their biological functions.
Ionizing radiation induces a variety of cellular responses, such as DNA damage and repair, cell cycle arrest, apoptosis, and carcinogenesis (10–15). However, it was also reported that low dose radiation triggers DNA repair, cell survival, and cell proliferation via the induction of cellular protective mechanisms (16–19). Ionizing radiation regulates cell survival by activating a variety of signaling cascades, including the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. Our previous study showed that the activation of AKT is associated with cell protection against ionizing radiation-induced apoptosis (20). AKT directly phosphorylates acinus and regulates its expression via the nuclear factor κB pathway, thereby controlling cell survival to ionizing radiation (20, 21). AKT control of diverse cellular responses may be dependent on the regulation of downstream substrates.
The CDC2-like kinase (CLK)2 family proteins are evolutionarily conserved nuclear dual-specificity kinases that act on both serine/threonine and tyrosine residues (22, 23). The human CLK family includes three highly conserved isoforms, CLK1, CLK2, and CLK3 (24–26). CLK1 was initially identified by its ability to autophosphorylate tyrosine residues (27, 28). CLK2 and CLK3 have been shown to phosphorylate serine/arginine-rich proteins of the spliceosomal complex, which control alternative splicing (29, 30). It has been suggested that CLK2 localization and activity are influenced by its phosphorylation status (31). Therefore, CLK2 may be regulated by a number of kinases and phosphatases. However, the upstream molecules of CLK family kinases are still largely unknown, but may play an important and evolutionarily conserved role in signal transduction.
Given the diverse roles of the AKT pathway in the cellular response to ionizing radiation, it is important to identify new physiological substrates of activated AKT and to elucidate their biological functions. In this study, we first show that CLK2 is a novel substrate for AKT and that CLK2 phosphorylation by activated AKT leads to cell survival following ionizing radiation.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Polyclonal anti-CLK2 (N-terminal) antibody was purchased from ABGENT (San Diego, CA), anti-phospho-Ser/Thr from Upstate Biotechnology (Lake Placid, NY), anti-phospho-AKT (Ser473), anti-AKT, anti-phospho-GSK-3α/β, and anti-MEK1/2 from Cell Signaling Technology, Inc. (Danvers, MA). Monoclonal anti-Myc tag and anti-phospho-MEK1/2 antibody was purchased from Cell Signaling Technology, Inc., anti-actin from Sigma, and anti-GAPDH from Santa Cruz (Santa Cruz, CA). LY294002 and wortmannin were purchased from Cell Signaling Technology, Inc., and insulin and other chemicals from Sigma.
Cell Culture
Human lung fibroblast CCD-18Lu cells and HeLa cells were purchased from the American Type Culture Collection (Rockville, MD). CCD-18Lu and HeLa cells were cultured in minimum essential medium (Invitrogen) and Dulbecco's modified Eagle's medium (DMEM, Invitrogen) containing 10% fetal bovine serum (Invitrogen) and maintained at 37 °C in a humidified incubator containing 95% air and 5% CO2.
Plasmid Construction and Transfection
The pUSEamp-AKT1 (wild type) and pUSEamp-myr-AKT1 (activated) were purchased from Upstate Biotechnology. The pcDNA3.1-myc-CLK2 was kindly provided by Dr. Sungkwan An. The dominant-negative mutants of human CLK2 cDNAs (S34A, S36A, and T127A) were generated by PCR with WT CLK2 cDNA as the template. Cells were transfected with appropriate plasmids using Lipofectamine Plus (Invitrogen), according to the manufacturer's protocol.
Irradiation and Assessment of Cell Survival
Cells were seeded into 35-mm dishes at a density of 1 × 105 cells per dish 1 day prior to irradiation. Cells were irradiated with a total dose of 0.05, 0.2, or 2 Gy at a dose rate of 0.8 Gy/min using a 137Cs γ-irradiator (IBL 437C, CIS Bio International Co., France). To measure the viability of the irradiated cells, MTT assays were performed according to the manufacturer's instructions (Sigma). For the determination of cell proliferation, colorimetric immunoassay was performed using Cell Proliferation ELISA, BrdU colorimetric assay kit (Roche Applied Science). For the quantification of apoptosis, DNA fragmentation was detected using HT TiterTACS Assay Kit according to the manufacturer's instructions (Trevigen, Inc., Gaithersburg, MD).
PAGE and Immunoblot Analysis
Cells were lysed with SDS lysis buffer containing 125 mm Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, and 0.004% bromphenol blue, then boiled for 10 min. Protein contents were measured using BCA Protein Assay Reagent (Pierce). Samples were diluted with the lysis buffer containing 1.28 m β-mercaptoethanol. Equal amounts of protein were loaded onto 8–10% SDS-polyacrylamide gels. Proteins were electrophoretically transferred to nitrocellulose membranes. The membranes were then blocked with 5% nonfat dry milk in PBS/Tween-20 (0.1%, v/v) at 4 °C overnight, then incubated with primary antibody for 3 h, followed by horseradish peroxidase-conjugated secondary antibody for 1 h. Immunoreactive proteins were visualized by enhanced chemiluminescence (Amersham Biosciences).
