Abstract
Osteoporosis is one of the most common bone pathologies. A number of novel molecules have been reported to increase bone formation including cysteine-rich protein 61 (CYR61), a ligand of integrin receptor, but mechanisms remain unclear. It is known that bone morphogenetic proteins (BMPs), especially BMP-2, are crucial regulators of osteogenesis. However, the interaction between CYR61 and BMP-2 is unclear. We found that CYR61 significantly increases proliferation and osteoblastic differentiation in MC3T3-E1 osteoblasts and primary cultured osteoblasts. CYR61 enhances mRNA and protein expression of BMP-2 in a time- and dose-dependent manner. Moreover, CYR61-mediated proliferation and osteoblastic differentiation are significantly decreased by knockdown of BMP-2 expression or inhibition of BMP-2 activity. In this study we found integrin αvβ3 is critical for CYR61-mediated BMP-2 expression and osteoblastic differentiation. We also found that integrin-linked kinase, which is downstream of the αvβ3 receptor, is involved in CYR61-induced BMP-2 expression and subsequent osteoblastic differentiation through an ERK-dependent pathway. Taken together, our results show that CYR61 up-regulates BMP-2 mRNA and protein expression, resulting in enhanced cell proliferation and osteoblastic differentiation through activation of the αvβ3 integrin/integrin-linked kinase/ERK signaling pathway.
Keywords: Bone Morphogenetic Protein (BMP), Cytokine, Differentiation, ERK, Integrin, Cysteine-rich Protein 61 (CYR61), Osteogenesis
Introduction
Bone is a mineralized tissue that underlies multiple mechanical and metabolic functions of the skeleton (1). Bone functions include maintaining blood calcium levels, providing mechanical support to soft tissues and serving as levers for muscle action, supporting hematopoiesis, and housing the brain and spinal cord (2). Formation and maintenance of bone tissue are regulated in a sophisticated fashion by bone-forming osteoblasts and bone-resorbing osteoclasts (3). Development and differentiation of these two cell types are under tight regulation by a number of endogenous substances such as hormones, growth factors, and cytokines (4). These factors are individually secreted through endocrine, paracrine/autocrine, and neurocrine systems, with subsequent interaction essential to the delicate balance between bone-forming and -resorbing cells in the marrow microenvironment. An imbalance between the two cell types leads to pathogenesis of certain bone diseases including osteopetrosis and osteoporosis (5, 6).
Osteoporosis is the most common human metabolic bone disorder characterized by progressive and age-dependent bone loss and increasing bone fracture risk. It is an important public health issue in postmenopausal women; if untreated, more than half of white women will experience fractures during their lifetime. Between 30 and 50% of women and 15–30% of men will suffer a fracture related to osteoporosis in their lifetime (7). Fractures increase morbidity and mortality and impose a financial burden on the community (8). A most compelling therapeutic need for osteoporosis at the present time is a drug that will substantially increase bone formation. The use of an anabolic agent that stimulates bone formation, restores trabecular bone microarchitecture, and rebuilds bone that has been lost, possibly in conjunction with an inhibitor of bone resorption, would be an ideal therapeutic approach for patients. Therefore, it is clearly necessary to identify novel molecular targets to develop bone-forming drugs aimed to combat osteoporosis by rebuilding the lost bone.
Multiple anabolic signaling pathways are positively involved in controlling bone formation, such as bone morphogenetic protein (BMP),3 Wnt, and Runx2 pathways. Among the BMP family, BMP2 is the best documented bone growth factor that stimulates osteoblast differentiation and bone formation (9). Genetic manipulation studies suggest that BMP2 is critical for postnatal bone formation (10). Aging studies have found that production and bone-forming activity of BMP2 are both significantly decreased in aged bones (11, 12). In human, BMP2 was recently recognized as an osteoporosis-associated gene through human polymorphism studies (13). Collectively, these findings suggest that decay of BMP2 function, which leads to an inhibition of bone formation, is one molecular mechanism that contributes to the development of osteoporosis with aging. Therefore, BMP2 has become an ideal target for drug development (14–17). BMP2 gene expression is transcriptionally regulated through various intracellular mechanisms that constitute a complex cross-talk between multiple signaling pathways, including estrogen receptors (18), PGE2 (19), HOXa13 (20), retinoic acid (21), 1,25(OH) vitamin D3 (19), hedgehog/Gli (22), Wnt/β-catenin (23) and BMP/Smad (24), responsible for regulation of osteoblast functions. However, the precise mechanisms by which some of these pathways, such as 1,25-(OH) vitamin D3 and Wnt/β-catenin, regulate BMP2 expression in osteoblasts need to be well elucidated. Recent evidence has indicated that cysteine-rich protein 61 (CYR61), a member of the connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family (25), may play a important role in regulating BMP2 expression in osteoblasts by mediating the effects of 1,25-(OH) vitamin D3 and Wnt/β-catenin on BMP2 genes.
CYR61 is the first cloned member of the CCN family, an immediate early gene family consisting of six members with homologous DNA sequence that exhibit diverse cellular functions such as regulation of cell division, chemotaxis, adhesion, motility, tumorigenesis, angiogenesis, and ion transport (26–34). Interestingly, in vitro evidence has suggested that CYR61, as an extracellular signaling molecule in bone (35), plays an essential role in maintaining normal osteoblast functions, including osteogenic commitment of mesenchymal cells (36, 37), proliferation and maturation of osteoblast precursor cells (37, 38), and migration of osteoblastic cells (37, 39). In osteoblastic cells, CYR61 mRNA expression is up-regulated by 1,25-(OH) vitamin D3 (36) and the canonical Wnt signaling (37), Both 1,25-(OH) vitamin D3 and Wnt signaling are important for osteoblast differentiation of mesenchymal stem cells. Because these two mechanisms were found to induce BMP2 gene expression in osteoblasts (19, 23) and BMP2 is a critical factor responsible for osteoblast differentiation, we hypothesized that, as a positive extracellular signaling protein, CYR61 controls osteoblast functions by regulating BMP2 gene expression in osteoblasts. This study was designed to test this hypothesis and also determine the precise signaling mechanisms involved in CYR61 regulation of BMP2 transcription in osteoblasts.
