Abstract
In the present study MRP2/ABCC2 and BSEP/ABCB11 expression were investigated in sandwich cultured (SC) human and rat hepatocytes exposed to the proinflammatory cytokines. The investigation was also done in lipopolysaccharide (LPS)-treated rats. In SC human hepatocytes, both absolute protein and mRNA levels of MRP2/ABCC2 were significantly down-regulated by TNF-α, IL-6, or IL-1β. In contrast to mRNA decrease, which was observed for BSEP/ABCB11, the protein amount was significantly increased by IL-6 or IL-1β. A discrepancy between the change in BSEP/ABCB11 mRNA and protein levels was encountered in SC human hepatocytes treated with proinflammatory cytokines. In SC rat hepatocytes, Mrp2/Abcc2 mRNA was down-regulated by TNF-α and IL-6, whereas the protein level was decreased by all three cytokines. Down-regulations of both Bsep/Abcb11 mRNA and protein levels were found in SC rat hepatocytes exposed to TNF-α or IL-1β. Administration of LPS triggered the release of the proinflammatory cytokines and caused the decrease of Mrp2/Abcc2 and Bsep/Abcb11 protein in liver at 24 h post-treatment; however, the Mrp2 and Bsep protein levels rebounded at 48 h post-LPS treatment. In total, our results indicate that proinflammatory cytokines regulate the expression of MRP2/Mrp2 and BSEP/Bsep and for the first time demonstrate the differential effects on BSEP/Bsep expression between SC human and rat hepatocytes. Furthermore, the agreement between transporter regulation in vitro in SC rat hepatocytes and in vivo in LPS-treated rats during the acute response phase demonstrates the utility of in vitro SC hepatocyte models for predicting in vivo effects.
Keywords: ABC Transporter, Cytokine, Gene Regulation, Hepatocyte, Inflammation, BSEP/ABCB11, MRP2/ABCC2
Introduction
Drug transporters facilitate the passage of many drugs across cellular barriers during the process of absorption, distribution, metabolism, and elimination. The hepatobiliary excretion of drugs and drug metabolites is one of the primary elimination routes for endogenous and exogenous compounds from blood circulation (1). Multidrug resistance-associated protein 2 (MRP2/ABCC2)4 and bile salt export pump (BSEP/ABCB11) are localized mainly on the canalicular membrane of hepatocytes and are responsible for elimination of conjugated bilirubin and bile acids as part of the hepatic detoxification process. The transporters are also simultaneously involved in hepatic excretion of diverse drugs and their metabolites into bile. Consequently, functional disruption of MRP2/ABCC25 and BSEP/ABCB11 may result in drug-induced liver injury (2), which is a major cause of withdrawal of drugs from the market (3).
Pharmacokinetics for numerous drugs has been reported to be significantly affected during inflammation (4). Proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 have been shown to regulate the expression of hepatic transporters during inflammation in rodents (5–8). Down-regulation of hepatic bile salt influx transporters such as the sodium-dependent taurocholate transporter (NTCP/SLC10A1) by inflammation has been characterized in both rodents and primary human hepatocytes (9, 10). The decreased expression of hepatic transporters could affect the hepatobiliary secretion of various physiological substances, which further indicates that alteration of transporter expression might play an important role in the pathogenesis of cholestasis (11–13). However, these previous investigations focus mainly on the effects of proinflammatory cytokines on hepatobiliary transporter expression either in vivo in rodents or in vitro using hepatoma cells or primary cultured hepatocytes. Primary cultured human hepatocytes have been reported to be capable of predicting clinically relevant regulation of CYP enzymes to some extent (14). Because of the recent accessibility of human hepatocytes, there has been a growing interest in the use of primary human hepatocytes for investigating regulation of cytochrome P450 enzymes and hepatobiliary transporters in an effort to predict in vivo effects (15, 16). Treatment of human hepatocytes with cytokines has also demonstrated alterations in the expression of various hepatic transporters including MRP2/ABCC2 and BSEP/ABCB11 (10, 17). However, the cellular polarity that allows uptake and efflux in vivo is disrupted rapidly when cells are isolated from the intact organ (18, 19). One of the major critiques for the primary cultured hepatocyte model is that the disruption of cellular polarity might affect the cellular responses to the stimulants. Indeed, discrepancies in efflux transporter regulation due to inflammation have been reported between human hepatocytes and human hepatoma cell lines and between human and rodent hepatocytes and undoubtedly complicate in vitro to in vivo extrapolation. In the present study the sandwich cultured (SC) human and rat hepatocytes were used to investigate the transporter regulation and to further establish the in vitro models for in vivo prediction.
MATERIALS AND METHODS
Chemicals and Reagents
HPLC grade acetonitrile, water, and methanol were purchased from Burdick & Jackson (Muskegon, MI) and EMD Chemicals, Inc. (Gibbstown, NJ), respectively. Hanks' balanced salt solution was purchased from Invitrogen. ProteoExtract Native Membrane Protein Extraction kit was purchased from Calbiochem. The protein quantification BCA kit and the in-solution digestion kit were purchased from Pierce. Trypsin was purchased from Promega (Madison, WI). MatrigelTM (phenol red free) and collagen I-coated 24-well plates were obtained from BD Biosciences. The hepatocyte plating medium (InVitroGROTM CP medium), culture medium (InVitroGROTM HI medium), and TorpedoTM antibiotic mix were purchased from Celsis IVT Technologies (Baltimore, MD). The completed plating media or culture media were prepared by mixing 1 ml of TorpedoTM antibiotic mix with 45 ml of InVitroGROTM CP medium or InVitroGROTM HI medium, respectively. Recombinant IL-1β, IL-6, and TNF-α were purchased from R&D System (Minneapolis, MN). The Milliplex rat cytokine immunoassay kit was obtained from Millipore Corp. (Billerica, MA). The RNeasy kit and RNase free DNase kit were purchased from Qiagen (Valencia, CA). SYBR supermix was obtained from Applied Biosystems (Foster City).