Immunoprecipitation and in Vitro Kinase Assay
Cells were lysed in 1 ml of ice-cold lysis buffer containing 20 mm Tris (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerolphosphate, 1 mm Na3VO4, 1 μg/ml of leupeptin, and 1 mm PMSF. After centrifugation, the supernatants were incubated with primary antibody at 4 °C for 2 h. Subsequently, protein A/G beads (Pierce) were added and incubated at 4 °C overnight. Immunoprecipitates were washed twice with lysis buffer and boiled for 3 min after addition of sample loading buffer. After centrifugation, the supernatants were used for immunoblot analysis.
For in vitro kinase assays, cell lysates were incubated with anti-CLK2 or anti-Myc antibody at 4 °C for 2 h and then protein A/G beads (Pierce) were added and incubated at 4 °C overnight. Immunoprecipitates were washed twice with lysis buffer and twice with kinase buffer. To measure CLK2 phosphorylation, in vitro kinase assays were performed using an AKT kinase assay kit with recombinant active AKT protein and immunoprecipitated CLK2, according to the manufacturer's instruction (Cell Signaling Technology, Inc.). Glycogen synthase kinase 3 (GSK-3) fusion protein was used as a positive control for the AKT kinase assays.
Transfection of Small Interfering RNA (siRNA) for AKT or CLK2
To knockdown AKT or CLK2 expression in CCD-18Lu cells, cells were transfected with siRNA SMARTpool AKT1 (Dharmacon Inc., Chicago, IL) or siRNA SMARTpool CLK2 (Dharmacon Inc.) using the Cell Line Nucleofector Kit R (Amaxa Inc., Gaithersburg, MD), according to the manufacturer's instructions. ON-TARGETplus GAPDH siRNA (Dharmacon Inc.) was used as a control.
RESULTS
AKT Binds to and Phosphorylates CLK2
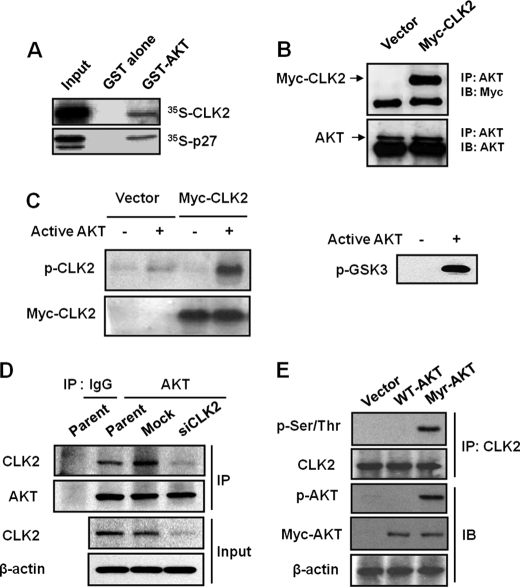
To identify novel substrates of AKT, we systematically screened the bioinformation data base for proteins containing an AKT consensus phosphorylation site. We identified CLK2 as a new candidate for AKT target protein. First, to determine whether AKT binds to CLK2, we prepared GST recombinant AKT protein and combined it in a reaction with 35S-labeled CLK2 protein. 35S-Labeled p27, one of the AKT-binding proteins, was used as a positive control for the in vitro binding assay. The results of GST pull-down suggested that CLK2 associates with AKT (Fig. 1A). To confirm the in vivo CLK2 binding to AKT, we transfected HeLa cells with myc-CLK2 and then immunoprecipitated endogenous AKT followed by Western blot analysis with anti-Myc antibody. Fig. 1B shows that CLK2 was co-immunoprecipitated with AKT, suggesting that AKT interacts directly with CLK2, in vivo.
FIGURE 1.
AKT binds to and phosphorylates CLK2. A, CLK2 co-precipitates with GST-AKT, in vitro. 35S-Labeled CLK2 (10 μl) produced from a CLK2 cDNA construct was used in an in vitro binding assay with GST-AKT. 35S-Labeled p27 was used as a positive control for the in vitro binding assay. B, CLK2 binds to endogenous AKT. HeLa cells were transfected with control vector or myc-CLK2. AKT was immunoprecipitated followed by Western blotting with anti-Myc antibody. C, CLK2 was phosphorylated by recombinant active AKT protein. In vitro AKT kinase assays were performed with immunoprecipitated (IP) CLK2 with anti-CLK2 antibody from HeLa cells overexpressing Myc-CLK2. Western blotting was performed with anti-phospho-Ser/Thr or anti-Myc antibodies. GSK-3 fusion protein was used as a positive control for the in vitro AKT kinase assays. D, endogenous CLK2 binds to endogenous AKT. HeLa cells were transfected with siNon-Target (Mock) or siRNA specific for CLK2 (siCLK2). After 24 h of transfection, AKT was immunoprecipitated followed by Western blotting with anti-CLK2 antibody. E, active AKT phosphorylates CLK2. HeLa cells were transiently transfected with vector, WT-AKT1, or Myr-AKT1. After 24 h of transfection, the phosphorylation of CLK2 was detected using immunoprecipitation with anti-CLK2 antibody followed by Western blotting with anti-phospho-Ser/Thr antibody. IB, immunoblot.