In the current work we found that CYR61 increases cell proliferation and osteoblastic differentiation; these results fit in with previous studies (36–39). We also provide the novel molecular mechanisms involved in CYR61-mediated osteogenic effects in this study. BMP-2-dependent phenomenon is critical for CYR61-induced osteogenic effects in fetal mouse pre-osteoblast MC3T3-E1 cells and primary cultured osteoblast cells. In addition, αvβ3 integrin/integrin-linked kinase (ILK)/extracellular signal-regulated kinase (ERK) signaling pathways are involved in CYR61-mediated induction of BMP-2 expression and subsequent cell proliferation and osteoblastic differentiation.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Anti-mouse BMP-2 antibody, BMP-2 ELISA kit, and noggin were purchased from R&D Systems (Minneapolis, MN). Anti-α-tubulin antibody, anti-pERK1/2 or ERK1 antibodies, anti-pAKT1/2/3 (ser-473) or total AKT-1 antibodies, anti-pJNK or JNK antibodies, anti-p-p38 or total p38 antibodies, and protein A/G beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant CYR61 protein was purchased from Abnova (Taipei, Taiwan). Chemicals, anti-β-actin antibody, and an Alkaline Phosphatase activity kit were purchased from Sigma. An in vitro osteogenesis kit and αvβ3 neutralizing antibody were purchased from Chemicom (Temecula, CA). Rabbit polyclonal antibody for ILK was purchased from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal antibody for glycogen synthase kinase 3β (GSK3β) and phosphor-GSK3β were purchased from Cell Signaling Technology (Beverly, MA).
Cell Culture
MC3T3-E1 cells were purchased from ATCC (Manassas, VA) and grown in αMEM (Invitrogen, catalog no. 001008-3DJ, Invitrogen) containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Medium was changed every 48 h. Murine primary osteoblastic cells (pOB cells) were prepared as described previously (40). Calvaria were dissected from murine fetuses, divided into small pieces, and treated with 0.1% type I collagenase solution for 10 min at 37 °C. The next two 20-min sequential collagenase digestions were pooled and filtered through 70-μm nylon filters (Falcon, NJ). Cells were grown on plastic cell culture dishes in 95% air, 5% CO2 with αMEM that was supplemented with 20 mm HEPES and 10% heat-inactivated fetal calf serum (FCS), 2 mm glutamine, penicillin (100 units/ml), and streptomycin (100 μg/ml). The characteristics of osteoblasts were confirmed by morphology and expression of AP enzymatic activity.
MTT Assay
Growth rates of recombinant CYR61 (rCYR61)-treated MC3T3-E1 cells were determined using MTT as a substrate. The MTT assay is based on the activity of mitochondrial dehydrogenases, which reduce the water-soluble tetrazolium salt to a purple insoluble formazan product. The amount of MTT formazan product was analyzed spectrophotometrically at a wavelength of 570 nm. Each individual experiment was repeated at least three times.
Colony Formation Assay
Aliquots of 1000 cells were seeded into six-well culture dishes. The next day cells were exposed to recombinant CYR61 protein in serum-free medium at the appropriate times. Cells were washed and further incubated with 2% FCS medium until further analysis. After incubation for 7 days, cells were washed with 1× PBS and fixed with methanol at room temperature for 10–20 min. After washing with 1x PBS again, cells were stained with 0.1% crystal violet, PBS, and colonies were counted. All experiments were carried out in triplicate.
Boyden Chamber Assay
Migration assays were performed using modified Boyden chambers with filter inserts for 24-well dishes containing 8-μm pores (Nucleopore Corp, Pleasanton, CA). Cells (2 × 104) were plated into 100 μl of complete αMEM in the upper chamber, and the lower chamber was filled with 1 ml of αMEM with various doses of rCYR61 or PBS. After 12 h in culture, cells were fixed in methanol for 15 min and stained with 0.05% crystal violet in PBS for 15 min. Cells on the upper side of filters were removed with cotton-tipped swabs, and filters were washed in PBS. Cells on the underside of filters were viewed and counted under a microscope (type 090-135.001, Leica Microsystems, Wetzlar, Germany). Each clone was plated in triplicate in each experiment, and each experiment was repeated at least three times.
Wound-healing Migration Assay
For the wound-healing migration assay, cells were seeded on 12-well plates at a density of 1 × 105 cells/well in culture medium. At 24 h after seeding, the confluent monolayer of culture was scratched with a fine pipette tip, and migration was visualized by microscope and magnification. The rate of wound closure was observed at the indicated time.
Differentiation Assay
To induce differentiation, confluent MC3T3-E1 cells were transferred to differentiation medium (αMEM supplemented with 10% FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.2 mm ascorbic acid (Sigma), and 10 mm β-glycerol phosphate (Sigma) Osteogenesis was determined by staining with Alizarin red solution. Cells were washed with PBS or Hanks' balanced salt solution and then fixed with 70% ethanol for 15 min. After fixation, cells were rinsed three times with distilled water for 10∼15 min each time. One ml of Alizarin red solution was added into the well followed by incubation at room temperature for at least 20 min. Excess dye was removed, and then cells were washed four times with deionized water. Differentiated cells containing mineral deposits stained bright red with Alizarin red solution. After photography, bound staining was eluted with 10% (w/v) cetylpyridinium chloride, and Alizarin red solution in samples was quantified by measuring absorbance at 550 nm and calculated according to a standard curve. One molar Alizarin solution selectively binds about 2 mol of calcium.
ALP Activity Assay
MC3T3-E1 cells were cultured in αMEM containing 50 μg/ml vitamin C and 10 mm β-glycerophosphate for 2 weeks, with medium changed every 3 days. After 14 days, cells were harvested in 1 ml of 0.2% Nonidet P-40, and the cell suspension was disrupted by sonication. After centrifugation at 1500 × g for 5 min, ALP activity in the supernatant was measured using the method of Lowry et al. (41).