Hepatocyte Sandwich Culture
Freshly isolated rat and human hepatocytes were purchased from CellzDirect (Pittsboro, NC). Hepatocyte sandwich culture was conducted as previously described (20). Briefly, upon arrival fresh hepatocytes were centrifuged at 500 rpm at 4 °C for 4 min. The cell pellet was resuspended with 5 ml of completed plating media to assess cell viability by trypan blue exclusion. The hepatocytes were further diluted to 0.7 million cells/ml with completed plating media and dispensed into 24-well BioCoat plates (0.5 ml/well) and allowed to attach for 2–4 h at 37 °C in a humidified incubator with 95/5% air/CO2. Unattached hepatocytes were removed, and the media were replaced with fresh completed plating medium. On day 2 the hepatocytes were washed once with warmed completed culture media and then overlaid with MatrigelTM at a concentration of 0.25 mg/ml in ice-cold completed culture media. On day 3 or 4, the hepatocytes were cultured with completed culture medium containing TNF-α (100 ng/ml), IL-1β (1 ng/ml), or IL-6 (100 ng/ml) for a 48- or 24-h incubation, respectively. Cytokine concentrations were chosen according to the preliminary dose dependence results (supplemental Figs. S1–S3) and literature reports (10, 16). From the preliminary experiments, general dose dependence effects of all three cytokines on MRP2/ABCC2 protein expression in SC human hepatocytes were confirmed, whereas only IL-6 showed the dose-dependent effects on BSEP/ABCB11 expression (supplemental Fig. S1 and S2). In HepG2 cell models, the dose-dependent effects of TNF-α on MRP2/ABCC2 protein expression were detected (supplemental Fig. S3). Considering the physiological relevance (21), a lower concentration of IL-1 was selected. The culture media with or without cytokines was refreshed every 24 h.
RNA Isolation and Quantitative Real Time-PCR
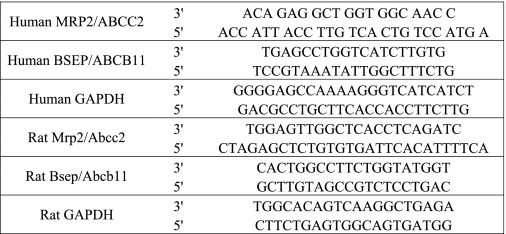
Total RNA was isolated from SC hepatocytes or rat liver tissues using the RNeasy kit. The obtained RNA was treated with RNase free DNase to remove the genomic DNA and quantified using a nano-UV spectrometer (NanoDrop Technology, Wilmington, DE). The first-strand cDNA was prepared from 200–500 ng of RNA using the Superscript III first-strand synthesis kit (Invitrogen) with random hexamer primers according to the manufacturer's suggested protocol. Negative controls were prepared without reverse transcriptase. All quantitative PCR reactions were prepared using SYBR PCR supermix with the synthesized first-strand cDNA and specific primer pairs (Table 1) and performed using an ABI-PRISM 7500 Fast Detection System (Applied Biosystems). The thermal cycling conditions were 10 min at 95 °C, then 40 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s. Quantification of relative gene expression was performed using the 2−ΔΔCt approach to calculate the -fold change normalized to housekeeping gene (GAPDH).
TABLE 1.
Primer pairs for quantitative RT-PCR of transporters and internal standard
Extraction of Membrane Protein and Proteomic Digestion
At 24 and 48 h post-treatment of proinflammatory cytokines, SC hepatocytes were harvested and washed with Hanks' balanced salt solution. The membrane protein of hepatocytes or rat liver tissue was extracted as described previously (22). Protein concentrations of extracted membrane fractions were determined using the BCA protein assay kit (Pierce). The samples were then subjected to overnight tryptic digestion in the presence of stable isotope labeled internal standard followed by LC-MS/MS quantitative analysis as described previously (22, 23). Briefly, 50 fmol of stable isotope-labeled peptide serving as internal standard was added into 30 μg of membrane fraction protein. The protein mixture was then reduced with 10 mm DTT and alkylated with iodoacetamide in 50 mm ammonium bicarbonate digestion buffer and digested by trypsin (22). At the end of digestion, samples were acidified with equal amounts of 0.2% formic acid in H2O (MRP2/Mrp2) or 1:1 acetonitrile/H2O (BSEP/Bsep), then centrifuged at 5000 rpm for 20 min before LC-MS/MS analysis. The synthetic peptides corresponding to MRP2/Mrp2 and BSEP/Bsep tryptic fragments (Celtek Bioscience, Nashville, TN) and the corresponding stable isotope-labeled peptides (Sigma) were synthesized for LC-MS/MS protein quantification. Data were processed by integrating the appropriate peak areas generated from the reconstructed ion chromatograms for the analyte peptides and the stable isotope-labeled internal standard peptides by Analyst 1.4.1 (Applied Biosystems).
Sprague-Dawley Rats Treated with Lipopolysaccharide (LPS)
Male Sprague-Dawley rats weighing 275–340 g were purchased from Charles River Laboratories (Wilmington, DE) and acclimated to their surroundings for approximately 1 week with food and water provided ad libitum. A minimum of 1 day before the study, animals were anesthetized with isoflurane (to effect) and then implanted with BASi vascular catheters (Bioanalytical Services, Inc., West Lafayette, IN) in the carotid artery and jugular vein. Animals were intravenously-dosed with LPS (1 mg/kg, Sigma) formulated in 0.9% normal saline (Baxter) via the jugular vein catheter. At the designated time points (24 or 48 h post LPS-treatment), the rats were euthanized with 30 mg/kg pentobarbitol (Sleepaway) injection intraperitoneally. The rats then were flushed with 200 ml of phosphate-buffered saline (1× PBS) via carotid artery catheters to remove the blood. The livers were then removed and kept at −80 °C until analysis. To determine the plasma level of proinflammatory cytokines, the blood samples were collected from a subset group of LPS-treated rats (n = 2) at 4 h post-treatment. This animal study was approved by the St. Louis Pfizer Institutional Animal Care and Use Committee. The animal care and use program is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Determination of TNF-α, IL-1β, and IL-6 in the Plasma of LPS-treated Rats
Proinflammatory cytokines were measured by ELISA according to the protocols suggested by the vendor. Briefly, plasma was diluted 10-fold using serum matrix provided in the kit. The diluted plasma or standards (50 μl) were added into the appropriate wells. Antibody-immobilized beads (25 μl) were added into each well. After overnight incubation at 4 °C, the liquid in the plate was gently removed by vacuum. The plate was washed 2 times with 200 μl washing buffer, and 25 μl of detection antibody was added. After a 2-h incubation at room temperature, streptavidin-phycoerythrin, provided in the kit, was added to the plate. The plate was incubated for 30 min at room temperature and then washed 4 times with washing buffer. The substrate solution was added (100 μl/well), and the plate was incubated at room temperature in the dark for 15 min. The chromogenic reaction was stopped by the addition of 100 μl/well of stop solution. The optical densities were read on a plate reader (Molecular Devices, Sunnyvale, CA) at 450 nm.