We then asked whether CLK2 is directly phosphorylated by AKT. We transfected myc-CLK2 into HeLa cells, and then immunoprecipitated CLK2 was incubated in vitro with recombinant active AKT. GSK-3 fusion protein was used as a positive control for the AKT kinase assay. We found that CLK2 was phosphorylated by AKT (Fig. 1C). We further examined the association between endogenous CLK2 and endogenous AKT. HeLa cells were transfected with siNon-Target (mock) or siRNA specific for CLK2 (siCLK2) and then the immunoprecipitation assay was performed. Endogenous CLK2 bound to endogenous AKT in parent and mock-control cells, but not in CLK2 knockdown cells (Fig. 1D). To assay for in vivo CLK2 phosphorylation, HeLa cells were transfected with vacant vector, wild type (WT-AKT), or myristoylated active Akt (Myr-AKT) cDNA. The phosphorylation of endogenous CLK2 was then estimated by immunoprecipitation from cells with anti-CLK2 antibody and followed by Western blot analysis with anti-phospho-Ser/Thr antibody. As shown in Fig. 1E, CLK2 phosphorylation was detected in HeLa cells overexpressing active AKT. These results suggest that CLK2 is a new substrate of AKT activation.
CLK2 Phosphorylation in Response to Ionizing Radiation Is Associated with AKT Activation
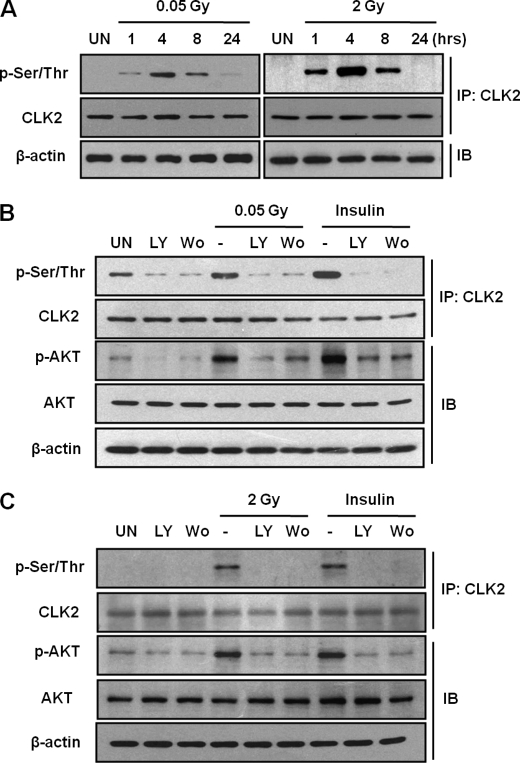
We previously reported that ionizing radiation induces AKT activation and peaks 4 h post-irradiation (20). We first sought to determine whether ionizing radiation induces CLK2 phosphorylation in normal human lung fibroblast CCD-18Lu cells. Thus, CLK2 phosphorylation was assessed after irradiation with either 0.05 or 2 Gy (Fig. 2A). Both 0.05 and 2 Gy induced the phosphorylation of CLK2, which peaked 4 h post-irradiation.
FIGURE 2.
The phosphorylation of CLK2 is induced by ionizing radiation and insulin. A, the CCD-18Lu cells were irradiated with 0.05 or 2 Gy of γ-rays and incubated for the indicated time periods. The phosphorylations of CLK2 were detected using immunoprecipitation (IP) with anti-CLK2 antibody followed by Western blotting with anti-phospho-Ser/Thr antibody. β-Actin was used as a loading control. B, effects of PI3K/AKT inhibition on 0.05 Gy or insulin-induced CLK2 phosphorylation. CCD-18Lu cells were pretreated with LY294002 (50 μm) or wortmannin (200 nm) for 1 h and then exposed to 0.05 Gy or insulin treatment (10 μg/ml). CLK2 phosphorylation was detected using immunoprecipitation with anti-CLK2 antibody followed by Western blotting with anti-phospho-Ser/Thr antibody. C, effects of PI3K/AKT inhibition on 2 Gy or insulin-induced CLK2 phosphorylation. IB, immunoblot.
To determine whether CLK2 phosphorylation following ionizing radiation is regulated by AKT activation, we pretreated CCD-18Lu cells with PI3K inhibitors, LY294002 (50 μm) or wortmannin (200 nm), followed by ionizing radiation or insulin (10 μg/ml). Both LY294002 and wortmannin treatment blocked AKT activation in response to low-dose radiation of 0.05 Gy or insulin, and markedly decreased 0.05 Gy- or insulin-induced CLK2 phosphorylation (Fig. 2B). Irradiation with 2 Gy γ-ray also induced CLK2 phosphorylation, which was inhibited by LY294002 or wortmannin (Fig. 2C).
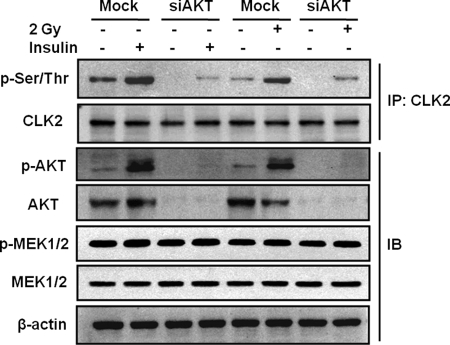
To confirm that endogenous AKT activation is related to CLK2 phosphorylation induced by radiation or insulin, we transfected CCD-18Lu cells with siNon-Target or siRNA specific for AKT (siAKT) directed against the AKT1 isoform. AKT was markedly depleted 24 h after siAKT transfection (Fig. 3). CLK2 protein levels were not changed in AKT knockdown cells compared with control cells transfected with siNon-Target. However, CLK2 phosphorylation in response to 2 Gy of γ-ray or insulin was decreased in AKT knockdown cells 4 h after irradiation or 15 min after insulin treatment (Fig. 3). To eliminate the possibility of the potential off-target effect by siAKT, we examined whether AKT activation is correlated with the regulation of p-MEK1/2 and MEK1/2 expression, which is an upstream molecule of mitogen-activated protein kinases (MAPKs). The expressions of both p-MEK1/2 and MEK1/2 were not changed by AKT knockdown (Fig. 3). These findings suggest that CLK2 phosphorylation in response to ionizing radiation is specifically regulated by AKT activation.