Total RNA Extraction and RT-PCR
Total RNA was isolated using Trizol reagent according to the manufacturer's instructions. Total RNA (5 μg) was reverse-transcribed into single-stranded cDNA using a Moloney murine leukemia-virus reverse transcriptase and random hexamers (Promega, Madison, WI). Primers were used at a final concentration of 0.5 μm. The reaction mixture was first denatured at 95 °C for 5 min. The cDNAs were amplified with the forward (F) and reverse (R) primers by PCR as described. The primer sequences for BMP-2 were 5′-CCAAGAGACATGTGAGGATT-3′ (F) and 5′-TTAGTGGAGTTCAGGTGGTC-3′ (R). The primer sequences for BMP-4 were 5′-ACGACTACTGGACACCAGAC-3′ (F) and 5′-GTTGGTTGAGTTGAGGTGAT-3′ (R). The primer sequences for BMP-6 were 5′-GACATCACAGCAACTAGCAA-3′ (F) and 5′-AGGAACACTCTCCATCACAG-3′ (R). The primer sequences for BMP-7 were 5′-GCTTACAGCTCTCTGTGGAG-3′ (F) and 5′-GGTTGATGAAGTGAACAAGT-3′ (R). The primer sequences for BMP-9 were 5′-TACCACTATGAGGGGATGAG-3′ (F) and 5′-CTATGAGCCACAGGAGAGTC-3′ (R). The primer sequences for GAPDH were 5′-CTCACTCAAGATTGTCAGCA-3′ (F) and 5′-GTCATCATACTTGGCAGGTT-3′ (R). PCR conditions were 30 cycles of 95 °C for 30 s, 52 °C for 30 s, and 72 °C for 1 min followed by 72 °C for 10 min. PCR products were visualized by ethidium bromide staining after agarose gel electrophoresis.
Quantitative Real-time PCR
Quantitative real-time PCR (qPCR) analysis was carried out using Taqman one-step PCR Master Mix (Applied Biosystems, Foster City, CA). One hundred nanograms of total cDNA were added per 25 μl reaction with sequence-specific primers and Taqman probes. Sequences for all target gene primers and probes were purchased commercially (GAPDH was used as internal control) (Applied Biosystems). The qPCR assays were carried out in triplicate on an ABI Prism 7900 sequence detection system. Cycling conditions were 10 min of polymerase activation at 95 °C followed by 40 cycles at 95 °C for 15 s and 60 °C for 60 s. The threshold was set above the non-template control background and within the linear phase of target gene amplification to calculate the cycle number at which transcript was detected (denoted CT).
Western Blot Analysis
MC3T3-E1 cells were incubated in serum-free αMEM during treatment with rCYR61 (100 ng/ml), and cells were lysed in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.5, 120 mm NaCl, 0.5% Nonidet P-40, 100 mm NaF, 200 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 1 μg/ml aprotinin) for 15 min on ice. Cellular lysates were prepared as described previously (48). An equal quantity of protein from cell lysates was resuspended in gel sample buffer, resolved by 10% SDS-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes (Millipore). After blocking, blots were incubated with specific primary antibodies, and after washing and incubating with secondary antibodies, immunoreactive proteins were visualized using an enhanced chemiluminescence detection system (GE Healthcare). Where indicated, membranes were stripped and reprobed with another antibody.
Assay of BMP-2
BMP-2 ELISA kits were used to detect BMP-2 levels. Briefly, cells were treated with various concentrations of rCYR61 for the indicated time. Culture medium was collected for measurement of BMP-2. Samples were placed in 96-well microtiter plates coated with monoclonal detective antibodies and incubated for 2 h at room temperature. After removing unbound material by washing with washing buffer (50 mm Tris, 200 mm NaCl, and 0.2% Tween 20), horseradish peroxidase-conjugated streptavidin was added to bind to the antibodies. Horseradish peroxidase catalyzed conversion of the chromogenic substrate (tetramethylbenzidine) to a colored solution, with color intensity proportional to the amount of protein present in the sample. The absorbance of each well was measured at 450 nm. Results are presented as the percentage of change in activity compared with activity in untreated controls.
ILK Kinase Assay
ILK enzymatic activity was assayed in MC3T3-E1 cells lysed in Nonidet P-40 buffer (0.5% sodium deoxycholate, 1% Nonidet P-40, 50 mm HEPES, pH 7.4, 150 mm NaCl) as previous reported (42). Briefly, ILK was immunoprecipitated with ILK antibody overnight at 4 °C from 250 μg of lysate. After immunoprecipitation, beads were resuspended in 30 liters of kinase buffer containing 1 mg of recombinant substrate (GSK3 fusion protein) and 200 m cold ATP, and the reaction was carried out for 30 min at 30 °C. Phosphorylated substrate was visualized by Western blot with phosphor-GSK3β antibody. Total GSK3β was detected with the appropriate antibody.
Statistical Analysis
Data are presented as the mean ± S.D. Student's t test was used to compare data between groups. All statistical tests included two-way analysis of variance. p values of less than 0.05 were considered to be statistically significant.