LC-MS/MS Quantitative Measurement of MRP2/ABCC2 and BSEP/ABCB11 Protein
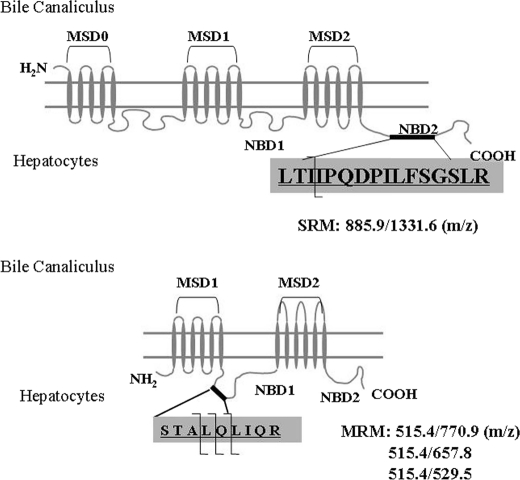
Transporter protein quantification was conducted on a triple quadrupole mass spectrometer (API4000, Applied Biosystems, Foster City, CA) coupled to a Shimadzu LC (SLC-10A) system (WoolDale, IL) with a HTS PAL Leap autosampler (Carrboro, NC). For MRP2/Mrp2, a Vydac EVERESTTM C18 column (2.1 × 100 mm, 5 μm in particle size 300 Å in pore size) was used. For BSEP/Bsep, an Agilent Eclipse XDB C18 column (2.1 × 150 mm, 5 μm in particle size, 80 Å in pore size) was used. A linear gradient elution program was conducted to achieve chromatographic separation with mobile phase A (0.1% formic acid in HPLC grade water) and mobile phase B (0.1% formic acid in acetonitrile). MRP2/Mrp2 gradient conditions were 5 to 35% B over a period of 30 min, and BSEP/Bsep gradient conditions were 5 to 25% B over a period of 20 min. A sample volume of 20 μl was injected onto the LC column at a flow rate of 0.4 ml/min. The parent-to-product transitions for the proteotypic peptide represent the doubly charged parent ion to the single charged product y ions for each transporter (Fig. 1). The instrument settings of the API4000 triple quadrupole mass spectrometer were: ion spray voltage, 4 kV; temperature, 400 °C. Declustering potential, collision energy, entrance potential, and collision cell exit potential were set as reported previously (22, 23).
FIGURE 1.
Diagram of MRP2/ABCC2 (upper panel)- and BSEP/ABCB11 (lower panel)-selective tryptic peptide for LC-MS/MS quantification. MSD, membrane-spanning domain; NBD, nucleotide binding domain; SRM, selected reaction monitoring; MRM, multiple reaction monitoring.
Data Analysis
Data are representative of a minimum of two in vitro experiments performed on different days. The in vivo experiments were preformed as n = 3 (n = 2 for subset group). Data are presented as the mean ± S.D. The differences were determined by ANOVA after post hoc analysis using Graphpad Prism Version 5.01 for windows (GraphPad Software, San Diego, CA). A p value less than 0.05 was regarded as statistically significant.
RESULTS
Modifications of MRP2/ABCC2 and BSEP/ABCB11 in SC Human Hepatocytes Exposed to TNF-α, IL-6, or IL-1β
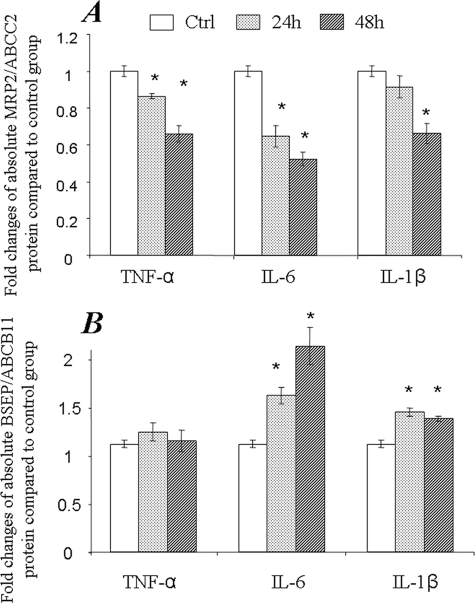
Quantitative mRNA detection (quantitative real time-PCR) has been a very popular and powerful tool in gene expression analysis studies (24, 25). Approaching in front, we examined the mRNA level of MRP2/ABCC2 and BSEP/ABCB11 in SC human and rat hepatocytes exposed to cytokines for 24 and 48 h. As shown in Fig. 2, mRNA expression of both MRP2/ABCC2 and BSEP/ABCB11 in SC human hepatocytes was significantly down-regulated by exposure to TNF-α, IL-1β, and IL-6. To determine whether the changes in mRNA level induced by cytokine treatments also occur at the protein level, LC-MS/MS-mediated absolute quantitative methods were utilized to measure MRP2/ABCC2 and BSEP/ABCB11 protein amounts in the inflammatory cytokine treated hepatocytes. Consistent with the changes in mRNA levels, TNF-α, IL-6, or IL-1β significantly decreased the absolute amount of MRP2/ABCC2 protein at 24 and 48 h post-treatment (Fig. 3A). However, in contrast to the down-regulation of mRNA, BSEP/ABCB11 protein was increased by IL-1β or IL-6 treatment, whereas no effect was detected with TNF-α treatment (Fig. 3B), indicating a discrepancy exists between BSEP/ABCB11 mRNA and protein levels in SC human hepatocytes exposed to cytokines.