FIGURE 3.
CLK2 phosphorylation in AKT knockdown CCD-18Lu cells. siNon-Target (Mock) or siRNA specific for AKT (siAKT) directed to the target sequence of human AKT1 cDNAs were transfected into CCD-18Lu cells. After 24 h, the cells were exposed to 2 Gy of radiation or insulin treatment (10 μg/ml) and lysed at 4 h after irradiation or 15 min after insulin treatment. CLK2 phosphorylation was detected using immunoprecipitation (IP) with anti-CLK2 antibody followed by Western blotting with anti-phospho-Ser/Thr antibody. Western blot was performed with indicated antibodies. IB, immunoblot.
Identification of Ser34 and Thr127 Residues of CLK2 as Novel Target Sites of Activated AKT
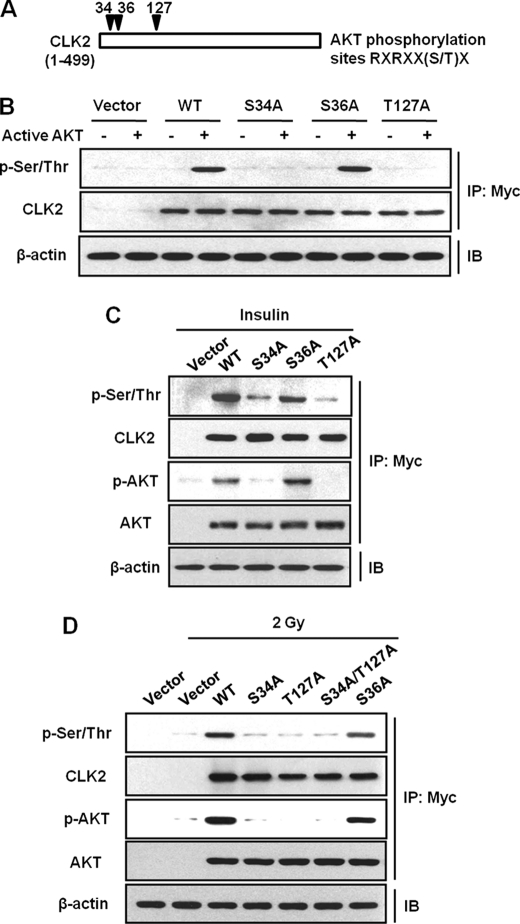
In exploring the sequence of CLK2 for putative AKT phosphorylation sites, we found that amino acids 29–35, RRRSRSW, 31–37, RSRSWSS, and 122–128, RRRSRTF, match the AKT consensus phosphorylation motif (Fig. 4A). To identify CLK2 phosphorylation sites, we generated several CLK2 point mutants in which Ser34, Ser36, and Thr127 were converted to alanine (S34A, S36A, and T127A). We transfected HeLa cells with vacant vector, WT, S34A, S36A, and T127A mutants, and then immunoprecipitated Myc-CLK2 was incubated with recombinant active AKT, in vitro. To monitor the phosphorylation status of CLK2, Western blot analysis was then performed with anti-phospho-Ser/Thr antibody (Fig. 4B). CLK2 phosphorylation by active AKT was blocked in S34A and T127A mutants, although CLK2 phosphorylation remained intact in the S36A mutant compared with the wild type (Fig. 4B). To confirm the CLK2 phosphorylation sites in vivo, CCD-18Lu cells were transfected with WT, S34A, S36A, and T127A mutants and then treated with insulin. The S34A and T127A mutants blocked the phosphorylation of CLK2 induced by insulin compared with wild type, although CLK2 phosphorylation was detected in the S36A mutant (Fig. 4C). In addition, interestingly, phosphorylated CLK2 bound to p-AKT, but the mutants of S34A and T127A did not bind to phosphorylated AKT. However, WT-CLK2 and the mutants of S34A, S36A, and T127A bound to AKT (Fig. 4C). We then generated a double mutant of CLK2 in which both Ser34 and Thr127 were converted to alanine (S34A/T127A) and transfected it into CCD-18Lu cells. When the cells were exposed to 2 Gy of γ-ray, CLK2 phosphorylation was not detected in the S34A, T127A, and S34A/T127A mutants (Fig. 4D). Although WT-CLK2 and all of the mutants (S34A, S36A, T127A, and S34A/T127A) bound to AKT, phosphorylated CLK2 at Ser34 and Thr127 sites bound to phosphorylated AKT (Fig. 4D). These results suggested that activated AKT induced by ionizing radiation or insulin binds to CLK2 and potently phosphorylates CLK2 at Ser34 and Thr127 sites.
FIGURE 4.
