Abstract
Pyk2 was identified as a Ca2+-dependent kinase, however, the regulation of Pyk2 by Ca2+ in T cells remains controversial. We found that Ca2+ mobilization preferentially induced Pyk2 phosphorylation in cytotoxic T lymphocytes (CTL). Furthermore, Pyk2 phosphorylation in CTL was not absolutely Ca2+ dependent but relied on the strength of T cell receptor stimulation. Ionomycin-stimulated Pyk2 phosphorylation did not require calmodulin activity, because phosphorylation was not inhibited by the calmodulin inhibitor W7, and we detected no Ca2+-regulated association between Pyk2 and calmodulin. Ca2+-stimulated Pyk2 phosphorylation was dependent on Src-family kinase activity, even at the Pyk2 autophosphorylation site. We sought to identify a Ca2+-regulated pathway that could trigger Pyk2 phosphorylation in T cells and found that ionomycin stimulated the production of reactive oxygen species and an H2O2 scavenger inhibited ionomycin-induced Pyk2 phosphorylation. Additionally, H2O2 induced strong Erk activation and ionomycin-stimulated Pyk2 phosphorylation was Erk dependent. These data support the conclusion that Ca2+ mobilization induces the production of reactive oxygen species, which in turn activate the Erk pathway, leading to Src-family kinase-dependent Pyk2 phosphorylation. Our data demonstrate that Pyk2 is not a Ca2+-dependent kinase in T cells but instead, increased intracellular Ca2+ induces Pyk2 phosphorylation through production of reactive oxygen species. These findings are consistent with the possibility that Pyk2 acts as an early sensor of numerous extracellular signals that trigger a Ca2+ flux and/or reactive oxygen species to amplify tyrosine phosphorylation signaling events.
Keywords: Calcium, Nonreceptor Tyrosine Kinase, Phosphotyrosine Signaling, Reactive Oxygen Species (ROS), Src
Introduction
Proline-rich tyrosine kinase 2 (Pyk2)5 (1), also known as CAKβ (cell adhesion kinase β) (2), RAFTK (related adhesion focal tyrosine kinase) (3), CADTK (calcium-dependent tyrosine kinase) (4), and FAK2 (5), are related to focal adhesion tyrosine kinase (FAK) and contains a large N-terminal FERM (4.1/ezrin/radixin/moesin) domain, a centrally located kinase domain, and a C-terminal focal adhesion targeting domain (6). Pyk2 is required for membrane polarization and cell migration in macrophages (7) and for microtubule-dependent podosome belt formation for bone resorption by osteoclasts (8). The varied functions of Pyk2 in these two different cell types might imply that the role of Pyk2 depends on the cellular context.
The catalytic activity of Pyk2 is regulated by phosphorylation. Pyk2 has four major sites of tyrosine phosphorylation, Tyr-402, Tyr-579, Tyr-580, and Tyr-881 (9). Tyrosine 402 is an autophosphorylation site that, once phosphorylated, can bind to the SH2 domain of Src family kinases (SFK) (9, 10). The current model for the activation of this kinase is that upon stimulation, Pyk2 becomes autophosphorylated as a result of trans-autophosphorylation, which allows for the recruitment of SFK (11). SFK phosphorylate additional tyrosine residues within the protein thus enhancing catalytic activity (Tyr-579/Tyr-580) and providing a new docking site (Tyr-881) for SH2 domain-containing proteins (11). It is not clear how Pyk2 is regulated; however recent evidence from structural studies on FAK have revealed that the N-terminal FERM domain binds to its kinase domain forming an autoinhibited structure (12, 13). Stimulation of FAK activity would be predicted to occur when a stimulus destabilized the interaction between the FERM and kinase domains, allowing for autophosphorylation and subsequent recruitment of SFK (12, 13).
Pyk2 phosphorylation is regulated by intracellular Ca2+ in many cell types such as neurons (1), epithelial cells (4), megakaryocytes (14), platelets (15), and cardiomyocytes (16) and was initially identified as a Ca2+-dependent kinase (4). Although it is known that Pyk2 can be regulated by Ca2+, the mechanism by which this occurs has not been fully elucidated. Regulation of Pyk2 by Ca2+ must be indirect since it does not contain Ca2+ binding motifs. A number of mechanisms have been shown to be used in various systems, including regulation by the calmodulin-dependent kinase CaMKII (17, 18), phosphorylation by nonmuscle myosin light-chain kinase (19), and direct interaction of the Pyk2 FERM domain with calmodulin (20). It is not clear at this point, however, if all of these are operational in all cell types or if there are indeed multiple ways of regulating Pyk2 depending on the cellular context.
In T cells, Pyk2 becomes activated in response to numerous extracellular signals including, but not limited to, integrin engagement, antigen receptor stimulation, activation of G-protein-coupled receptors, including chemokine receptors, and cytokine binding (6, 9). However, the function of Pyk2 during T cell activation remains unknown. Additionally, whether Pyk2 is regulated by Ca2+ in T cells remains controversial. One group found no Ca2+-induced activation of Pyk2 since treatment of Jurkat T cells and murine thymocytes with the calcium ionophore ionomycin did not induce Pyk2 phosphorylation (21). Furthermore, activation of Pyk2 did not require Ca2+ because stimulation of T cells with cross-linked anti-CD3 in the presence of EGTA to chelate extracellular calcium had no effect on Pyk2 phosphorylation (21, 22). Consistent with these results, another group reported that stimulation of human T lymphoblasts with either the calcium ionophore A23187 or thapsigargin, an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases or SERCA pumps that refill ER stores, did not stimulate Pyk2 kinase activity (23). In contrast, two other studies found that treatment of Jurkat T cells with EGTA, or stimulating the cells in Ca2+-free medium, partially reduced TCR-induced Pyk2 phosphorylation (24, 25). Thus, there appears to be no consensus regarding the regulation of Pyk2 by Ca2+ in T cells.
To examine the contribution of calcium to Pyk2 regulation in T cells, we used well-characterized non-transformed cytotoxic T lymphocyte (CTL) clones to determine if Ca2+ could stimulate Pyk2 activation and if Ca2+ is required for TCR-stimulated Pyk2 activation. Here, we present evidence that Pyk2 phosphorylation can be regulated by Ca2+ in CTL clones and ex vivo activated T cells and that TCR-stimulated Pyk2 activation can either be Ca2+ dependent or independent, varying with the method used for TCR stimulation. We further examined the role of Ca2+ in regulating Pyk2 function in T cells and found that Ca2+-stimulated Pyk2 phosphorylation is primarily regulated by SFK and through the production of hydrogen peroxide-induced activation of Erk. These data suggest that Pyk2 might sense the activation of numerous extracellular signals that trigger a Ca2+ flux and/or reactive oxygen species and may serve to amplify tyrosine phosphorylation signaling events in lymphocytes.
EXPERIMENTAL PROCEDURES
Cells
The murine CD8+ T cell clone AB.1 is a non-transformed, antigen- and IL-2-dependent CTL clone that has cytolytic activity with specificity for Kb alloantigen (26). The clones were stimulated weekly, as described previously (26), with irradiated allogeneic C57BL/6 splenocytes and recombinant IL-2 and were used for experiments 4–6 days after stimulation. Activated T cells were generated by culturing C57BL/6 splenocytes with 2 μg/ml concanavalin A, a T cell mitogen, for 48 h in 10% FBS in RPMI. The mouse B lymphoma WEHI-231 was kindly provided by Dr. M. R. Gold (University of British Columbia, Vancouver, BC), the Jurkat human T leukemia cell line was obtained from American Type Culture Collection and the rat natural killer cell tumor RNK was provided by Dr. K. P. Kane (University of Alberta, Edmonton, AB) and were maintained in RPMI supplemented with 10% FBS. NIH-3T3 cells were obtained from Dr. J. C. Stone (University of Alberta, Edmonton, AB) and maintained in DMEM supplemented with 10% FBS. The CD8+ T cell hybirdoma B3Z (27), a generous gift from Dr. N. Shastri (University of California, Berkley, CA), was cultured in DMEM with 8% FBS.