RESULTS
Recombinant CYR61 Protein Induced Differentiation in MC3T3-E1 Cells
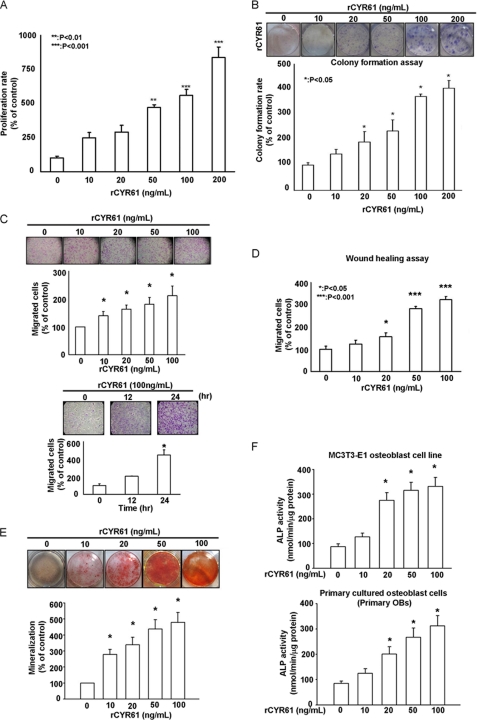
In this study we investigated the role of CYR61 in osteoblast proliferation and differentiation. We found that treatment with rCYR61 protein significantly increased proliferation of mouse osteoblast MC3T3-E1 cells in a dose-dependent manner to ∼200 ng/ml (Fig. 1A), after which we found that higher concentrations of rCYR61 decrease the proliferation of MC3T3-E1 cells (supplemental Fig. 1A). The above data imply that CYR61 may has a biphasic effect on MC3T3-E1 cell proliferation. The different results of CYR61 on MC3T3-E1 cell proliferation reported by our study and that Craig et al. (61) may be caused by the different experimental conditions and the biphasic effect of CYR61. Colony formation assay produced similar results (Fig. 1B). We also treated MC3T3-E1 cells with rCYR61 in a time-dependent manner (0, 24, 48, 72 h) and found that CYR61-induced MC3T3-E1 cells proliferation was detectable at 48 h as well as 72 h (supplemental Fig. 1B). Because osteoblastic differentiation is a complicated process that includes proliferation and migration of osteoblasts, we also tested the migrative ability of MC3T3-E1 cells after treatment with rCYR61. As shown in Fig. 1, C and D, rCYR61 significantly induced migration of MC3T3-E1 cells in both a Boyden chamber assay and in wound healing analyses in a time- and dose-dependent manner. In the in vitro differentiation assay, mineralization of MC3T3-E1 osteoblasts and primary cultured osteoblasts was demonstrated by Alizarin red staining assay and ALP activity assay after rCYR61 treatment (Fig. 1, E and F). These results indicate that CYR61 induces osteogenic differentiation in MC3T3-E1 osteoblasts and primary osteoblasts.
FIGURE 1.
Recombinant CYR61 protein induces proliferation, migration, and osteoblastic differentiation of MC3T3-E1 cells. A, treatment with rCYR61 increased proliferation per MTT assay is shown. After treatment at various doses, growth rates were measured by MTT assay. B, treatment with rCYR61 increased proliferation per colony counts. After 7 days colonies were stained with crystal violet and counted. C, migration ability of MC3T3-E1 cells increased after rCYR61 treatment. Cells that migrated were stained with crystal violet and counted (upper). Cells were incubated with 100 ng/ml rCYR61 in Transwell plates for indicated times. Cells that migrated were stained with crystal violet and counted (lower). D, treatment with rCYR61 increased wound-healing migration. Still images were captured at the indicated times after wounding, and then cells were counted. E, cells were incubated with rCYR61 in the indicated doses. After 14 days cells were stained with Alizarin red (upper). The quantitative data of mineralization ability are shown in the lower panel. F, ALP activity assay identified osteoblastic differentiation of MC3T3-E1 and primary cultured osteoblasts after rCYR61 treatment. Cells were incubated with rCYR61 in the indicated doses. After 14 days, cells were collected to determine ALP activity. The upper panel indicates enzyme activity of MC3T3-E1 cells; the lower panel indicates enzyme activity of primary cultured osteoblasts (OB). Each experiment was performed in triplicate, and results represent the mean ± S.D. of three independent experiments. The asterisks indicate a significant difference (*, p < 0.05; **, p < 0.01; ***, p < 0.001) between rCYR61 treatment and vehicle treatment cells.
CYR61 Induced BMP-2 Production in Osteoblast Cells
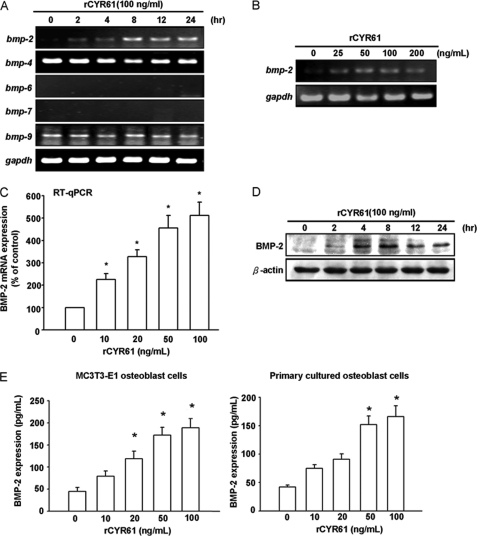
Given the crucial role of BMPs in osteoblastic differentiation, we tested whether CYR61 mediated alteration of osteoblast proliferation and differentiation through regulation of BMP expression. According to Cheng et al. (43), BMP-2, -6, and -9 may be the most potent molecules that induce osteoblast lineage-specific differentiation. Therefore, we explored possible target gene expression during rCYR61-induced osteoblastic differentiation. We examined expression levels of members of the BMP family by RT-PCR in MC3T3-E1 osteoblast cells in response to rCYR61 treatment. Treatment with rCYR61 stimulated BMP-2 expression in a time-dependent manner by RT-PCR and Western blot analysis (Fig. 2, A and D). A significant increase in BMP-2 mRNA could be detected as early as 2 h after rCYR61 treatment. Elevation of BMP-2 mRNA was maximal at 8 h and lasted until 24 h after treatment. Expression level of other BMP mRNAs, such as BMP-4, -6, -7, and -9, were not significantly affected by rCYR61 (Fig. 2A). In addition, the stimulatory effect of rCYR61 on BMP-2 expression was dose-dependent in the range between 10 and 200 ng/ml (Fig. 2, B and C). The BMP-2 protein expression level in response to rCYR61 treatment was increased at 4∼24 h after treatment (Fig. 2D). Furthermore, we found rCYR61 induced BMP-2 secretion into cultured medium of both MC3T3-E1 cells and primary osteoblasts by BMP-2 ELISA assay (Fig. 2E). These data indicate that rCYR61 induces expression of BMP-2 in osteoblast cells in a time- and dose-dependent manner.