AKT phosphorylates Ser34 and Thr127 of CLK2. A, diagram of CLK2 with putative AKT phosphorylation sites. Amino acids 29–35, RRRSRSW, 31–37, RSRSWSS, and 122–128, RRRSRTF, that correspond to the AKT consensus phosphorylation motifs are labeled with arrows. B, the CLK2 phosphorylation sites targeted by active AKT. Wild-type (WT) CLK2 and S34A, S36A, and T127A mutants were transfected into HeLa cells. AKT kinase assays were performed with immunoprecipitated (IP) WT-CLK2 or mutants as the substrates. The WT-CLK2 and S36A mutant were phosphorylated by active AKT, but S34A and T127A mutants were not. C, the sites of CLK2 phosphorylation induced by insulin. Insulin-induced CLK2 phosphorylation in both WT-CLK2 and S36A, but not in S34A and T127A mutants. D, ionizing radiation phosphorylated Ser34 and Thr127 of CLK2. IB, immunoblot.
CLK2 Is Responsible for Cell Survival to Ionizing Radiation
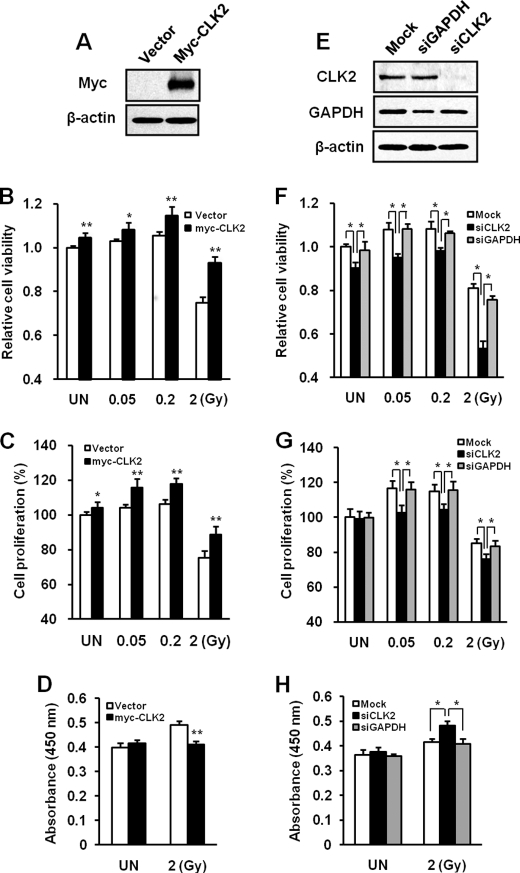
To examine the role of CLK2 on cell survival after irradiation, CCD-18Lu cells were transfected with control vector or myc-CLK2. Western blot was performed to confirm Myc-CLK2 overexpression (Fig. 5A). At 24 h post-transfection, cells were irradiated with γ-rays of 0.05, 0.2, and 2 Gy and cell viability was assayed 48 h later. CLK2 overexpression significantly increased cell growth in untreated and 0.05 or 0.2 Gy-irradiated cells and inhibited cell death in 2 Gy-irradiated cells compared with the vector control (Fig. 5B). We confirmed that CLK2 overexpression enhanced cell proliferation in untreated and irradiated cells (Fig. 5C) and blocked apoptosis induced by high dose radiation of 2 Gy (Fig. 5D).
FIGURE 5.
The effects of CLK2 on radiation sensitivity of CCD-18Lu cells. Cells were transfected with either vacant vector or myc-CLK2. At 24 h post-transfection, cells were exposed to 0.05–2 Gy of γ-rays and cell viability was assayed 48 h later. A, the overexpression of Myc-CLK2 at 72 h post-transfection. B, the cell viability of CLK2 overexpressing CCD-18Lu cells to ionizing radiation was determined by MTT assay. C, cell proliferation of CLK2 overexpressing cells after irradiation. D, effect of CLK2 overexpression on 2 Gy-induced apoptosis. DNA fragmentation was detected with HT TiterTACS Assay Kit for the quantification of apoptosis. B–D, data represent mean ± S.D. (n = 3) and were analyzed by the t test. Data showed a significant difference compared with vector control at the indicated dose (*, p < 0.01; **, p < 0.001). E, cells were transfected with siNon-Target, siCLK2, or siGAPDH and then Western blot was performed 72 h later. F, cells were exposed to 0.05–2 Gy of γ-rays at 24 h post-transfection. The radiation sensitivity in CLK2 knockdown cells was detected by MTT assay at 48 h after irradiation. G, the cell proliferation after irradiation in CLK2 knockdown cells. H, effect of CLK2 knockdown on 2 Gy-induced apoptosis. The apoptosis was assessed as described in panel D. F–H, data represent mean ± S.D. (n = 3) and were analyzed by Tukey's test for multiple comparison in analysis of variance at the indicated dose (*, p < 0.05). UN, untreated control.
We also investigated whether the depletion of endogenous CLK2 affects radiation sensitivity in CCD-18Lu cells. Cells were transfected with siNon-Target or siRNAs against CLK2 (siCLK2) or GAPDH (siGAPDH), which is used as a control to eliminate the possibility of the potential off-target effect. Western blot was performed to detect the level of target gene depletion by siRNAs (Fig. 5E). At 24 h post-transfection, cells were irradiated with γ-rays of 0.05, 0.2, or 2 Gy and cell viability was assayed 48 h later. GAPDH knockdown did not affect cell viabilities after low dose radiation of 0.05 or 0.2 Gy compared with the mock control, but slightly increased cell death in response to high dose radiation of 2 Gy (Fig. 5F). However, the suppression of endogenous CLK2 significantly inhibited cell growth after irradiation of γ-rays compared with siGAPDH (Fig. 5, F and G) and enhanced apoptosis induced by 2 Gy (Fig. 5H). This results show that CLK2 is correlated with the regulation of cell survival to ionizing radiation.