Antibodies and Reagents
The source and purification of antibodies from hybridomas producing 145–2C11 (anti-CD3ϵ) and PY72.10.5 (anti-phosphotyrosine) have been described previously (28). The polyclonal anti-Pyk2 antibodies F298 (amino acids 2–12) and F245 (amino acids 720–862) were generated in our laboratory and have been described previously (29). The Pyk2/CAKβ specific monoclonal antibody use for immunoblotting was purchased from BD Biosciences (Mississauga, ON). The Pyk2 phosphospecific antibodies pY402, pY579, pY580, and pY881 were purchased from BIOSOURCE International (Camarillo, CA). Anti-phospho-Pyk2 Y881 was also purchased from Sigma. The mouse monoclonal antibody specific for calmodulin was purchased from Upstate (Lake Placid, NY). Antibodies specific for activated phospho-Erk and Erk were obtained from Cell Signaling Technology (Pickering, ON). Goat anti-hamster IgG and anti-mouse IgG-HRP were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Protein A-HRP was purchased from Pierce. Protein A-Sepharose was purchased from Amersham Biosciences (Piscataway, NJ). Ionomycin, BAPTA-AM, KN-62, PP2, PP3, W7, and W5 were purchased from Calbiochem. Thapsigargin and U0126 were purchased from Sigma. CM-H2DCFDA was purchased from Molecular Probes-Invitrogen (Carlsbad, CA).
Cell Stimulation
Cells were harvested, washed three times with PBS, resuspended at 107 cells/ml PBS and chilled on ice. Either ionomycin, thapsigargin, or DMSO (carrier control) was added to 107 cells, and the cells were incubated at 37 °C for 10 min, unless otherwise indicated. After 10 min, the cells were pelleted and lysed in 1% Nonidet P-40 lysis buffer (1% Nonidet P-40, 10 mm Tris (pH 7.5), 150 mm NaCl, and 1 mm sodium vanadate) for 20 min on ice. Post-nuclear lysates were used for immunoprecipitation. When included, cells were pretreated with the indicated inhibitor for 15 min on ice prior to stimulation. A sample of post-nuclear lysate containing 4 × 105 cell equivalents was used for lysates, where indicated. For cross-linked anti-CD3 stimulation, cells were resuspended at 1 × 107 cells/ml PBS and chilled on ice. One ml of cells was incubated with 10 μg/ml 145–2C11 for 15 min on ice. After 15 min, the cells were pelleted and resuspended in PBS and 5 μg/ml goat anti-hamster was added to cross-link the 145-2C11 antibody. Cells were incubated at 37 °C for the indicated time, after which the cells were pelleted, resuspended in 1% Nonidet P-40 lysis buffer and lysed on ice for 20 min. Post-nuclear lysates were then used for immunoprecipitation. For stimulation with plate-bound anti-CD3 clones were resuspended at 2 × 107 cells/ml in PBS and chilled on ice. 107 cells were added to BSA-blocked control or 145–2C11-coated dishes as previously described (30), and incubated at 37 °C for the indicated time. Cells were lysed in the dishes for 20 min by addition of 1.5% Nonidet P-40 lysis buffer. Post-nuclear lysates were used for immunoprecipitation.
Immunoprecipitation and Immunoblots
Post-nuclear cell lysates containing 107 cell equivalents were incubated with anti-Pyk2 antisera (F298 or F245) for 15 min on ice prior to the addition of protein A Sepharose. After incubation with rotation at 4 °C for 2 h, the beads were washed three times in 1% Nonidet P-40 lysis buffer, denatured by boiling in Laemmli reducing sample buffer and loaded onto SDS-PAGE gels. Calmodulin immunoprecipitates contained 2 × 107 cell equivalents and were performed overnight prior to washing with lysis buffer. Membranes were probed with the indicated primary antibody and HRP-coupled secondary antibody then visualized by enhanced chemiluminescence (ECL) (PerkinElmer Life Science Products, Boston, MA). When multiple immunoblots were performed on the same membrane, the membrane was stripped between each blot. Anti-phosphotyrosine blots were always performed first and loading controls were generally completed last.
Measurement of ROS Production
AB.1 cells were washed twice with PBS then resuspended at 4 × 106 per ml in PBS. Cells were stimulated with the indicated agent at 37 °C for 10 min. If cells were treated with ebselen, it was done so at room temperature for 20 min prior to stimulation. Immediately following stimulation, CM-H2DCFDA (Invitrogen C6827) was added at a final concentration of 0.5 μm and cells were incubated for 15 min at 37 °C. Cells were washed twice with PBS containing 1% FBS before data acquisition on a FACS calibur.
RESULTS
Intracellular Calcium Stimulates Pyk2 Phosphorylation in T Cells
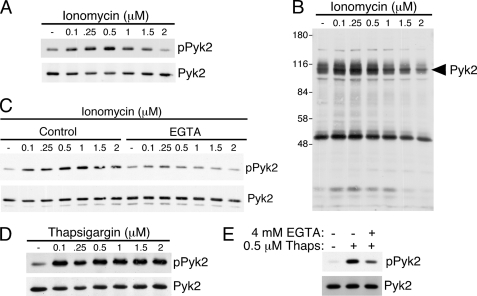
To ascertain the influence of a rise in intracellular Ca2+ concentration on Pyk2 phosphorylation in T cells, we subjected the CD8+ non-transformed CTL clone AB.1 to increasing concentrations of ionomycin (0.1–2 μm), lysed the cells and prepared Pyk2 immunoprecipitations. Phosphotyrosine immunoblotting of the Pyk2 immunoprecipitates revealed that Pyk2 becomes phosphorylated after treatment with ionomycin (Fig. 1A). Interestingly, lower concentrations of ionomycin (less than or equal to 1 μm) caused the greatest induction of phosphorylation whereas the highest concentration of 2 μm did not stimulate Pyk2 phosphorylation, implying that it is not merely the presence of intracellular free Ca2+ that is regulating Pyk2 phosphorylation because the higher concentrations of ionomycin also induced increased intracellular Ca2+ (data not shown). This decrease in Pyk2 phosphorylation at higher ionomycin concentrations might explain why 7 μm ionomycin was not stimulatory for T cells in a previous study (23). When total cell lysates were probed for anti-phosphotyrosine, there were only limited increases in overall tyrosine phosphorylation with protein(s) migrating with Pyk2 constituting the predominant ionomycin-induced tyrosine phosphorylated proteins (Fig. 1B). Of note, there is a protein at around 50 kDa that undergoes a slight increase in tyrosine phosphorylation at 0.1 and 0.25 μm of ionomycin and is decreased below basal phosphorylation at 2 μm (Fig. 1B). Stimulation of CTL clones with ionomycin in the presence of EGTA to chelate extracellular Ca2+ substantially reduced tyrosine phosphorylation of Pyk2 (Fig. 1C) suggesting that an influx of extracellular Ca2+ is required for maximal phosphorylation.
FIGURE 1.
Stimulation of CTL clones with agents that increase intracellular Ca2+ concentration induce Pyk2 phosphorylation. A, AB.1 CTL clones were stimulated with the indicated concentration of ionomycin or DMSO as a carrier control for 10 min. The cells were lysed, and Pyk2 was immunoprecipitated from post-nuclear lysates. The immunoprecipitates were subjected to SDS-PAGE followed by immunoblottting with anti-phosphotyrosine. The membrane was then stripped and reprobed for Pyk2. B, samples of the post-nuclear lysates from A were immunoblotted for anti-phosphotyrosine. C, AB.1 cells were stimulated with ionomycin in the presence or absence of 4 mm EGTA. Pyk2 immunoprecipitates were probed with anti-phosphotyrosine then anti-Pyk2. D, AB.1 were treated with the indicated concentration of thapsigargin or DMSO as a carrier control for 10 min. Pyk2 immunoprecipitates were probed for phosphotyrosine and then reprobed for Pyk2. E, AB.1 cells were stimulated with 0.5 μm thapsigargin for 10 min in the presence or absence of 4 mm EGTA and Pyk2 immunoprecipitates were subjected to immunoblotting with anti-phosphotyrosine followed by anti-Pyk2.