FIGURE 2.
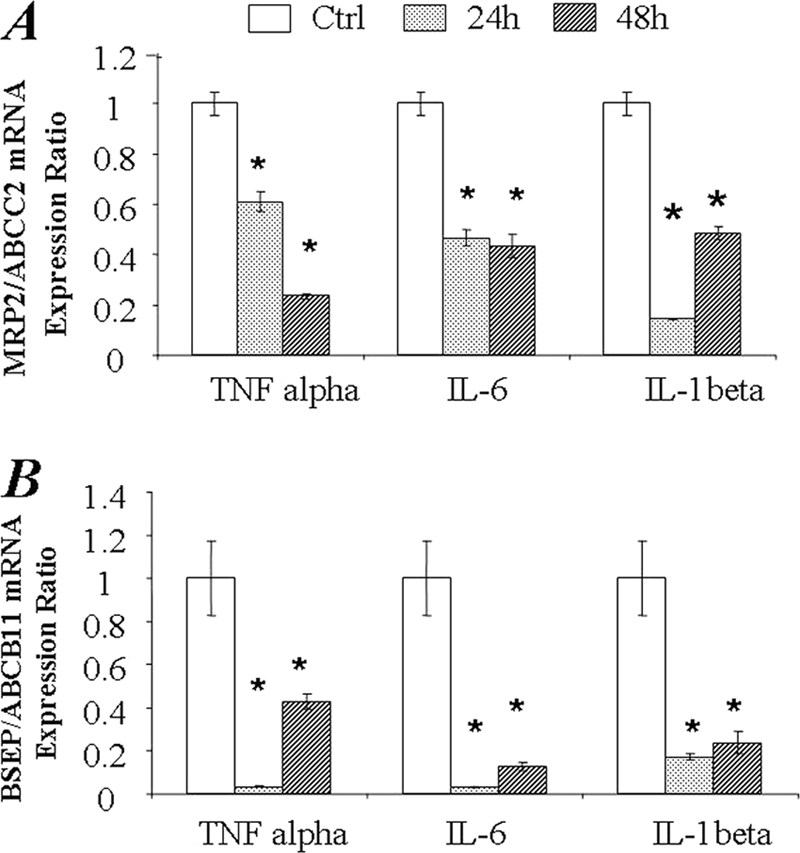
Changes of MRP2/ABCC2 and BSEP/ABCB11 mRNA expression in SC human hepatocytes exposed to TNF-α, IL-6, and IL-1β. The mRNA expression was normalized to GAPDH. The data represent the relative -fold of mRNA expression of MRP2/ABCC2 (A) or BSEP/ABCB11 (B) to control cultures; data represent the mean ± S.D. (n = 3). *, statistically significant (p ≤ 0.05 by ANOVA).
FIGURE 3.
Changes of MRP2/ABCC2 and BSEP/ABCB11 protein expression in SC human hepatocytes exposed to TNF-α, IL-6, and IL-1β. The membrane protein fraction was prepared from SC human hepatocytes from 2–3 wells of a 24-well plate and subjected to LC-MS/MS SRM analysis for absolute quantification of MRP2/ABCC2 (A) or BSEP/ABCB11 (B) protein. The data represent the mean ± S.D. (n = 3). *, statistically significant (p ≤ 0.05 by ANOVA). SRM, selected reaction monitoring.
Modifications of Mrp2/Abcc2 and Bsep/Abcb11 in SC Rat Hepatocytes Exposed to TNF-α, IL-6, or IL-1β
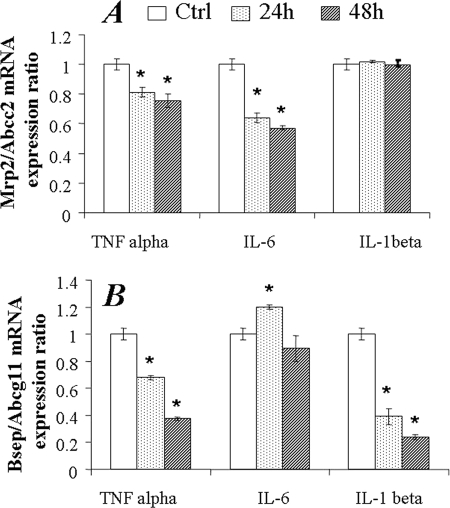
The regulation of Mrp2/Abcc2 and Bsep/Abcb11 transporters was also examined in SC rat hepatocytes exposed to TNF-α, IL-1β, or IL-6. TNF-α and IL-6 significantly down-regulated Mrp2/Abcc2 mRNA expression at 24 and 48 h; however, the effect of IL-1β on Mrp2/Abcc2 mRNA expression was not detected (Fig. 4A). TNF-α and IL-1β significantly decreased Bsep/Abcb11 mRNA levels at both 24 and 48 h in a time-dependent manner (Fig. 4B). At 24 h post-IL-6 treatment, a statistically significant increase of Bsep/Abcb11 mRNA was detected. However, no significant changes were found at 48 h. In line with mRNA changes, Mrp2/Abcc2 protein was significantly down-regulated by TNF-α, IL-1β, or IL-6, both at 24 and 48 h post-treatment (Fig. 5A). Bsep/Abcb11 protein was decreased by TNF-α at 24 and 48 h or by IL-1β at 48 h (Fig. 5B). Surprisingly, in contrast to the findings with human SC hepatocytes, the up-regulation of Bsep/Abcb11 protein by IL-6 was not detected in SC rat hepatocytes (Fig. 5B).
FIGURE 4.
Changes of Mrp2 and Bsep/Abcb11 mRNA expression in SC rat hepatocytes exposed to TNF-α, IL-6, and IL-1β. The mRNA expression was normalized to GAPDH. The data represents the relative -fold of mRNA expression of Mrp2 (A) or Bsep/Abcb11 (B) to control cultures, mean ± S.D. (n = 3). *, statistically significant (p ≤ 0.05 by ANOVA).
FIGURE 5.