FIGURE 2.
Recombinant CYR61 induces BMP-2 expression. A and B, RT-PCR for mRNA of different BMP family members is shown. The coding regions of cDNA were used as probes as indicated under “Experimental Procedures.” A GAPDH probe was used as an internal control for RNA quantity. C, MC3T3-E1 cells were incubated with rCYR61 for 8 h, after which mRNA expression of BMP-2 was determined using qPCR. D, shown is Western blot analysis of BMP-2 protein expression in MC3T3-E1 cells. Total proteins were extracted from cells and probed with polyclonal antibody specific for BMP-2. Each lane contains 80 μg of total protein. The internal loading control was β-actin. E, shown is an ELISA assay of BMP-2 protein expression in MC3T3-E1 cells (left) and primary cultured osteoblasts (right). MC3T3-E1 cells were incubated with the indicated dose of rCYR61 for 24 h. The protein secreted into conditioned medium was determined by BMP-2 ELISA assay kit. All the results except those in D are expressed as the means ± S.E. Each assay was performed in three separate experiments. The asterisk indicates p < 0.05 between rCYR61 treatment and vehicle treatment cells.
BMP-2 Is Critical for CYR61-mediated Osteoblastic Differentiation
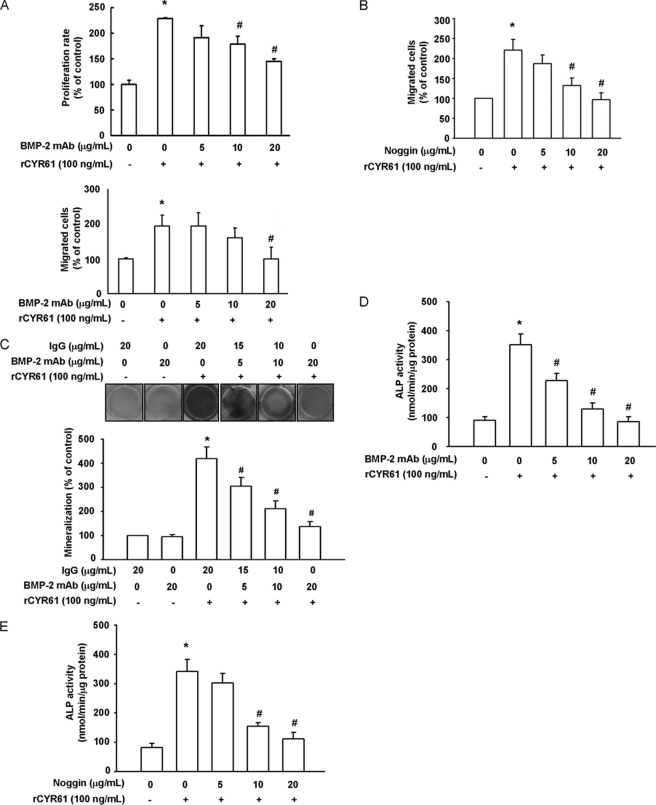
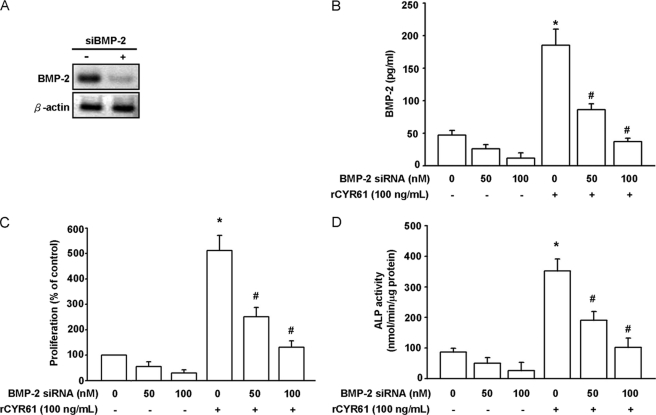
To determine whether induction of BMP-2 is critical for CYR61-mediated osteogenesis, the inhibitory effect of BMP-2 neutralizing antibody on rCYR61-induced MC3T3-E1 osteoblast cell migration, proliferation, and differentiation was investigated. Our data showed that rCYR61-induced osteoblast proliferation and migration activity were significantly decreased after treatment with neutralizing BMP-2 antibodies for indicated times and dosages (Fig. 3A). Further evidence showed that CYR61-induced migration of osteoblast cells was also inhibited by the BMP-2 inhibitor Noggin (Fig. 3B). These observations suggest that CYR61-induced osteoblast proliferation and migration may be due to BMP-2 expression. In the in vitro differentiation assay, CYR61-induced bone mineralization was inhibited by neutralizing BMP-2 antibodies in a dose-dependent manner (Fig. 3C). In an ALP activity assay, both BMP-2 neutralizing antibody and Noggin inhibited rCYR61-induced ALP activity in osteoblast cells (Fig. 3, D and E). To further confirm the role of BMP-2 in rCYR61-mediated osteoblastic function, MC3T3-E1 cells were treated with BMP-2-specific siRNA or control siRNA. Western blot and ELISA analysis showed that expression levels of BMP-2 protein were significantly suppressed by transfection with BMP-2 siRNA (Fig. 4, A and B). Treatment with BMP-2 siRNA also reduced CYR61-induced proliferation and differentiation of MC3T3-E1 osteoblast cells (Fig. 4, C and D). The above data show that rCYR61 induces proliferation, migration, and differentiation of osteoblast cells via a BMP-2-dependent pathway.
FIGURE 3.