We next used thapsigargin to examine the effect of increasing the concentration of intracellular Ca2+on Pyk2 phosphorylation. Thapsigargin causes eventual emptying of Ca2+ from intracellular stores by inhibiting SERCA pumps in the ER that are responsible for pumping Ca2+ back into the ER (31). Phosphotyrosine immunoblots of Pyk2 immunoprecipitations performed on lysates of AB.1 cells treated with different concentrations of thapsigargin (0.1–2 μm) demonstrate that Pyk2 is inducibly phosphorylated after treatment with thapsigargin (Fig. 1D). Unlike ionomycin, there was no decrease in the phosphorylation of Pyk2 at higher concentrations of thapsigargin. Stimulation with thapsigargin in the presence of EGTA to chelate extracellular Ca2+ substantially reduced, but did not abrogate, Pyk2 phosphorylation (Fig. 1E) suggesting that intracellular stores of Ca2+ are sufficient to partially activate Pyk2 in CTL.
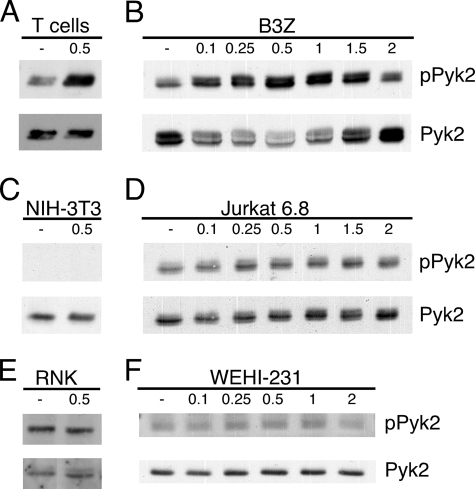
To determine if the inconsistencies in the literature with respect to the regulation of Pyk2 phosphorylation by intracellular Ca2+ are partly due to the cell type used in the assay, we tested a panel of T cells, other hematopoietic cell lines and a fibroblast line for Pyk2 phosphorylation after ionomycin or thapsigargin stimulation. Cells that demonstrated an induction of Pyk2 phosphorylation in response to a Ca2+ flux include activated mouse splenic T cells treated with thapsigargin (Fig. 2A) and B3Z cells (CD8+ T cell hybridoma, Fig. 2B) treated with ionomycin. Cells that showed limited or no significant induction of Pyk2 phosphorylation in response to ionomycin treatment were NIH-3T3 (fibroblast, Fig. 2C), Jurkat T cells (T leukemia, Fig. 2D), RNK (NK leukemia, Fig. 2E) and WEHI-231 (B lymphoma, Fig. 2F). These results suggest that not all cells that express Pyk2 display a robust induction of Pyk2 phosphorylation in response to stimulation with Ca2+-mobilizing agents. Also, whether a cell is of hematopoietic origin or adherent does not seem to be an indicator of whether a cell is likely to regulate Pyk2 phosphorylation by a Ca2+ flux (Fig. 2 and data not shown). Cell transformation also does not appear to account for the differences in Pyk2 phosphorylation in response to increases in intracellular Ca2+ (Fig. 2 and data not shown). These data suggest that the pathway that leads to Ca2+-triggered Pyk2 phosphorylation is differentially regulated between different cell types implying that Pyk2 is not universally regulated by Ca2+.
FIGURE 2.
Pyk2 tyrosine phosphorylation is not induced by Ca2+ in all Pyk2-expressing cells. ConA-activated splenic T cell blasts were stimulated with 0.5 μm thapsigargin (A) and B3Z (B), NIH-3T3 (C), Jurkat 6.8 T cells (D), RNK (E), or WEHI-231 (F) were treated with the indicated concentration of ionomycin for 10 min. Pyk2 was immunoprecipitated and probed by immunoblotting for phosphotyrosine followed by Pyk2.
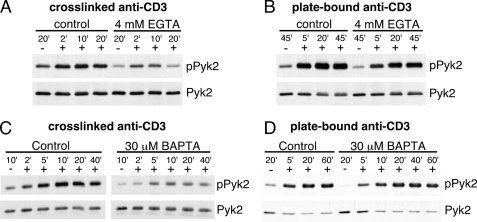
The Requirement for Calcium for Pyk2 Phosphorylation during TCR Stimulation Depends on the Stimulation Conditions
TCR triggering induces a rise in intracellular free Ca2+in T cells. We, and others (21, 24, 29, 32, 33) have shown that TCR engagement also induces phosphorylation of Pyk2. To determine if extracellular Ca2+ is required for the induction of Pyk2 phosphorylation after TCR stimulation, AB.1 were stimulated with cross-linked antibodies specific for CD3, a component of the TCR complex, in the presence of 4 mm EGTA. Cross-linked anti-CD3 is known to stimulate signaling in these cells but is not sufficient to trigger degranulation of the cytolytic molecules (30). Although there is some TCR induced Pyk2 phosphorylation in the presence of EGTA, it was substantially reduced compared with the control group (Fig. 3A) suggesting that cross-linked TCR stimulated Pyk2 phosphorylation is largely Ca2+ dependent. Interestingly, when stimulating AB.1 with plate-bound solid phase anti-CD3, which generally stimulates more robust and sustained signals and triggers degranulation (30), 4 mm EGTA had only a limited impact on Pyk2 phosphorylation (Fig. 3B). This concentration of EGTA inhibited Ca2+-dependent degranulation by these clones (Ref. 34 and data not shown) and blocked the flux of extracellular Ca2+ (data not shown).
FIGURE 3.
The calcium requirement for TCR-stimulated Pyk2 phosphorylation depends on the method of stimulation. AB.1 were left unstimulated (−) or stimulated (+) with cross-linked anti-CD3 (A) or plate-bound immobilized anti-CD3 (B) for the indicated time, in the presence or absence of 4 mm EGTA. The unstimulated control (−) for the cross-linked anti-CD3 contains secondary cross-linking antibody alone and for the plate-bound stimulation the cells were plated on BSA-blocked plastic. Cells were lysed and Pyk2 immunoprecipitates were probed with anti-phosphotyrosine followed by anti-Pyk2. AB.1 cells were pretreated with either 30 μm BAPTA or vehicle control (DMSO) for 15 min on ice then stimulated with cross-linked anti-CD3 (C) or immobilized anti-CD3 (D) for the indicated time. Pyk2 immunoprecipitations were performed and probed for phosphotyrosine then Pyk2.
It is possible that the intracellular stores of Ca2+ are sufficient to support Pyk2 phosphorylation under conditions of plate-bound anti-CD3 stimulation, which provides a more robust signal through the TCR (30), so we examined Pyk2 phosphorylation after CD3 stimulation in the presence of the cell-permeable intracellular Ca2+ chelator BAPTA-AM. The concentration of BAPTA used in these experiments was 30 μm as this concentration was determined to inhibit Ca2+-dependent degranulation, but not overall tyrosine phosphorylation (data not shown). Pyk2 phosphorylation after cross-linked anti-CD3 stimulation in the presence of 30 μm BAPTA was substantially inhibited compared with the control (Fig. 3C), similar to the inhibition by EGTA (Fig. 3A). In contrast, 30 μm BAPTA (Fig. 3D) or 60 μm BAPTA (data not shown) did not inhibit, but did slightly delay, immobilized anti-CD3-induced Pyk2 phosphorylation. These data suggest that Pyk2 phosphorylation is not always Ca2+ dependent and may contribute to the conflicting data regarding the Ca2+-requirement for TCR-triggered Pyk2 activation in the literature (21, 24, 25). These results also imply that Pyk2 is not solely dependent on Ca2+ for its activation and that other factors contribute to the induction of Pyk2 phosphorylation during immobilized anti-CD3 stimulation.