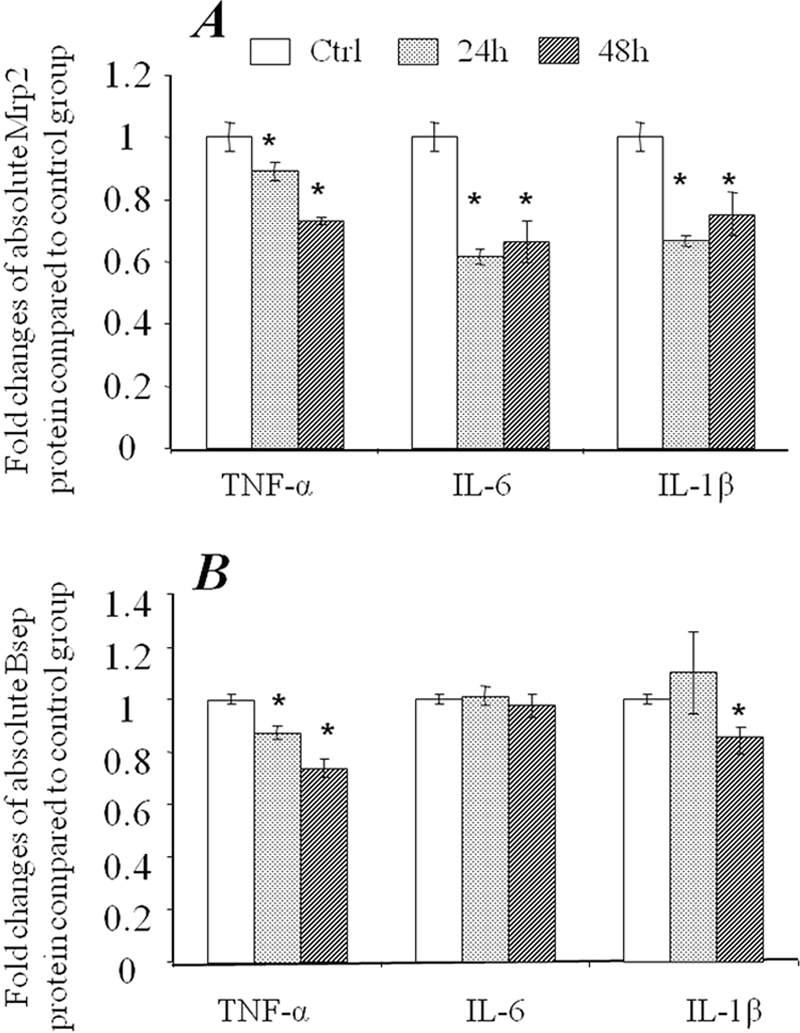
Changes of Mrp2/Abcc2 and Bsep/Abcb11 protein expression in SC rat hepatocytes exposed to TNF-α, IL-6, and IL-1β. The membrane protein fraction was prepared from SC human hepatocytes from 2–3 wells of a 24-well plate and subjected to LC-MS/MS SRM analysis for absolute quantification of Mrp2/Abcc2 (A) or Bsep (B) protein. The data represent the mean ± S.D. (n = 3). *, statistically significant (p ≤ 0.05 by ANOVA).
Hepatic Mrp2/Abcc2 and Bsep/Abcb11 Expression in LPS-treated Rats
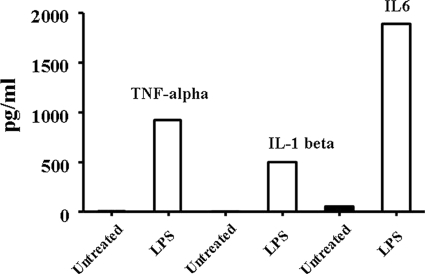
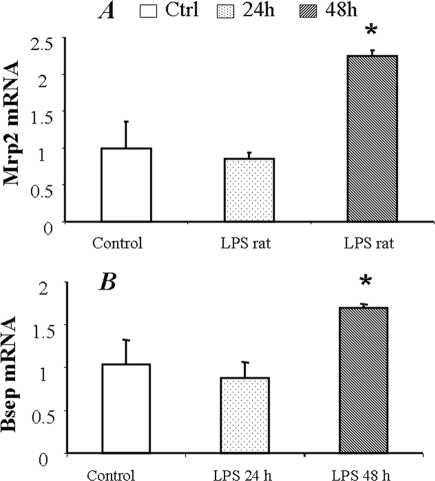
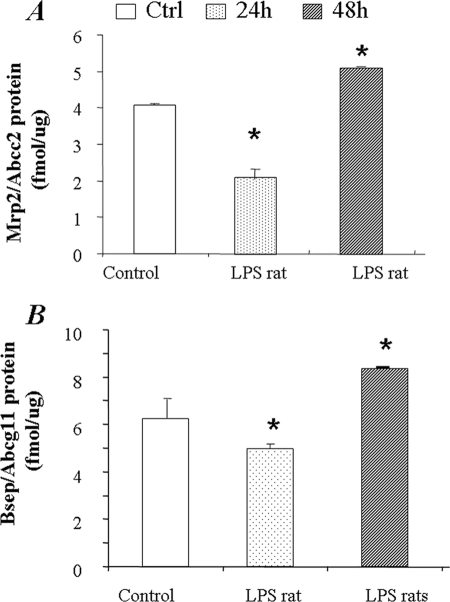
Regulation of Mrp2/Abcc2 and Bsep/Abcb11 expression was further evaluated in vivo in LPS-treated (1 mg/kg) rats. Liver tissues were collected at 24 and 48 h post-LPS injection. LPS challenge significantly increased the plasma levels of TNF-α, IL-1β, and IL-6, as determined by ELISA (Fig. 6). Mrp2/Abcc2 and Bsep/Abcb11 mRNA was slightly decreased at 24 h post LPS treatment; however, no statistical significance was detected as compared with the control group (Fig. 7, A and B). The Mrp2/Abcc2 and Bsep/Abcb11 proteins were significantly decreased in liver at 24 h (Fig. 8, A and B). Both Mrp2/Abcc2 and Bsep/Abcb11 mRNA expression in rat liver were significantly increased at 48 h post-LPS treatments (Fig. 7, A and B). In agreement with mRNA changes, Mrp2/Abcc2 and Bsep/Abcb11 proteins also increased significantly at 48 h post LPS treatment (Fig. 8, A and B). During the ARP (24 h post LPS treatment), the suppression of Mrp2/Abcc2 and Bsep/Abcb11 protein in vitro in SC rat hepatocytes exposed to proinflammatory cytokines was reproduced in vivo in LPS-treated rats.