BMP-2 is critical for CYR61-mediated osteoblastic differentiation. A, cells were incubated with rCYR61 and BMP-2 neutralizing antibodies for the indicated times in 24-well plates. Growth rates were measured by MTT assay. B, cells were incubated with rCYR61- and BMP-2-neutralizing antibodies or with Noggin for the indicated doses in Transwell plates. Migrated cells were stained and counted. C, MC3T3-E1 cells were incubated with rCYR61- and BMP-2-neutralizing antibodies in the indicated doses. Cells were stained with Alizarin red (upper). The quantitative data are shown in the lower panel. D, MC3T3-E1 cells were incubated with rCYR61- and BMP-2-neutralizing antibodies in indicated doses. Cells were assayed with ALP activity assay kit. E, MC3T3-E1 cells were incubated with rCYR61 and Noggin in the indicated doses. Cells were assayed with ALP activity assay kit. Each assay was performed in three independent experiments. The asterisk indicates p < 0.05 between rCYR61 treatment and vehicle treatment cells. The # symbol indicates a significant difference (p < 0.05) between BMP-2 monoclonal antibody treatment and rCYR61 treatment cells.
FIGURE 4.
Knockdown of BMP-2 inhibits CYR61-induced osteoblastic differentiation. A, MC3T3-E1 cells were transfected with BMP-2 or control siRNA for 24 h, after which protein levels of BMP-2 were examined using Western blot analysis. B, MC3T3-E1 cells were transfected with BMP-2 or control siRNA for 24 h followed by stimulation with CYR61 for 24h, after which secreted protein levels of BMP-2 were examined using an ELISA assay. C, MC3T3-E1 cells were transfected with BMP-2 or control siRNA for 24 h followed by stimulation with CYR61 for 24 h, then proliferation was measured with an MTT assay. D, MC3T3-E1 cells were transfected with BMP-2 or control siRNA for 24 h followed by stimulation with CYR61, after which osteoblastic differentiation was measured with ALP activity assay after 14 days. Each assay was performed in three independent experiments. The asterisk indicates p < 0.05 between rCYR61 treatment and vehicle treatment cells. The # symbol indicates a significant difference (p < 0.05) between BMP-2 monoclonal antibody treatment and rCYR61 treatment cells.
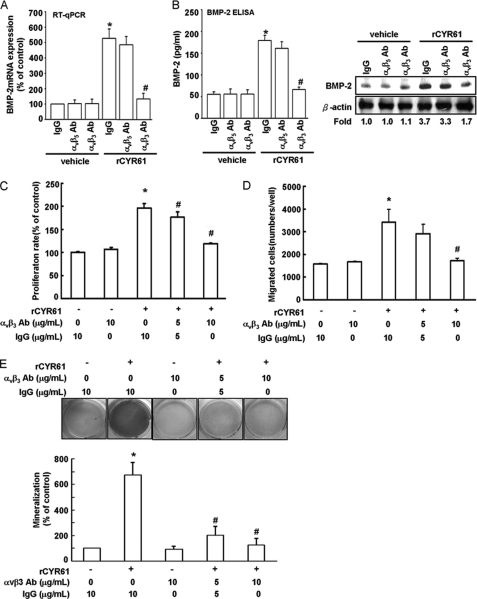
CYR61-induced BMP-2 Expression and Subsequent Osteogenesis Were Dependent on Integrin αvβ3 Receptor
According to previous study, MC3T3-E1 cells express integrin receptor αv, α2, β1, and β3, and CYR61 is the ligand of integrin α6β1, αvβ3, αvβ5, and αIIbβ3 (44). This implies that CYR61 may affect MC3T3-E1 cells through integrins αvβ3 or αvβ5. To examine this hypothesis, MC3T3-E1 cells were treated with anti-αvβ3 and anti-αvβ5 inhibitory monoclonal antibodies to evaluate which integrin was involved in CYR61-induced BMP-2 expression and subsequent osteogenesis. Pretreatment of cells for 30 min with anti-αvβ3 but not anti-αvβ5 inhibitory monoclonal antibody markedly inhibited CYR61-induced BMP-2 mRNA and protein expression using qPCR analysis (Fig. 5A), ELISA assay, and Western blot analysis (Fig. 5B). Treatment with cyclic (RGD) peptides, which has been reported to bind αvβ3 at high affinity and block its function (45), but not treatment with low αvβ3-binding affinity (RAD) peptides, also inhibited CYR61-induced BMP-2 protein expression (supplemental Fig. 2). CYR61-induced increases in proliferation and migration ability as well as differentiation activity of MC3T3-E1 osteoblast cells were decreased by treatment with integrin αvβ3 inhibitory antibody in a dose-dependent manner (Fig. 5, C–E). This evidence taken together shows that αvβ3 integrin is critical for CYR61-induced bone differentiation through a BMP-2-dependent pathway.
FIGURE 5.
Integrin αvβ3 is involved in CYR61-induced BMP-2 expression and osteoblastic differentiation. A, cells were pretreated with αvβ3, αvβ5, and IgG control antibody (10 μg/ml) for 30 min followed by stimulation with CYR61 (100 ng/ml) for 24 h, then mRNA levels of BMP-2 were determined using qPCR assay. B, cells were pretreated with αvβ3, αvβ5, and IgG control antibody (10 μg/ml) for 30 min followed by stimulation with CYR61 (100 ng/ml) for 24 h, then protein levels of BMP-2 were determined using ELISA assay and Western blot analysis. C, cells were incubated with rCYR61 and αvβ3 neutralizing antibodies at the indicated doses in 24-well plates. Growth rates were measured by MTT assay. D, cells were incubated with rCYR61 and αvβ3 neutralizing antibodies for the indicated times in Transwell plates. Migrated cells were stained and counted. E, cells were incubated with rCYR61 and αvβ3 neutralizing antibodies for the indicated number of days. Cells were stained with Alizarin red. The quantitative data of mineralization ability are shown in the lower panel. Each assay was performed in three independent experiments. The asterisk indicates p < 0.05 between rCYR61 treatment and vehicle treatment cells. The # indicates a significant difference (p < 0.05) between αvβ3 antibody treatment and rCYR61 treatment cells.