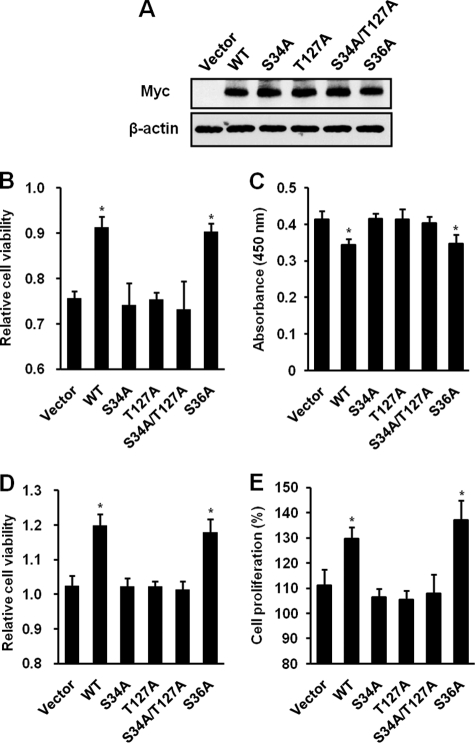
Phosphorylation of CLK2 on Ser34 and Thr127 Is Correlated with Cell Viability after Irradiation
To investigate whether CLK2 phosphorylation affects radiation sensitivity, CCD-18Lu cells were transfected with control vector, WT-CLK2, or CLK2 mutants. We first confirmed the overexpression levels of WT-CLK2 and CLK2 mutants (Fig. 6A). At 24 h after transfection, cells were irradiated with γ-rays of 2 Gy and cell viability was assayed 48 h later. As shown in Fig. 6B, vector control cells exposed to 2 Gy had higher levels of cell death compared with untreated cells. The overexpression of WT-CLK2 or S36A mutant blocked radiation-induced cell death, but S34A, T127A, and S34A/T127A mutants did not have any effect on radiation-induced cell death. To confirm whether the inhibition of cell death by CLK2 phosphorylation is correlated with the regulation of apoptosis, we performed DNA fragmentation assay (Fig. 6C). Vector control cells exposed to 2 Gy showed apoptosis compared with untreated vector control, but the overexpression of WT-CLK2 or S36A mutant blocked 2 Gy-induced apoptosis (Fig. 6C). To expand our study of the role of CLK2 phosphorylation in low dose radiation sensitivity, we performed cell viability (Fig. 6D) and cell proliferation assays (Fig. 6E) after irradiation of 0.05 Gy. Interestingly, overexpression of WT-CLK2 or the S36A mutant increased cell proliferation after irradiation of 0.05 Gy, but S34A, T127A, and S34A/T127A mutants did not have any effect on cell growth, suggesting that CLK2 phosphorylation correlates with cell proliferation after low dose radiation (Fig. 6, D and E). These results demonstrate that CLK2 phosphorylation in response to ionizing radiation plays important roles in the regulation of cell survival.
FIGURE 6.
The regulation of the sensitivity of CCD-18Lu cells to ionizing radiation is dependent on CLK2 phosphorylation. A, CCD-18Lu cells were transfected with vacant vector, WT-CLK2, or CLK2 mutants and the overexpressions of WT-CLK2 and CLK2 mutants were detected. B, the viabilities of vector, WT-CLK2, or mutant-CLK2 overexpressing cells were measured using MTT assays 48 h after 2 Gy of γ-ray. C, effect of CLK2 phosphorylation on 2 Gy-induced apoptosis. DNA fragmentation was detected with HT TiterTACS Assay Kit for the quantification of apoptosis. The absorbance (450 nm) of untreated vector control was 0.34. D, the viabilities of vacant vector, WT-CLK2, or mutant-CLK2 overexpressing cells after exposure to 0.05 Gy of γ-ray were measured by MTT assay. E, the cell proliferation after irradiation of 0.05 Gy in vacant vector, WT-CLK2, or mutant-CLK2 CCD-18Lu cells. Data represent mean ± S.D. (n = 3) and were analyzed by Dunnett's test for multiple comparison in analysis of variance to irradiated vector control (*, p < 0.05).
DISCUSSION
Protein phosphorylation by AKT is an important regulatory mechanism in the control of diverse cellular functions, such as cell cycle, survival, and signal transduction pathways. In this study, we identified and characterized CLK2 as a novel substrate of AKT. AKT directly binds and phosphorylates CLK2, in vitro and in vivo. We found that ionizing radiation induces CLK2 phosphorylation via AKT activation, in vivo. Furthermore, we demonstrated that CLK2 phosphorylation plays a critical role in cell proliferation following low dose radiation and prevents cell death following high dose radiation. To the best of our knowledge, this is the first report of the role of CLK2 as a novel target of AKT in controlling the cellular response to ionizing radiation.