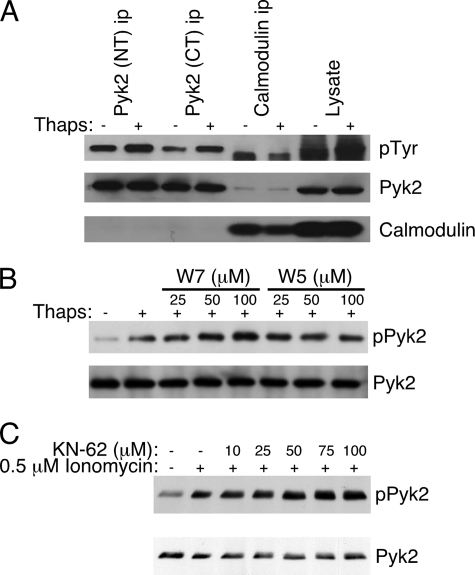
Calcium-stimulated Pyk2 Phosphorylation Does Not Require Calmodulin
It was recently reported that calmodulin associates with the FERM domain of Pyk2 in a Ca2+-dependent manner, which promoted dimerization of Pyk2, and the authors proposed that this was the mechanism by which Ca2+ could regulate Pyk2 autophosphorylation (20). We first wanted to determine if Pyk2 associates with calmodulin in T cells and if this interaction was enhanced upon Ca2+ mobilization. We stimulated AB.1 with 1 μm thapsigargin for 10 min, lysed the cells and prepared Pyk2 and calmodulin immunoprecipitates. To control for potential interference of calmodulin binding by the antibodies, we used two different Pyk2-specific antibodies, NT that recognizes a peptide at the N terminus and CT that recognizes a sequence in the C-terminal domain (35). These immunoprecipitates were then probed with antibodies specific for phosphotyrosine, Pyk2 and calmodulin. We detected no calmodulin in the Pyk2 immunoprecipitates either with or without thapsigargin treatment, even though the thapsigargin clearly stimulated Pyk2 phosphorylation (Fig. 4A). A small amount of Pyk2 in the calmodulin immunoprecipitates was visible, however this was very low and did not increase after thapsigargin treatment (Fig. 4A). There was a tyrosine-phosphorylated protein with a faster mobility than Pyk2 that was recovered in the calmodulin immunoprecipitate, however this was not Pyk2 since it was not detected in the Pyk2 immunoblot (Fig. 4A). We also treated cells with the calmodulin antagonist W7, and its non-inhibitory analog W5, to determine if Ca2+-triggered Pyk2 phosphorylation required calmodulin activity. Pretreatment of the CTL clone with W7 prior to thapsigargin stimulation did not result in inhibited Pyk2 phosphorylation (Fig. 4B). In fact, we consistently detected an increase in Pyk2 phosphorylation with increasing concentrations of W7 (Fig. 4B and data not shown). We detected no significant change in the level of Pyk2 phosphorylation after pre-treatment with the inactive analog W5 (Fig. 4B). These data suggest that calmodulin does not play a significant role in the Ca2+-induced activation of Pyk2 in CTL clones. It has been suggested that CaMKII may be an intermediate in the Ca2+-dependent activation of Pyk2 (17, 18). To determine if CaMKII is required for Ca2+-induced Pyk2 phosphorylation in CTL, we treated the cells with KN-62, an inhibitor of CaMKII. KN-62 had no effect on ionomycin-stimulated Pyk2 phosphorylation in CTL (Fig. 4C), consistent with lack of inhibition observed with the calmodulin inhibitor.
FIGURE 4.
Calcium-induced Pyk2 phosphorylation does not require calmodulin. A, AB.1 were stimulated with 1 μm thapsigargin for 10 min then lysed. Immunoprecipitates were prepared with antibodies specific for either the N terminus (NT) or C terminus (CT) of Pyk2 or with antibodies specific for calmodulin. A single membrane was first probed with anti-phosphotyrosine then stripped and simultaneously probed for Pyk2 and calmodulin. B, AB.1 cells were pretreated with the indicated concentration of the calmodulin inhibitor W7 or its inactive analog W5 for 20 min prior to stimulation of the cells for 10 min with 0.5 μm thapsigargin. Pyk2 immunoprecipitates were prepared then sequentially probed with anti-phosphotyrosine then anti-Pyk2. C, Pyk2 immunoprecipitates from cells stimulated with ionomycin in the presence of increasing concentrations of KN-62 probed with anti-phosphotyrosine then anti-Pyk2.
Calcium-induced Pyk2 Phosphorylation Is SFK Dependent
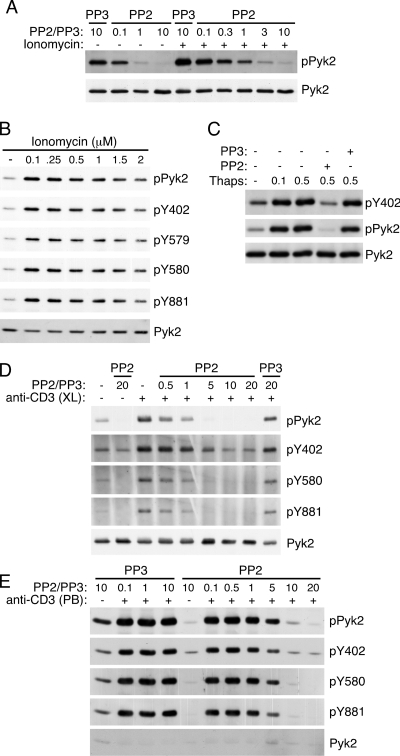
SFK are known to be involved in the phosphorylation and activation of Pyk2 (11). Furthermore, it has been found that stimulating vascular smooth muscle cells with ionomycin in the presence of the SFK inhibitor PP2 decreased the overall phosphorylation of Pyk2 (17). To determine whether SFK may be involved in Ca2+-induced Pyk2 phosphorylation, AB.1 cells were stimulated with ionomycin in the presence of 10 μm PP2 or its inactive analog PP3. In the presence of PP2, both basal and ionomycin-induced Pyk2 phosphorylation were reduced (Fig. 5A). This suggested that SFK are likely playing a role in Ca2+-induced Pyk2 phosphorylation. It is thought that Pyk2 undergoes autophosphorylation on Tyr-402, which in turn recruits SFK to bind via its SH2 domain and stimulate phosphorylation of Tyr-579/Tyr-580 and Tyr-881 (6). Consistent with a role for SFK in Ca2+-induced Pyk2 phosphorylation, all of the tyrosine residues phosphorylated by SFK were tyrosine phosphorylated in response to stimulatory concentrations ionomycin (Fig. 5B). There were also no differences in the kinetics of ionomycin-induced phosphorylation of the different tyrosine residues (data not shown). Based on the current model for Pyk2 regulation, it is predicted that only the Tyr-579/Tyr-580 and Tyr-881 sites would be inhibited by PP2 after ionomycin treatment and that Tyr-402 phosphorylation would be SFK independent. However, ionomycin-induced phosphorylation was inhibited by PP2 at Tyr-402 while the inactive analog PP3 had no effect on the phosphorylation (Fig. 5C). Because we demonstrated differences in the Ca2+ requirement for Pyk2 phosphorylation depending on the method of TCR/CD3 stimulation (Fig. 3) we also examined the requirement for SFK in cross-linked (Fig. 5D) and plate-bound (Fig. 5E) anti-CD3 stimulation. As was recently shown for soluble TCR stimulation of human T cell leukemias (22), we found that both methods of stimulation required SFK activity for Pyk2 phosphorylation at all phosphorylation sites, including Tyr-402. However, there appears to be low but detectable levels of Tyr-402 phosphorylation, both before and after stimulation, in the presence of 20 μm PP2 (Fig. 5, D and E), suggesting there is low-level SFK-independent phosphorylation of Tyr-402. These data implicate SFK in the phosphorylation at all sites, including Tyr-402 suggesting that Ca2+ might stimulate SFK activity in cells to trigger Pyk2 phosphorylation.
FIGURE 5.