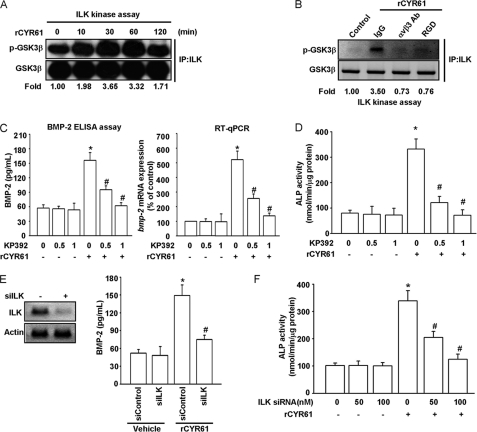
ILK/ERK Pathway Is Required for CYR61-induced BMP-2 Expression and Osteoblastic Differentiation
ILK has been shown to be capable of regulating integrin-mediated signaling and biological functions such as cell spreading, migration, invasion, and proliferation (46). To see whether ILK is involved in CYR61-mediated signaling and osteogenesis, we first examined ILK activity after CYR61 treatment with use of an ILK kinase assay. As shown in Fig. 6A, using GSK3β as substrate (47), ILK kinase activity increased in a time-dependent manner in response to rCYR61 stimulation, reaching a maximum between 30 to 60 min. Pretreatment with integrin αvβ3 inhibitory antibody or RGD peptide significantly decreased CYR61-induced ILK activity (Fig. 6B). Treatment with the ILK inhibitor KP392 decreased BMP-2 protein and mRNA expression (Fig. 6C). KP392 also dramatically inhibited CYR61-induced osteoblast-specific ALP activity (Fig. 6D). Afterward, we performed genetic knockdown of ILK expression using ILK-specific siRNA in MC3T3-E1 cells; we found knockdown of ILK had an inhibitory effect on rCYR61-induced BMP-2 protein secretion (Fig. 6E) and ALP activity (Fig. 6F).
FIGURE 6.
ILK activity is involved in CYR61-induced BMP-2 expression and osteoblastic differentiation. A, cells were incubated with rCYR61 (100 ng/ml) for the indicated times. Cell lysates were collected and analyzed by immunoprecipitation (IP)/immunoblotting (anti-ILK/anti-p-GSK3β). The internal loading control was GSK3β. The quantitative data are shown in the bottom. B, cells were pretreated with αv β3 antibody (10 μg/ml) and RGD (100 nm) for 30 min followed by stimulation with CYR61 (100 ng/ml) for the indicated times. Cell lysates were then collected and analyzed by immunoprecipitation/immunoblotting (anti-ILK/anti-p-GSK3β). The internal loading control was GSK3β. Quantitative data are shown in the bottom. C, cells were incubated with rCYR61 (100 ng/ml) and ILK inhibitor KP392 at the indicated doses. The mRNA levels of BMP-2 were determined using qPCR (left), and the protein levels were determined using ELISA assay (right). D, cells were incubated with rCYR61 (100 ng/ml) and ILK inhibitor KP392 at the indicated doses. Osteoblastic differentiation was examined using an ALP activity assay. E, cells were transfected with control and ILK siRNA for 24 h. ILK protein level was determined using Western blot analysis (left panel). BMP-2 protein level was determined using an ELISA assay (right panel). F, cells were transfected with ILK siRNA at indicated doses and incubated with CYR61 (100 ng/ml) for the indicated times. Osteoblastic differentiation was determined using an ALP activity assay. Each assay was performed in at least three independent experiments. The asterisk indicates p < 0.05 between rCYR61 treatment and vehicle treatment cells. The # symbol indicates significant difference (p < 0.05) between KP392 or siILK treatment and rCYR61 treatment cells.
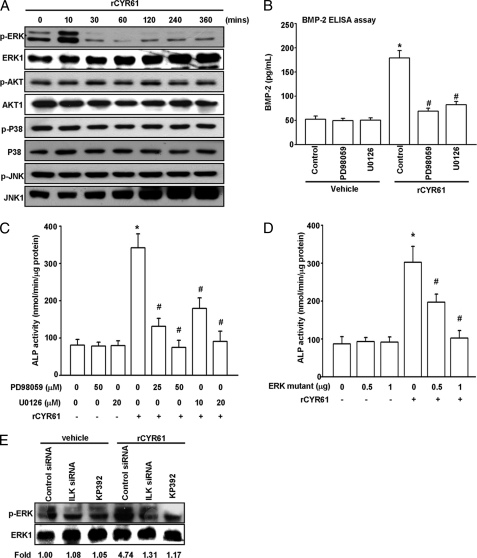
It has been reported that BMP-2 expression in osteoblasts is regulated by MAPK and PI3K/Akt pathways (48, 49). Thus, we used Western blotting assay to check the phosphorylation status of some candidate signaling molecules, including JNK, p38, ERK, and Akt. As shown in Fig. 7A, treatment of MC3T3-E1 cells with rCYR61 increased ERK phosphorylation but not phosphorylation of JNK, p38, or Akt. Moreover, pretreatment with U0126 and PD98059 (MEK inhibitor) deceased CYR61-stimulated BMP-2 protein expression by ELISA assay (Fig. 7B). We further examined whether the ERK pathway is involved in CYR61-mediated osteoblastic function of MC3T3-E1 cells. CYR61-induced MC3T3-E1 osteoblastic differentiation was inhibited not only by treatment with ERK inhibitors but also by transfection of an ERK inactive mutant form-expressing vector (Fig. 7, C and D). Furthermore, we found that CYR61-mediated ERK phosphorylation was inhibited by treatment with ILK-specific siRNA or the ILK chemical inhibitor KP392 (Fig. 7E). These results indicate that the αvβ3 integrin/ILK/ERK pathways are involved in CYR61-induced BMP-2 up-regulation and subsequent osteoblastic differentiation in MC3T3-E1 osteoblast cells.