CLK2 is a member of the CLK family of kinases, which contains an serine/arginine-rich domain (23, 29). The CLK family kinases phosphorylate protein-tyrosine phosphatase PTP-1B and serine/arginine-rich proteins, such as ASF/SF2 (32, 33). Although CLK2 was reported to regulate its nuclear localization by autophosphorylating serine 141 (31), CLK2 phosphorylation might be regulated by upstream kinases that influence its localization and activity (31, 34). However, the regulatory machinery for CLK2 phosphorylation and its function still remain to be elucidated. We discovered that CLK2 protein contains the AKT consensus phosphorylation motif, RXRXX(S/T)X, which suggested it is a novel AKT substrate. We demonstrated that AKT binds directly to CLK2 and phosphorylates it in vitro. The overexpression of active AKT-induced CLK2 phosphorylation in HeLa cells, suggesting that CLK2 is a genuine AKT substrate.
We previously reported that AKT is potently activated in response to ionizing radiation and regulates ionizing radiation-induced apoptosis (20). To identify whether ionizing radiation-induced AKT activation is related to CLK2 phosphorylation, we measured CLK2 phosphorylation after irradiation. Both 0.05 and 2 Gy induced CLK2 phosphorylation, which peaked at 4 h post-irradiation. The inhibition of AKT activation by LY294002 or wortmannin blocked radiation-induced CLK2 phosphorylation. Insulin was also shown to induce CLK2 phosphorylation, which was inhibited by PI3K inhibitors. Moreover, CLK2 phosphorylation in response to radiation or insulin was blocked in AKT knockdown cells, suggesting that CLK2 phosphorylation is regulated by activated AKT, in vivo. However, as shown in Fig. 4, CLK2 possesses three putative AKT consensus phosphorylation motifs, Ser34, Ser36, and Thr127; however, Ser36 was not phosphorylated by active AKT. Both Ser34 and Thr127 were phosphorylated by active AKT, in vitro, and phosphorylation of these sites was induced by radiation or insulin, in vivo. In addition, phosphorylated CLK2 at these sites bound to phosphorylated AKT. When both residues Ser34 and Thr127 were mutated to alanine, CLK2 phosphorylation in response to radiation was no different from the single mutant forms, S34A or T127A.
The CLK family kinases have been suggested to play an important role in the regulation of mRNA splicing, which is critical to the control of cellular gene expression (30, 35). CLK1 was shown to activate the mitogen-activated protein kinase signaling cascade, including ERKs and pp90Rsk (36). Although the importance of the CLK family in the control of gene expression and signal transduction has been suggested, its biological function is unclear. Here, we found that CLK2 overexpression increased cell growth induced by low dose radiation (0.05 or 0.2 Gy) and blocked cell death induced by high dose radiation (2 Gy). Moreover, the depletion of endogenous CLK2 by siRNA also decreased cell viability following both low and high dose irradiations. Our findings suggest that CLK2 is correlated with cell proliferation and cell protection against high dose radiation. Furthermore, we have demonstrated that CLK2 phosphorylation is correlated with the regulation of cell survival after irradiation. Interestingly, the overexpression of WT-CLK2 and the S36A mutant blocked 2 Gy-induced cell death, but S34A, T127A, and S34A/T127A mutants did not have any protective effect. In addition, CLK2 phosphorylation affected cell proliferation after low dose radiation. Our data suggest that CLK2 phosphorylation on Ser34 and Thr127 in response to radiation plays important roles in the regulation of cell survival.
CLKs were previously shown to change cellular localization depending on phosphorylation status. CLK2 localization and catalytic activity influenced by phosphorylation may be associated with the regulation of downstream molecules. We have sought to determine the change of CLK2 localization and subsequent regulation of CLK2 targets in response to ionizing radiation. However, although much more work is required to understand the biological function of CLK2, we have identified CLK2 as a direct target of AKT and characterized the role of CLK2 phosphorylation in the cellular response to ionizing radiation. As far as we know, this study provides the first evidence that sensitivity to ionizing radiation is, at least in part, regulated by CLK2 phosphorylation via activated AKT.
This work was supported by Grant E09NS02 from Korea Hydro & Nuclear Power Co., Ltd. and Grant R-2006-1-043 from the Ministry of Knowledge Economy, Republic of Korea.