Pyk2 phosphorylation induced by ionomycin is dependent on SFK activity. A, Pyk2 immunoprecipitates from cells stimulated with 0.5 μm ionomycin in the presence of the indicated concentration (in μm) of PP2 or the inactive analog PP3 sequentially probed with anti-phosphotyrosine then Pyk2. B, Pyk2 immunoprecipitates from AB.1 cells stimulated with the indicated concentration of ionomycin then probed with anti-phosphotyrosine, Pyk2 phosphorylation site specific antibodies then anti-Pyk2. C, AB.1 were pretreated with 10 μm PP2 or PP3 then stimulated with either 0.1 μm or 0.5 μm thapsigargin prior to lysis. Pyk2 immunoprecipitates were sequentially probed with anti-pY402, anti-phosphotyrosine, and anti-Pyk2. D, Pyk2 immunoprecipitates from cells stimulated with cross-linked anti-CD3 antibodies (+) or cross-linking antibodies alone (−) for 10 min in the presence of the indicated concentration (in μm) of PP2 or the inactive analog PP3 sequentially probed with anti-phosphotyrosine, the indicated phosphospecific antibody followed by Pyk2. E, as in D except the cells were stimulated (+), or not (−), with plate-bound anti-CD3 for 20 min.
Ionomycin Induces H2O2 Production in T Cells, Which Stimulates Pyk2 Phosphorylation
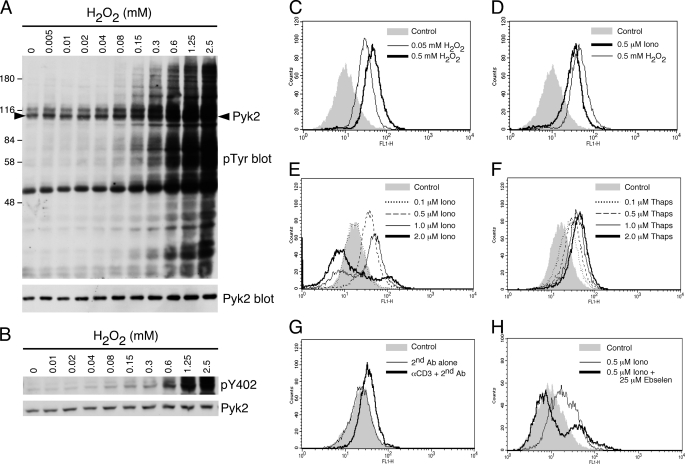
TCR stimulation of T cells triggers the production of reactive oxygen species (ROS) via a Ca2+-dependent mechanism (36). Furthermore, H2O2 can inhibit phosphatases leading to increased phosphorylation of proteins (37–39). Moreover, H2O2 was demonstrated to trigger Pyk2 phosphorylation in vascular smooth muscle cells (40) and induction of Pyk2 phosphorylation could be inhibited by ROS scavengers (40, 41), suggesting that Pyk2 can be regulated by ROS. To confirm that H2O2 does indeed induce Pyk2 phosphorylation in the AB.1 CTL clone, we titrated H2O2 on AB.1 cells then determined the degree of tyrosine phosphorylation. The overall pattern of phosphorylation stimulated with H2O2 (Fig. 6A), particularly at lower concentrations (i.e. 0.04–0.3 mm), was remarkably similar to the overall pattern of phosphorylation stimulated by the lower concentrations of ionomycin (Fig. 1B). Additionally, there was H2O2-stimulated phosphorylation of a protein that corresponds to Pyk2 in the total cell lysates (Fig. 6B) that we have confirmed to be Pyk2 by immunoprecipitation (data not shown) and immunoblotting with a pY402-specific antibody (Fig. 6B).
FIGURE 6.
H2O2 stimulates Pyk2 phosphorylation and inducers of Pyk2 phosphorylation stimulate production of reactive oxygen species in CTL. A, AB.1 were stimulated with the indicated concentration of H2O2 for 10 min then lysed. Shown are total cell lysates probed with anti-phosphotyrosine followed by Pyk2. The arrowhead in the top blot indicates the position of Pyk2. B, as in A except cell lysates were probed sequentially with antibodies specific for pY402-Pyk2 and Pyk2. C, AB.1 cells were left as a control or stimulated with 0.05 mm or 0.5 mm H2O2 for 10 min at 37 °C and assessed for the presence of ROS after incubation for 15 min with the indicator dye H2DCFDA, as described under “Experimental Procedures.” D, AB.1 cells were treated with DMSO carrier control, 0.5 μm ionomycin or 0.5 mm H2O2 for 10 min at 37 °C and assessed for ROS. E, AB.1 cells were treated with DMSO control (corresponding to the highest amount added to the cells as carrier) or with increasing ionomycin concentrations as indicated prior to ROS assessment. F, as for E except cells were treated with various concentrations of thapsigargin. G, AB.1 cells were left untreated or treated with secondary cross-linking antibody alone or in combination with anti-CD3 and assessed for the presence of ROS. H, AB.1 cells were pretreated with or without 25 μm ebselen then stimulated with 0.5 μm ionomycin for 10 min at 37 °C prior to ROS detection. The control contains the highest amount of DMSO added to the cells. All data are representative of at least three independent experiments.
We determined if ionomycin induced H2O2 production in T cells by making use of the cell permeable oxidation-sensitive dye CM-H2DCFDA (42) detected by flow cytometry. We confirmed that the dye was able to detect ROS in our cells by treating them with 0.05 mm or 0.5 mm H2O2 (Fig. 6C). Ionomycin (0.5 μm) induced a substantial shift in H2DCFDA fluorescence (Fig. 6D), roughly comparable to the level stimulated with 0.05 mm H2O2 (Fig. 6C). We performed a titration of ionomycin and thapsigargin to determine if increasing concentrations of these agents resulted in increasing ROS production. Surprisingly, 0.5 μm ionomycin resulted in the highest level of ROS production with increasing levels yielding lower levels of ROS, even below background (Fig. 6E). In contrast, thapsigargin titration resulted in slightly increasing levels of ROS production (Fig. 6F). This exactly reflects the Pyk2 phosphorylation induced with these two reagents (Fig. 1). It is not clear why the ROS levels should decrease with increasing ionomycin concentration since the Ca2+ would be increasing with increasing concentration and is likely due to some other effect of ionomycin not directly related to the intracellular Ca2+ flux. We also confirmed that cross-linked anti-CD3 stimulation of these cells stimulates ROS production (Fig. 6G), as previously shown (36). Finally, the ionomycin-induced H2O2 production is substantially inhibited in most cells in the presence of the H2O2 scavenger ebselen (Fig. 6H).
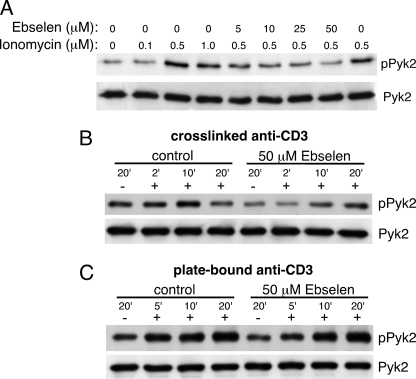
To determine if ionomycin-induced Pyk2 phosphorylation is dependent on the production of H2O2, AB.1 cells were pretreated with or without ebselen then stimulated with ionomycin. Ionomycin-induced phosphorylation was decreased with increasing concentrations of ebselen with 50 μm ebselen completely inhibiting the ionomycin-induced phosphorylation (Fig. 7A). Additionally, we found that 50 μm ebselen substantially reduced and delayed cross-linked anti-CD3 induced Pyk2 tyrosine phosphorylation (Fig. 7B), but had no apparent effect on plate-bound anti-CD3 stimulation. These data demonstrate that H2O2 production is required only under circumstances where Ca2+ is also required for Pyk2 tyrosine phosphorylation. Taken together, these data suggest that increased intracellular Ca2+ results in increased H2O2 production, which in turn results in increased Pyk2 phosphorylation.
FIGURE 7.
Ebselen inhibits ionomycin- and cross-linked anti-CD3-induced Pyk2 phosphorylation. A, AB.1 cells were pretreated with the indicated concentration of ebselen, or carrier control, for 20 min prior to stimulation for 10 min with the indicated concentration of ionomycin. After stimulation, cells were lysed and immunoprecipitates were probed first with anti-phosphotyrosine followed by anti-Pyk2. AB.1 cells were pretreated with 50 μm ebselen or DMSO carrier control for 20 min prior to stimulation with cross-linked (B) or plate-bound (C) anti-CD3 for the indicated time, as in Fig. 3. After stimulation, Pyk2 immunoprecipitations and immunoblots were performed as in A.