FIGURE 6.
Proinflammatory cytokine level in plasma of LPS-treated rats. Plasma was collected 6 h post-LPS treatment. Pooled plasma was subjected to cytokine measurements using ELISA kits. The data represent the average of two measurements.
FIGURE 7.
Changes of Mrp2/Abcc2 and Bsep/Abcb11 mRNA expression in LPS-treated rats. The total mRNA was extracted from liver of LPS-treated rats. Mrp2/Abcc2 and Bsep/Abcb11 mRNA expression was normalized to GAPDH. The data represent the relative -fold of mRNA expression of Mrp2/Abcc2 (A) or Bsep (B) to control rats, mean ± S.D. (n = 3). *, statistically significant (p ≤ 0.05 by ANOVA).
FIGURE 8.
Changes of Mrp2 and Bsep protein in the liver of LPS-treated rats. The membrane protein fraction was prepared from liver tissues of LPS-treated rats and was subjected to LC-MS/MS SRM analysis for absolute quantification of Mrp2/Abcc2 (A) or Bsep (B) protein. The data represent the mean ± S.D. (n = 3). *, statistically significant (p ≤ 0.05 by ANOVA).
DISCUSSION
Because of the well known links between inflammation and proinflammatory cytokines such as TNF-α, IL-1β, and IL-6, the present study was designed to analyze the effects of these cytokines on the expression of major human hepatic transporters in SC hepatocytes by monitoring the changes of both mRNA and protein levels. Although data obtained from in vivo administration of cytokines in rodents yields valuable information on gene regulation, in vitro models used for analyzing the effects of proinflammatory cytokines on human hepatic drug transporters are essential due to the limited knowledge of interspecies differences with respect to responses to cytokines. Primary human hepatocytes represent the gold standard of in vitro models for analyzing drug metabolism and transport mechanisms. However, it remains largely unknown whether in vitro/in vivo correlation of transporter regulation by proinflammatory cytokines could be established in primary cultured hepatocytes due to the lack of the proper three-dimensional distribution of transporter proteins. Recently, the use of SC hepatocytes has become a valuable tool, allowing repolarization of hepatocytes and development of intact bile canaliculi, thereby providing the proper three-dimensional orientation and localization of efflux transporters like MRP2/ABCC2 and BSEP/ABCB11 (26, 27). The restoration of transporter orientation in in vitro hepatocyte cultured models is desired for investigating the regulation of hepatic transporters and vectorial transport activities (41). In addition, our previous report characterized the time-dependent changes of hepatobiliary transporter expression in SC hepatocytes (20). The primary purpose of the present study was to characterize the effects of TNF-α, IL-1β, and IL-6 on the expression of MRP2/ABCC2 and BSEP/ABCB11 in SC rat and human hepatocytes. Second, we investigated the in vitro/in vivo correlation between SC rat hepatocytes and LPS-treated rats, which in turn is fundamental to build confidence for in vitro to in vivo predictions of human hepatic transporter regulation using the SC human hepatocyte model.
The gene expression of cytochrome P450 enzymes and drug transporters is regulated during inflammatory processes (29), which affects absorption, disposition, metabolism, and elimination of many drugs (30). Mechanisms of gene regulation mediated by proinflammatory cytokines are proposed to involve both pre-translational and post-translational processes, resulting in changes of the corresponding mRNA and protein levels and enzyme/transporter activities (30, 31). Suppression of mRNA and protein levels of pregnane X receptor and other nuclear receptors including farnesoid X activated receptor and constitutive androstane receptor have been reported in LPS-treated mice or proinflammatory cytokine-treated human hepatocytes (32–34), suggesting that these nuclear receptors are involved in enzyme/transporter gene regulation. In fact, mRNA expression of hepatic efflux transporters is found to be highly repressed in endotoxin-induced inflammation rodent models (35). In SC human hepatocytes, both MRP2/ABCC2 and BSEP/ABCB11 mRNA expression were reduced by the exposure to three proinflammatory cytokines: TNF-α, IL-1β or IL-6. In SC rat hepatocytes, a decrease in Mrp2/Abcc2 mRNA was observed in hepatocytes exposed to TNF-α and IL-6, whereas BSEP/ABCB11 mRNA expression was decreased by TNF-α or IL-1β exposure. The results regarding mRNA suppression are consistent with previous results related to suppressive effects on transporter mRNA expression found in primary cultured human hepatocytes (7, 36). Interestingly, the BSEP/ABCB11 protein in human SC hepatocytes exposed to IL-6 or IL-1β was significantly increased (Fig. 3). In addition, we also observed a poor correlation between the modifications of mRNA and protein levels in hepatocytes exposed to proinflammatory cytokines. These cytokines might be involved in the decrease of BSEP/ABCB11 transcription with an increase of BSEP/ABCB11 translation, indicating that both pre- and post-transcriptional regulation might be involved in the process of regulation. In this regard, quantitative real time-PCR and absolute quantification of transporter proteins have been promising tools in the investigation of both pre- and post-transcriptional regulatory mechanisms.
In agreement with findings obtained using in vitro SC rat hepatocytes, a significant decrease of Mrp2/Abcc2 and Bsep/Abcb11 protein was detected in the rat at 24 h post LPS treatment. Interestingly, Mrp2/Abcc2 and Bsep/Abcb11 protein levels rebounded at 48 h, suggesting that the down-regulation of transporter proteins in LPS-treated rats was found during ARP, which usually lasts 24 h and encompasses initial tissue injury resulting in cytokine release and activation of signaling cascades. In fact, the proinflammatory cytokines decrease sharply after peaking at 2 h (37). Therefore, the dynamic phase of proinflammatory cytokines in plasma might explain the difference between in vitro and in vivo observations at 48 h post-treatment. It is known that the end of ARP usually shows the restoration of homeostasis; however, failure of the ARP to subside can develop into the chronic inflammatory phase (38). In this regard additional investigations are needed to elucidate the regulation of hepatic transporters during chronic inflammation.