- CLK
- CDC2-like kinases
- GSK-3
- glycogen synthase kinase 3
- Myr-AKT
- myristoylated active Akt
- siAKT
- small interfering AKT
- Gy
- gray
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1.Gottlob K., Majewski N., Kennedy S., Kandel E., Robey R. B., Hay N. (2001) Genes Dev. 15, 1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Downward J. (1998) Curr. Opin. Cell Biol. 10, 262–267 [DOI] [PubMed] [Google Scholar]
- 3.Nicholson K. M., Anderson N. G. (2002) Cell Signal. 14, 381–395 [DOI] [PubMed] [Google Scholar]
- 4.Gu Q., Wang D., Wang X., Peng R., Liu J., Jiang T., Wang Z., Wang S., Deng H. (2004) Radiat. Res. 161, 692–702 [DOI] [PubMed] [Google Scholar]
- 5.Söderlund K., Pérez-Tenorio G., Stål O. (2005) Int. J. Oncol. 26, 25–32 [PubMed] [Google Scholar]
- 6.Viniegra J. G., Martínez N., Modirassari P., Losa J. H., Parada Cobo C., Lobo V. J., Luquero C. I., Alvarez-Vallina L., Ramón y Cajal S., Rojas J. M., Sánchez-Prieto R. (2005) J. Biol. Chem. 280, 4029–4036 [DOI] [PubMed] [Google Scholar]
- 7.Kim S. J. (1998) Biochem. Mol. Biol. Int. 46, 187–196 [DOI] [PubMed] [Google Scholar]
- 8.Lu P. J., Hsu A. L., Wang D. S., Yan H. Y., Yin H. L., Chen C. S. (1998) Biochemistry 37, 5738–5745 [DOI] [PubMed] [Google Scholar]
- 9.Marchisio M., Bertagnolo V., Colamussi M. L., Capitani S., Neri L. M. (1998) Biochem. Biophys. Res. Commun. 253, 346–351 [DOI] [PubMed] [Google Scholar]
- 10.Krasilnikov M., Adler V., Fuchs S. Y., Dong Z., Haimovitz-Friedman A., Herlyn M., Ronai Z. (1999) Mol. Carcinog. 24, 64–69 [DOI] [PubMed] [Google Scholar]
- 11.Pollycove M., Feinendegen L. E. (2003) Hum. Exp. Toxicol. 22, 290–306 [DOI] [PubMed] [Google Scholar]
- 12.Mishra K. P. (2004) J. Environ. Pathol. Toxicol. Oncol. 23, 61–66 [DOI] [PubMed] [Google Scholar]
- 13.Wang Z. B., Liu Y. Q., Zhang Y., Li Y., An X. X., Xu H., Guo Y., Jin W., Jiang Z. J., Cui Y. F. (2007) Cell Biol. Int. 31, 1353–1358 [DOI] [PubMed] [Google Scholar]
- 14.Choi K. M., Kang C. M., Cho E. S., Kang S. M., Lee S. B., Um H. D. (2007) Oncol. Rep. 17, 1183–1188 [PubMed] [Google Scholar]
- 15.Portess D. I., Bauer G., Hill M. A., O'Neill P. (2007) Cancer Res. 67, 1246–1253 [DOI] [PubMed] [Google Scholar]
- 16.Wang G. J., Cai L. (2000) Toxicol. Sci. 53, 369–376 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K., Kodama S., Watanabe M. (2001) Cancer Res. 61, 5396–5401 [PubMed] [Google Scholar]
- 18.Li W., Wang G., Cui J., Xue L., Cai L. (2004) Exp. Hematol. 32, 1088–1096 [DOI] [PubMed] [Google Scholar]
- 19.Kim C. S., Kim J. M., Nam S. Y., Yang K. H., Jeong M., Kim H. S., Lim Y. K., Kim C. S., Jin Y. W., Kim J. (2007) J. Radiat. Res. 48, 407–415 [DOI] [PubMed] [Google Scholar]
- 20.Park H. S., Yun Y., Kim C. S., Yang K. H., Jeong M., Ahn S. K., Jin Y. W., Nam S. Y. (2009) Eur. J. Cell Biol. 88, 563–575 [DOI] [PubMed] [Google Scholar]
- 21.Hu Y., Yao J., Liu Z., Liu X., Fu H., Ye K. (2005) EMBO J. 24, 3543–3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindberg R. A., Quinn A. M., Hunter T. (1992) Trends Biochem. Sci. 17, 114–119 [DOI] [PubMed] [Google Scholar]
- 23.Douville E., Duncan P., Abraham N., Bell J. C. (1994) Cancer Metastasis Rev. 13, 1–7 [DOI] [PubMed] [Google Scholar]
- 24.Johnson K. W., Smith K. A. (1991) J. Biol. Chem. 266, 3402–3407 [PubMed] [Google Scholar]
- 25.Hanks S. K., Quinn A. M. (1991) Methods Enzymol. 200, 38–62 [DOI] [PubMed] [Google Scholar]
- 26.Hanes J., von der Kammer H., Klaudiny J., Scheit K. H. (1994) J. Mol. Biol. 244, 665–672 [DOI] [PubMed] [Google Scholar]
- 27.Ben-David Y., Letwin K., Tannock L., Bernstein A., Pawson T. (1991) EMBO J. 10, 317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howell B. W., Afar D. E., Lew J., Douville E. M., Icely P. L., Gray D. A., Bell J. C. (1991) Mol. Cell Biol. 11, 568–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayler O., Stamm S., Ullrich A. (1997) Biochem. J. 326, 693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan P. I., Stojdl D. F., Marius R. M., Scheit K. H., Bell J. C. (1998) Exp. Cell Res. 241, 300–308 [DOI] [PubMed] [Google Scholar]
- 31.Nayler O., Schnorrer F., Stamm S., Ullrich A. (1998) J. Biol. Chem. 273, 34341–34348 [DOI] [PubMed] [Google Scholar]
- 32.Moeslein F. M., Myers M. P., Landreth G. E. (1999) J. Biol. Chem. 274, 26697–26704 [DOI] [PubMed] [Google Scholar]
- 33.Ngo J. C., Chakrabarti S., Ding J. H., Velazquez-Dones A., Nolen B., Aubol B. E., Adams J. A., Fu X. D., Ghosh G. (2005) Mol. Cell 20, 77–89 [DOI] [PubMed] [Google Scholar]
- 34.Lee K., Du C., Horn M., Rabinow L. (1996) J. Biol. Chem. 271, 27299–27303 [DOI] [PubMed] [Google Scholar]
- 35.Colwill K., Pawson T., Andrews B., Prasad J., Manley J. L., Bell J. C., Duncan P. I. (1996) EMBO J. 15, 265–275 [PMC free article] [PubMed] [Google Scholar]
- 36.Myers M. P., Murphy M. B., Landreth G. (1994) Mol. Cell Biol. 14, 6954–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]