Ionomycin-stimulated Pyk2 Phosphorylation Is Erk Dependent in T Cells
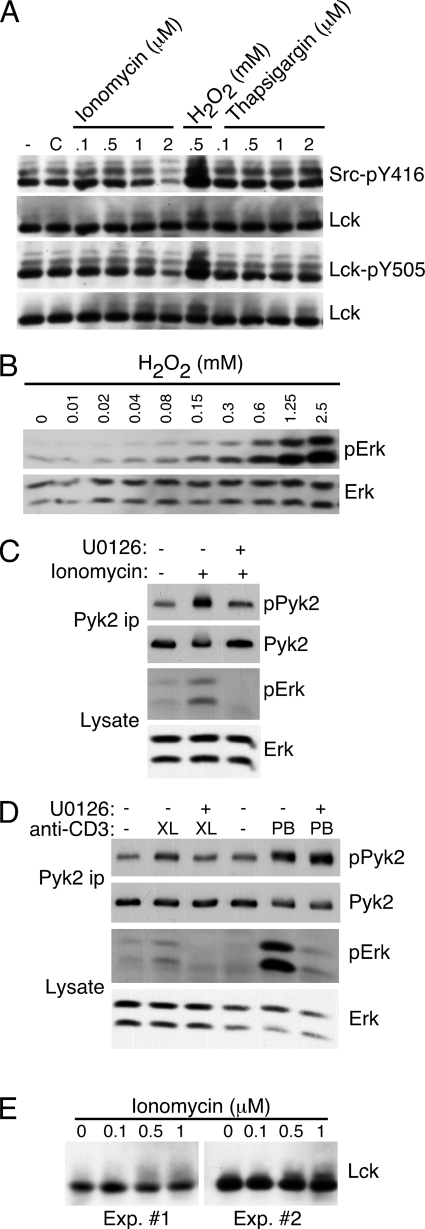
One possibility for how H2O2 might regulate Pyk2 phosphorylation is through the activation of SFK. It has been shown that 5 mm H2O2 induces Lck activation (43). We used phosphorylation-site specific antibodies to examine the phosphorylation of both the autophosphorylation site and the C-terminal inhibitory site of Lck, the predominant SFK expressed in these cells, after ionomycin or thapsigargin treatment. We could detect no changes in the phosphorylation of either site after thapsigargin treatment (Fig. 8A). Interestingly, we saw decreased phosphorylation of Lck phosphorylation at both sites with increasing ionomycin concentrations, with a substantial decrease at 2 μm (Fig. 8A). This is consistent with the decrease in the phosphorylation of the ∼52-kDa band observed in Fig. 1B, which corresponds to Lck, and could explain why at higher ionomycin, but not thapsigargin, concentrations, Pyk2 phosphorylation is reduced. Identical results were obtained using these antibodies in flow cytometry-based assays (data not shown). We did detect increased phosphorylation at both sites using 0.5 mm H2O2 (Fig. 8A), as was previously demonstrated at 5 mm (43).
FIGURE 8.
Erk phosphorylation is stimulated by H2O2 or ionomycin and is required for optimal ionomycin-stimulated Pyk2 phosphorylation. A, AB.1 were stimulated with the indicated concentration of ionomycin, H2O2 or thapsigargin for 10 min. (−) indicates untreated cells and C indicates cells treated with DMSO carrier. Cell lysates were prepared and duplicate blots were probed sequentially with antibodies specific for Src-pY416 then Lck or Lck-pY505 followed by Lck. B, AB.1 were stimulated with the indicated concentration of H2O2 for 10 min. Cell lysates were prepared and probed sequentially with antibodies specific for phospho-Erk then Erk. C, AB.1 were stimulated with 0.5 μm ionomycin for 10 min in the presence or absence of 10 μm U0126. Cell lysates and Pyk2 immunoprecipitates were prepared and probed with the indicated antibody. D, AB.1 were stimulated with cross-linked (XL) anti-CD3 for 10 min or with plate-bound (PB) anti-CD3 for 20 min in the presence or absence of 10 μm U0126. Cell lysates and Pyk2 immunoprecipitates were prepared and probed with the indicated antibody. E, AB.1 were stimulated with the indicated concentration of ionomycin for 10 min and cell lysates probed with anti-Lck. Shown are blots from two separate experiments.
Because ionomycin- or thapsigargin-induced H2O2 production does not appear to directly regulate SFK, we sought to identify a pathway through which Pyk2 might be regulated downstream of H2O2 production. It has previously been shown that cross-linked anti-CD3 stimulated Erk activation required the production of H2O2 in T cells (36). Furthermore, ionomycin induces Erk activation in Jurkat T cells (44). We therefore determined if there was a link between H2O2, Ca2+-stimulated Erk activation, and Pyk2 phosphorylation. CTL were stimulated with different concentrations of H2O2 and probed with antibodies specific for phospho-Erk demonstrating that phosphorylation of Erk is stimulated at relatively low concentrations of H2O2 (Fig. 8B). Stimulation of CTL with ionomycin also stimulated Erk activation (Fig. 8C), consistent with the previous study in Jurkat T cells (44). Surprisingly, ionomycin-stimulated Pyk2 phosphorylation was Erk dependent as it was inhibited by the MEK inhibitor U0126 (Fig. 8C), suggesting that Ca2+-stimulated Pyk2 activation is an Erk-dependent pathway. We also examined the requirement for Erk for Pyk2 phosphorylation in response to cross-linked or plate-bound anti-CD3 stimulation and found, consistent with the calcium requirement, that only stimulation with cross-linked anti-CD3, but not plate-bound anti-CD3, was inhibited by U0126 (Fig. 8D). Therefore Erk is required for Pyk2 activation only under circumstances where Ca2+ is also required. These data indicate that H2O2 can lead to Erk activation, which is required for Ca2+-dependent Pyk2 phosphorylation in CTL. Because we found that SFK activity was required for ionomycin-induced Pyk2 activation even though we could not detect changes in SFK tyrosine phosphorylation, we determined if there was a possible link between Erk and SFK after ionomycin treatment. It has been reported that Erk-mediated phosphorylation of Lck after T cell activation can be detected as a shift in the relative mobility of Lck (45, 46). We detect a mobility shift of an admittedly small fraction of Lck after ionomycin treatment (Fig. 8, E and A), opening the possibility that Erk could be phosphorylating Lck upon ionomycin treatment. Taken together, these data demonstrate that optimal Pyk2 phosphorylation induced by either ionomycin or cross-linked anti-CD3 is dependent on the Erk pathway.
DISCUSSION
Herein we demonstrated that Ca2+ can induce Pyk2 phosphorylation in T cells, but is not absolutely required for TCR stimulated Pyk2 phosphorylation. The requirement for Ca2+ in TCR/CD3-stimulated Pyk2 phosphorylation appears to depend on the strength of the signal received through the TCR because cross-linked anti-CD3, which triggers a relatively transient and weak signal (30), requires Ca2+ for Pyk2 phosphorylation, whereas plate-bound antibody, which triggers a robust and sustained signal (30), is largely unaffected by extracellular or intracellular Ca2+ chelation. These results suggest that Pyk2 is not an obligatory Ca2+-dependent enzyme in T cells. We also found that either ionomycin or thapsigargin could stimulate Pyk2 phosphorylation in CTL clones as well as in ex vivo activated T cells, suggesting that increases in intracellular Ca2+ can activate Pyk2 phosphorylation in normal T cells. However, this is not universally the case, since not all cell lines tested responded to ionomycin or thapsigargin stimulation with increased Pyk2 phosphorylation.
It is still not known how Ca2+ regulates Pyk2. Calmodulin has been shown to bind to the N-terminal FERM domain of Pyk2, potentially resulting in the release of the autoinhibition and activation of Pyk2 (20). While this pathway may be operational upon vasopressin stimulation of the rat fetus-derived fibroblast cell line WFB (20), in T cells, we detected no association of Pyk2 with calmodulin that was increased with stimulation of a Ca2+ flux, and a calmodulin antagonist did not abolish thapsigargin-stimulated Pyk2 phosphorylation (Fig. 4). It appears that there are additional mechanisms of Pyk2 regulation by Ca2+ in T cells and likely other cell types.