In the present study we observed differences in BSEP/ABCB11 protein regulation between SC human and rat hepatocytes in response to cytokine treatments. Proinflammatory cytokines have been shown to activate signaling pathways such as mitogen-activated protein kinase (MAPKs) in primary human hepatocytes (10). IL-1β or IL-6 are thought to be involved in the activation of ERK, JNK, and p38 MAPK phosphorylation (39). The discrepancies in the responses of BSEP/Bsep between SC rat and human hepatocytes might be attributed to differences in the signaling pathways between the two species. For examples, MRP2/ABCC2 and BSEP/ABCB11 mRNA levels are not affected in LPS-treated human liver slices, suggesting that post-transcriptional mechanisms play a more prominent role in LPS-induced regulation of human MRP2/ABCC2 and BSEP/ABCB11 as compared with the rat transporter proteins (40). Moreover, mRNA levels in trophoblast cultures are not always found to closely correlate with transporter protein expression (28). Collectively, caution must be exercised when utilizing only rodent models to predict clinical events, which in turn demonstrate the importance of developing human in vitro hepatocyte models for in vivo prediction.
In the in vitro SC hepatocyte models, proinflammatory cytokines TNF-α, IL-1β, and IL-6 were found to reduce the absolute amount of MRP2/Mrp2 protein in both human and rat hepatocytes. However, we noticed a difference when comparing the cytokine-mediated transporter expression changes in SC human hepatocytes in this study to primary cultured human hepatocyte studies reported in the literature. In our study all three cytokines (TNF-α, IL-1β, and IL-6) suppressed both mRNA and protein expression of MRP2/ABCC2 post 24- and 48- h treatment. However, in two other studies using primary cultured human hepatocytes, IL-6 decreased both mRNA and protein expression of MRP2/ABCC2, whereas TNF-α did not change either mRNA or protein expression of MRP2/ABCC2 (10, 16, 17). In the studies using non-SC human hepatocytes (10, 16), TNF-α and IL-1β decreased BSEP/ABCB11 mRNA expression, IL-1β also decreased BSEP/ABCB11 protein expression, and the effect of TNF-α on BSEP/ABCB11 protein expression was not assessed. In contrast, IL-6 did not alter BSEP/ABCB11 mRNA expression. The discrepancies between primary cultured human hepatocytes and SC human hepatocytes manifested in this study might merit further investigation to illustrate which in vitro culture condition best represents the in vivo transporter expression changes.
In summary, using a combination of real-time PCR and LC-MS/MS-mediated absolute protein quantification, we have shown that proinflammatory cytokines significantly affect the expression of hepatobiliary transporters in SC hepatocytes and LPS-treated rats. The noteworthy findings include opposing changes in mRNA and protein levels, suggesting that both pre- and post-transcriptional regulation mechanisms exist. The agreement of transporter regulation in vitro in SC rat hepatocytes and in vivo in LPS-treated rats during the ARP emphasizes the potential utility of the SC hepatocyte model for in vivo prediction. Furthermore, the difference in BSEP/Bsep regulation between SC rat and human hepatocytes might reflect differences in the response of species-dependent signing pathways. Additional investigations are needed to further elucidate the in vitro-in vivo correlation and the complicated regulation during chronic inflammation.
Supplementary Material
Acknowledgments
We thank Drs. James Polli, Larissa M. Balogh, and Jeffrey C. Stevens for helpful comments and suggestions on our manuscript. We also thank Steve Wene and Lesley A. Albin for help in conducting animal studies.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
The use of upper case letters in transporter nomenclature denotes the human protein, i.e. MRP2/ABCC2. Lower case letters indicate the transporter derives from a preclinical species, i.e. Mrp2/Abcc2.
- MRP2/ABCC2
- multidrug resistance protein 2
- ARP
- acute response phase
- BSEP/ABCB11
- bile salt export pump
- SC
- sandwich cultured
- ANOVA
- analysis of variance
- Ctrl
- control.
REFERENCES
- 1.Arias I. M. (1993) Gastroenterology 104, 1558–1560 [DOI] [PubMed] [Google Scholar]
- 2.Funk C., Pantze M., Jehle L., Ponelle C., Scheuermann G., Lazendic M., Gasser R. (2001) Toxicology 167, 83–98 [DOI] [PubMed] [Google Scholar]
- 3.Liss G., Lewis J. H. (2009) Expert. Opin. Drug. Metab. Toxicol. 5, 843–860 [DOI] [PubMed] [Google Scholar]
- 4.Slaviero K. A., Clarke S. J., Rivory L. P. (2003) Lancet Oncol. 4, 224–232 [DOI] [PubMed] [Google Scholar]
- 5.Donner M. G., Schumacher S., Warskulat U., Heinemann J., Häussinger D. (2007) Am. J. Physiol. Gastrointest Liver Physiol. 293, G1134–G1146 [DOI] [PubMed] [Google Scholar]
- 6.Siewert E., Dietrich C. G., Lammert F., Heinrich P. C., Matern S., Gartung C., Geier A. (2004) Biochem. Biophys. Res. Commun. 322, 232–238 [DOI] [PubMed] [Google Scholar]