Because calmodulin-dependent responses were not required for Ca2+-triggered activation of Pyk2 in T cells, we set out to identify a Ca2+-induced pathway that could regulate Pyk2 tyrosine phosphorylation in T cells. We showed that ionomycin-induced Pyk2 phosphorylation was SFK dependent, but it is not obvious how an SFK pathway might be regulated through Ca2+. It was demonstrated that antigen receptor signaling in B cells is controlled through a regulatory circuit between Ca2+ and ROS to amplify receptor-generated responses (47). It was therefore possible that an intracellular Ca2+ flux could trigger the production of ROS, specifically H2O2, which would inhibit phosphatases and increase Pyk2 phosphorylation. We found that concentrations of ionomycin that trigger H2O2 production (Fig. 6E) also triggered Pyk2 phosphorylation (Fig. 1A). Furthermore, the ROS scavenger ebselen inhibited ionomycin-induced Pyk2 phosphorylation (Fig. 7), thus supporting our hypothesis that ionomycin induces H2O2 production in T cells, which in turn stimulates Pyk2 phosphorylation. We further speculate that this can explain why in some cells a Ca2+ flux does not result in Pyk2 phosphorylation (Fig. 2), because ROS production can be differently regulated.
We sought to determine how H2O2 might lead to Pyk2 activation in T cells. We initially explored the possibility that the H2O2 generated after ionomycin stimulation would lead to preferential activation of SFK, which we showed is required for ionomycin-induced Pyk2 phosphorylation (Fig. 5), however no increased SFK activation, as measured by phosphorylation of the autophosphorylation site, could be detected after stimulation with ionomycin or thapsigargin (Fig. 8A). We also could not detect changes in Lck activity in these cells after TCR stimulation (data not shown), a situation known to require Lck activity, which is consistent with a recent study showing that there are no detectable changes in the activity of Lck and Fyn after TCR stimulation (48).
What then is regulated by H2O2 upstream of Pyk2? Erk kinase activity can be stimulated by ionomycin in Jurkat T cells (44) and we showed that ionomycin (Fig. 8C) and thapsigargin (data not shown) stimulates Erk activation in CTL clones. It has also been demonstrated that activation of Erk downstream of the TCR is H2O2 dependent, suggesting that H2O2 can regulate Erk activity (36). We found that ionomycin as well as low concentrations of H2O2, similar to those that induce Pyk2 phosphorylation but not substantially increased overall tyrosine phosphorylation, result in Erk activation (Fig. 8B). We further demonstrated that ionomycin-induced Pyk2 tyrosine phosphorylation is blocked with an Erk inhibitor (Fig. 8C), a finding that was unexpected. It is not clear how Erk might regulate Pyk2 tyrosine phosphorylation. One possibility is that Erk directly phosphorylates Pyk2, inducing a conformational change resulting in increased Pyk2 autophosphorylation. However, we have no biochemical evidence to support direct phosphorylation of Pyk2 by Erk. Rather, we suggest that Erk phosphorylates SFK to either alter its SH2 binding specificity (45) or maintain SFK in an active confirmation by preventing negative feedback by the SHP-1 protein-tyrosine phosphatase (49), allowing for its recruitment to Pyk2 for downstream phosphorylation.
It is thought that Tyr-402 of Pyk2 is an autophosphorylation site that allows for SFK recruitment, which in turn results in phosphorylation of the other major tyrosine phosphorylation sites on Pyk2 (11). It was recently demonstrated that Tyr-402 phosphorylation downstream of TCR stimulation is SFK dependent (22), a finding we confirmed and extended to ionomycin stimulation. How can the apparent SFK-dependence of phosphorylation of all sites be reconciled with the current model of Pyk2 regulation by SFK? We found a fraction of Pyk2 is basally phosphorylated on Tyr-402 that is SFK independent as evidenced by the relatively high levels of Tyr-402 phosphorylation, compared with the other phosphorylation sites, in the presence of PP2 (Fig. 5, D and E). We predict that this low level of phosphorylation is sufficient to recruit SFK, which would then rapidly phosphorylate and activate this small basally Tyr-402-phosphorylated pool of Pyk2. This would in turn initiate a positive feedback loop where SFK would phosphorylate and activate Pyk2 leading to autophosphorylation of additional Pyk2 molecules on Tyr-402 resulting in increased recruitment of SFK. With this model, the increased phosphorylation upon ionomycin treatment would be due to increased Pyk2 autophosphorylation downstream of SFK, rendering the Ca2+-induced phosphorylation of Tyr-402 entirely SFK dependent. It is of course possible that the current model of Pyk2 activation by SFK is incorrect and that under certain circumstances, Tyr-402 phosphorylation is entirely SFK dependent, as has been proposed by Collins et al. (22).
Our data demonstrate that under conditions where there is strong, directional TCR stimulation (i.e. plate-bound anti-CD3), activation of Pyk2 requires SFK but can occur independent of Ca2+ and Erk activation. In contrast, when suboptimal signals are provided (i.e. cross-linked anti-CD3 or ionomycin), both a Ca2+ flux and Erk activation are required to facilitate SFK phosphorylation of basally Tyr-402-phosphorylated Pyk2. When T cells are stimulated with plate-bound anti-CD3 or antigen presented on antigen-presenting cells, there is a strong induction of tyrosine phosphorylation and recruitment of SH2 domain-containing proteins to the contact site thus favoring interactions between signaling molecules such as SFK and Pyk2. Upon cross-linked anti-CD3 or ionophore treatment, there would be limited recruitment of molecules to the site of stimulation since there is no directionality to these signals and, in the case of ionophore treatment, essentially no induction of global tyrosine phosphorylation (Fig. 1B). Furthermore, upon cross-linked anti-CD3 treatment, there would be comparatively more transient induction of tyrosine phosphorylation (30) resulting in more transient formation of signaling complexes. Under these conditions of stimulation, we suggest that Erk might phosphorylate SFK to facilitate its interaction through its SH2 domain with the limited number of Tyr-402 autophosphorylated Pyk2 molecules. It is also possible that Erk phosphorylation might act in other ways, either directly or indirectly, to improve the ability of these two kinases to interact in a cellular context.
In summary, we have shown that Pyk2 can be activated by both Ca2+-dependent and -independent pathways in the same cells. We further demonstrated that Ca2+-stimulated activation of Pyk2 in T cells requires Erk and that both Pyk2 and Erk activation occurs downstream of H2O2 production. Our data also demonstrate that all stimulation pathways are dependent on SFK for propagation of Pyk2 phosphorylation and activation. It is possible that Pyk2 could be involved in the process of signal spreading between different cellular receptors and perhaps adhesion molecules. We hypothesize that Pyk2 is a sensor of extracellular signals that trigger a Ca2+ influx and/or ROS production allowing for amplification of signaling responses through numerous receptors. This might explain why it has been difficult to assign an obligatory role of Pyk2 in many signaling pathways in which it is thought to be operational, because it may amplify rather than mediate receptor-triggered responses. This does not diminish its contribution to various receptor systems, but does underscore the need to consider cellular context when attempting to understand Pyk2 function and regulation.
Acknowledgment
We thank Dr. Kevin Kane for helpful discussion and critical review of the manuscript.
This work was supported by Canadian Cancer Society Grant 017111.
- Pyk2
- proline-rich tyrosine kinase 2
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- CTL
- cytotoxic T lymphocyte
- FAK
- focal adhesion kinase
- ROS
- reactive oxygen species
- SFK
- Src family kinase
- TCR
- T cell receptor.