- 7.Hartmann G., Cheung A. K., Piquette-Miller M. (2002) J. Pharmacol. Exp. Ther. 303, 273–281 [DOI] [PubMed] [Google Scholar]
- 8.Geier A., Dietrich C. G., Voigt S., Ananthanarayanan M., Lammert F., Schmitz A., Trauner M., Wasmuth H. E., Boraschi D., Balasubramaniyan N., Suchy F. J., Matern S., Gartung C. (2005) Am. J. Physiol. Gastrointest. Liver Physiol. 289, G831–G841 [DOI] [PubMed] [Google Scholar]
- 9.Green R. M., Beier D., Gollan J. L. (1996) Gastroenterology 111, 193–198 [DOI] [PubMed] [Google Scholar]
- 10.Le Vee M., Gripon P., Stieger B., Fardel O. (2008) Drug Metab. Dispos. 36, 217–222 [DOI] [PubMed] [Google Scholar]
- 11.Zollner G., Fickert P., Zenz R., Fuchsbichler A., Stumptner C., Kenner L., Ferenci P., Stauber R. E., Krejs G. J., Denk H., Zatloukal K., Trauner M. (2001) Hepatology 33, 633–646 [DOI] [PubMed] [Google Scholar]
- 12.Trauner M., Boyer J. L. (1999) Curr. Opin. Gastroenterol. 15, 217–228 [DOI] [PubMed] [Google Scholar]
- 13.Trauner M., Fickert P., Stauber R. E. (1999) J. Gastroenterol. Hepatol. 14, 946–959 [DOI] [PubMed] [Google Scholar]
- 14.Zhang X., Schmitt C., Grange S., Terao K., Miya K., Kivitz A., Marino M. (2009) Clin. Pharmacol. Ther. 85, S59 [Google Scholar]
- 15.Gómez-Lechón M. J., Donato M. T., Castell J. V., Jover R. (2004) Curr. Drug. Metab. 5, 443–462 [DOI] [PubMed] [Google Scholar]
- 16.Vee M. L., Lecureur V., Stieger B., Fardel O. (2009) Drug Metab. Dispos. 37, 685–693 [DOI] [PubMed] [Google Scholar]
- 17.Le Vee M., Lecureur V., Moreau A., Stieger B., Fardel O. (2009) Drug Metab. Dispos. 37, 2228–2235 [DOI] [PubMed] [Google Scholar]
- 18.Roelofsen H., Bakker C. T., Schoemaker B., Heijn M., Jansen P. L., Elferink R. P. (1995) Hepatology 21, 1649–1657 [PubMed] [Google Scholar]
- 19.Bow D. A., Perry J. L., Miller D. S., Pritchard J. B., Brouwer K. L. (2008) Drug Metab. Dispos. 36, 198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N., Bi Y. A., Duignan D. B., Lai Y. (2009) Mol. Pharm. 6, 1180–1189 [DOI] [PubMed] [Google Scholar]
- 21.Lee G., Piquette-Miller M. (2003) J. Pharm. Sci. 92, 2152–2163 [DOI] [PubMed] [Google Scholar]
- 22.Li N., Nemirovskiy O. V., Zhang Y., Yuan H., Mo J., Ji C., Zhang B., Brayman T. G., Lepsy C., Heath T. G., Lai Y. (2008) Anal. Biochem. 380, 211–222 [DOI] [PubMed] [Google Scholar]
- 23.Li N., Palandra J., Nemirovskiy O. V., Lai Y. (2009) Anal. Chem. 81, 2251–2259 [DOI] [PubMed] [Google Scholar]
- 24.Hilgendorf C., Ahlin G., Seithel A., Artursson P., Ungell A. L., Karlsson J. (2007) Drug Metab. Dispos. 35, 1333–1340 [DOI] [PubMed] [Google Scholar]
- 25.Goh L. B., Spears K. J., Yao D., Ayrton A., Morgan P., Roland Wolf C., Friedberg T. (2002) Biochem. Pharmacol. 64, 1569–1578 [DOI] [PubMed] [Google Scholar]
- 26.Ghibellini G., Bridges A. S., Generaux C. N., Brouwer K. L. (2007) Drug Metab. Dispos. 35, 345–349 [DOI] [PubMed] [Google Scholar]
- 27.Bi Y. A., Kazolias D., Duignan D. B. (2006) Drug Metab. Dispos. 34, 1658–1665 [DOI] [PubMed] [Google Scholar]
- 28.Evseenko D. A., Paxton J. W., Keelan J. A. (2006) Am. J. Physiol. Regul Integr Comp. Physiol. 290, R1357–R1365 [DOI] [PubMed] [Google Scholar]
- 29.Renton K. W. (2001) Pharmacol Ther. 92, 147–163 [DOI] [PubMed] [Google Scholar]
- 30.Renton K. W. (2004) Curr. Drug. Metab. 5, 235–243 [DOI] [PubMed] [Google Scholar]
- 31.Morgan E. T., Li-Masters T., Cheng P. Y. (2002) Toxicology 181–182, 207–210 [DOI] [PubMed] [Google Scholar]
- 32.Teng S., Piquette-Miller M. (2005) J. Pharmacol Exp. Ther. 312, 841–848 [DOI] [PubMed] [Google Scholar]
- 33.Beigneux A. P., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. (2002) Biochem. Biophys. Res. Commun. 293, 145–149 [DOI] [PubMed] [Google Scholar]
- 34.Pascussi J. M., Gerbal-Chaloin S., Pichard-Garcia L., Daujat M., Fabre J. M., Maurel P., Vilarem M. J. (2000) Biochem. Biophys. Res. Commun. 274, 707–713 [DOI] [PubMed] [Google Scholar]
- 35.Morgan E. T., Goralski K. B., Piquette-Miller M., Renton K. W., Robertson G. R., Chaluvadi M. R., Charles K. A., Clarke S. J., Kacevska M., Liddle C., Richardson T. A., Sharma R., Sinal C. J. (2008) Drug Metab. Dispos. 36, 205–216 [DOI] [PubMed] [Google Scholar]
- 36.Evseenko D. A., Paxton J. W., Keelan J. A. (2007) Drug Metab. Dispos. 35, 595–601 [DOI] [PubMed] [Google Scholar]
- 37.Turnbull A. V., Rivier C. L. (1998) Endocrinology 139, 119–127 [DOI] [PubMed] [Google Scholar]
- 38.Petrovic V., Teng S., Piquette-Miller M. (2007) Mol. Interv. 7, 99–111 [DOI] [PubMed] [Google Scholar]
- 39.Fardel O., Le Vée M. (2009) Expert. Opin. Drug. Metab. Toxicol. 5, 1469–1481 [DOI] [PubMed] [Google Scholar]
- 40.Elferink M. G., Olinga P., Draaisma A. L., Merema M. T., Faber K. N., Slooff M. J., Meijer D. K., Groothuis G. M. (2004) Am. J. Physiol. Gastrointest Liver Physiol. 287, G1008–G1016 [DOI] [PubMed] [Google Scholar]
- 41.Li N., Singh P., Mandrell K. M., Lai Y. (2010) Mol. Pharm. 7, 630–641 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.