REFERENCES
- 1.Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. (1995) Nature 376, 737–745 [DOI] [PubMed] [Google Scholar]
- 2.Sasaki H., Nagura K., Ishino M., Tobioka H., Kotani K., Sasaki T. (1995) J. Biol. Chem. 270, 21206–21219 [DOI] [PubMed] [Google Scholar]
- 3.Avraham S., London R., Fu Y., Ota S., Hiregowdara D., Li J., Jiang S., Pasztor L. M., White R. A., Groopman J. E., Avraham H. (1995) J. Biol. Chem. 270, 27742–27751 [DOI] [PubMed] [Google Scholar]
- 4.Yu H., Li X., Marchetto G. S., Dy R., Hunter D., Calvo B., Dawson T. L., Wilm M., Anderegg R. J., Graves L. M., Earp H. S. (1996) J. Biol. Chem. 271, 29993–29998 [DOI] [PubMed] [Google Scholar]
- 5.Herzog H., Nicholl J., Hort Y. J., Sutherland G. R., Shine J. (1996) Genomics 32, 484–486 [DOI] [PubMed] [Google Scholar]
- 6.Ostergaard H. L., Lysechko T. L. (2005) Immunol. Res. 31, 267–282 [DOI] [PubMed] [Google Scholar]
- 7.Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D. P., Sheetz M. P., Schlessinger J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10740–10745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil-Henn H., Destaing O., Sims N. A., Aoki K., Alles N., Neff L., Sanjay A., Bruzzaniti A., De Camilli P., Baron R., Schlessinger J. (2007) J. Cell Biol. 178, 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avraham H., Park S. Y., Schinkmann K., Avraham S. (2000) Cell Signal 12, 123–133 [DOI] [PubMed] [Google Scholar]
- 10.Li X., Dy R. C., Cance W. G., Graves L. M., Earp H. S. (1999) J. Biol. Chem. 274, 8917–8924 [DOI] [PubMed] [Google Scholar]
- 11.Park S. Y., Avraham H. K., Avraham S. (2004) J. Biol. Chem. 279, 33315–33322 [DOI] [PubMed] [Google Scholar]
- 12.Cai X., Lietha D., Ceccarelli D. F., Karginov A. V., Rajfur Z., Jacobson K., Hahn K. M., Eck M. J., Schaller M. D. (2008) Mol. Cell. Biol. 28, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lietha D., Cai X., Ceccarelli D. F., Li Y., Schaller M. D., Eck M. J. (2007) Cell 129, 1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiregowdara D., Avraham H., Fu Y., London R., Avraham S. (1997) J. Biol. Chem. 272, 10804–10810 [DOI] [PubMed] [Google Scholar]
- 15.Raja S., Avraham S., Avraham H. (1997) J. Biol. Chem. 272, 10941–10947 [DOI] [PubMed] [Google Scholar]
- 16.Hirotani S., Higuchi Y., Nishida K., Nakayama H., Yamaguchi O., Hikoso S., Takeda T., Kashiwase K., Watanabe T., Asahi M., Taniike M., Tsujimoto I., Matsumura Y., Sasaki T., Hori M., Otsu K. (2004) J. Mol. Cell. Cardiol. 36, 799–807 [DOI] [PubMed] [Google Scholar]
- 17.Ginnan R., Singer H. A. (2002) Am. J. Physiol. Cell Physiol. 282, C754–C761 [DOI] [PubMed] [Google Scholar]
- 18.Guo J., Meng F., Fu X., Song B., Yan X., Zhang G. (2004) Neurosci. Lett. 355, 177–180 [DOI] [PubMed] [Google Scholar]
- 19.Xu J., Gao X. P., Ramchandran R., Zhao Y. Y., Vogel S. M., Malik A. B. (2008) Nat. Immunol. 9, 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohno T., Matsuda E., Sasaki H., Sasaki T. (2008) Biochem. J. 410, 513–523 [DOI] [PubMed] [Google Scholar]
- 21.Qian D., Lev S., van Oers N. S., Dikic I., Schlessinger J., Weiss A. (1997) J. Exp. Med. 185, 1253–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins M., Tremblay M., Chapman N., Curtiss M., Rothman P. B., Houtman J. C. (2010) J. Leukoc. Biol. 87, 691–701 [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Fernández J. L., Sánchez-Martín L., de Frutos C. A., Sancho D., Robinson M., Sánchez-Madrid F., Cabañas C. (2002) J. Leukoc. Biol. 71, 520–530 [PubMed] [Google Scholar]
- 24.Ganju R. K., Hatch W. C., Avraham H., Ona M. A., Druker B., Avraham S., Groopman J. E. (1997) J. Exp. Med. 185, 1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuchida M., Manthei E. R., Knechtle S. J., Hamawy M. M. (1999) Eur. J. Immunol. 29, 2354–2359 [DOI] [PubMed] [Google Scholar]
- 26.Blakely A., Gorman K., Ostergaard H., Svoboda K., Liu C. C., Young J. D., Clark W. R. (1987) J. Exp. Med. 166, 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karttunen J., Sanderson S., Shastri N. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 6020–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostergaard H. L., Lou O., Arendt C. W., Berg N. N. (1998) J. Biol. Chem. 273, 5692–5696 [DOI] [PubMed] [Google Scholar]
- 29.Berg N. N., Ostergaard H. L. (1997) J. Immunol. 159, 1753–1757 [PubMed] [Google Scholar]
- 30.Berg N. N., Puente L. G., Dawicki W., Ostergaard H. L. (1998) J. Immunol. 161, 2919–2924 [PubMed] [Google Scholar]
- 31.Mason M. J., Garcia-Rodriguez C., Grinstein S. (1991) J. Biol. Chem. 266, 20856–20862 [PubMed] [Google Scholar]
- 32.Dikic I., Schlessinger J. (1998) J. Biol. Chem. 273, 14301–14308 [DOI] [PubMed] [Google Scholar]
- 33.Katagiri T., Takahashi T., Sasaki T., Nakamura S., Hattori S. (2000) J. Biol. Chem. 275, 19645–19652 [DOI] [PubMed] [Google Scholar]
- 34.Ostergaard H., Clark W. R. (1987) J. Immunol. 139, 3573–3579 [PubMed] [Google Scholar]
- 35.Ma E. A., Lou O., Berg N. N., Ostergaard H. L. (1997) Eur. J. Immunol. 27, 329–335 [DOI] [PubMed] [Google Scholar]
- 36.Devadas S., Zaritskaya L., Rhee S. G., Oberley L., Williams M. S. (2002) J. Exp. Med. 195, 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng T. C., Fukada T., Tonks N. K. (2002) Mol. Cell 9, 387–399 [DOI] [PubMed] [Google Scholar]
- 38.Reth M. (2002) Nat. Immunol. 3, 1129–1134 [DOI] [PubMed] [Google Scholar]
- 39.Tonks N. K. (2005) Cell 121, 667–670 [DOI] [PubMed] [Google Scholar]
- 40.Frank G. D., Motley E. D., Inagami T., Eguchi S. (2000) Biochem. Biophys. Res. Commun. 270, 761–765 [DOI] [PubMed] [Google Scholar]
- 41.Daou G. B., Srivastava A. K. (2004) Free Radic. Biol. Med. 37, 208–215 [DOI] [PubMed] [Google Scholar]
- 42.Crow J. P. (1997) Nitric Oxide 1, 145–157 [DOI] [PubMed] [Google Scholar]
- 43.Hardwick J. S., Sefton B. M. (1997) J. Biol. Chem. 272, 25429–25432 [DOI] [PubMed] [Google Scholar]
- 44.Franklin R. A., Atherfold P. A., McCubrey J. A. (2000) Mol. Immunol. 37, 675–683 [DOI] [PubMed] [Google Scholar]
- 45.Joung I., Kim T., Stolz L. A., Payne G., Winkler D. G., Walsh C. T., Strominger J. L., Shin J. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 5778–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watts J. D., Sanghera J. S., Pelech S. L., Aebersold R. (1993) J. Biol. Chem. 268, 23275–23282 [PubMed] [Google Scholar]
- 47.Singh D. K., Kumar D., Siddiqui Z., Basu S. K., Kumar V., Rao K. V. (2005) Cell 121, 281–293 [DOI] [PubMed] [Google Scholar]
- 48.Nika K., Soldani C., Salek M., Paster W., Gray A., Etzensperger R., Fugger L., Polzella P., Cerundolo V., Dushek O., Höfer T., Viola A., Acuto O. (2010) Immunity 32, 766–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stefanová I., Hemmer B., Vergelli M., Martin R., Biddison W. E., Germain R. N. (2003) Nat. Immunol. 4, 248–254 [DOI] [PubMed] [Google Scholar]