FIGURE 7.
CYR61-induced ERK phosphorylation is essential for BMP-2 expression and osteoblastic differentiation. A, cells were plated at 5 × 105 in 6-cm plates. After a 24-h starvation, rCYR61 at 100 ng/ml was added to MC3T3-E1 cells for the indicated times. Whole cell extracts were prepared, and 40 μg total protein of lysate was subjected to Western blot analysis using antibody against phosphorylated ERK (p-ERK), phosphorylated AKT (p-Akt), phosphorylated JNK (p-JNK), and phosphorylated p38 (p-p38). The Western blot was stripped and probed with antibody against total ERK, AKT, JNK, p38 (ERK1, AKT1, JNK1, and p38) to confirm that the same amounts of whole-cell extracts were analyzed. B, cells were incubated with rCYR61 (100 ng/ml) and MEK inhibitor PD98059 and U0126 at the indicated doses. BMP-2 protein level was determined using an ELISA assay. C, cells were incubated with rCYR61 (100 ng/ml) and MEK inhibitor PD98059 and U0126 at the indicated doses. Osteoblastic differentiation was determined using an ALP activity assay. D, cells were transfected with ERK-mutated vector at indicated doses followed by stimulation with rCYR61 (100 ng/ml). Osteoblastic differentiation was determined using an ALP activity assay. E, cells were transfected with ILK siRNA or KP392 at the indicated doses and incubated with CYR61 (100 ng/ml) for the indicated times. The phosphorylation activity of ERK was determined using Western blot analysis. Each assay was performed in at least three independent experiments. The asterisk indicates p < 0.05 between rCYR61 treatment and vehicle treatment cells. The # symbol indicates significant difference (p < 0.05) between inhibitors or ERK mutant vector treatment and rCYR61 treatment cells.
DISCUSSION
In this study with osteoblasts, elevation of BMP-2 mRNA and protein levels followed recombinant CYR61 protein treatment through integrin αvβ3 receptor induction of the ILK and ERK signaling pathway. Our findings provide the first evidence that BMP-2-dependent osteoblastic differentiation may be regulated by CYR61, providing a link and molecular mechanism between CCN family and BMP family proteins in the physiology of bone.
CCN proteins are known to be involved in development, homeostasis, and repair of mesenchymal tissues. CCN proteins have four modules; they are an insulin-like growth factor-binding protein domain (module I), a Von Willebrand factor domain (module II), a thrombospondin-homology domain (module III), and a cysteine knot, heparin binding domain (module IV). Because the amino acid sequences of human CYR61 protein are very similar to mouse CYR61 protein (35), our experimental model seems to be appropriate. It has been reported that BMP-2 can induce expression of several CCN family proteins such as CYR61, Connective Tissue Growth Factor, WISP1, and WISP2 (50). Our results show for the first time that CYR61 can also act as an inducer to enhance BMP-2 expression in osteoblast cells (Fig. 2). Taking together our data and the data of others, we hypothesize there might be a feedback regulatory loop between CYR61 and BMP-2 or further CCN family and BMP family members. Feedback mechanism and other biological functions require further examination.
BMPs play an important role in bone tissue formation and remodeling (51). It has been well documented that stimulation of osteoblastic differentiation is characterized mainly by increased expression of AP, type I collagen, and osteocalcin (51). BMP-2 has been shown to activate SMAD signaling, but it inhibits the p38 MAPK and PI3K/p70 S6K signaling pathway, which is involved in osteoblastic differentiation (52, 53). Our study indicates that production of BMP-2 increased in rCYR61-treated MC3T3-E1 cells and that treatment with BMP-2-neutralizing antibody or BMP-2-specific siRNA can inhibit MC3T3-E1 osteoblastic cell proliferation, migration, and differentiation.
Integrin-linked kinase (4) was discovered in 1996; its kinase activity is stimulated by integrins and soluble mediators including growth factors and chemokines (46). The same paper reported that the function of ILK in bone formation is promotion of chondrocyte proliferation, adhesion, and spreading. However, the role of ILK in osteoblastic differentiation is still unclear (46). We present in this study the first evidence to show that ILK also plays a critical role in CYR61-mediated BMP-2 expression and subsequent osteoblastic differentiation (Fig. 6). Previous research showed that ILK was not required for some specific conditions, such as cyclic strain-mediated ERK1/2 activation (54). In our study we found ILK is involved in CYR61-mediated ERK activation. This discrepancy may be due to different cell types as well as different experimental conditions. It has been reported that ILK modulates cell spreading, migration, and cytoskeletal organization by activating p21-Activated Kinase interactive exchange factor (α-PIX, also known as ARHGEF6), a guanine-nucleotide exchange factor for Rac1 and Cdc42 (55). Other studies have indicated that Rac/Cdc42 activates the ERK pathway and promotes cell migration (56). Considering previous studies and our current data raises the possibility that ILK might regulate ERK through indirect interaction.
The ERK1/2 signaling pathway is important in osteoblast cell proliferation and differentiation (57, 58). A number of studies have reported that ERK is an important mediator of BMP-2-induced osteoblastic differentiation and that inhibition of ERK1/2 results in suppression of differentiation markers (59). Suzuki et al. (67) reported that ERKs play an essential role in cell replication during osteoblastic differentiation. In our study phosphorylation of ERK1/2 rapidly increased within 10 min of rCYR61 treatment and then decreased by 30 min (Fig. 7A). Furthermore, MEK inhibitors PD98059 and U0126 both inhibited BMP-2 protein production (Fig. 7B). Thus, we demonstrated that CYR61-induced BMP-2 expression is regulated via the ERK pathway.
The murine homologue of CYR61 is an extracellular matrix-associated protein that modulates basic fibroblast growth factor signaling, angiogenesis, and binds to integrin αvβ3 (44).In the human breast cancer cell line MCF-7, overexpression of CCN1/Cyr61 up-regulates αvβ3 and binds to it to activate ERK1/2 MAPK, thus promoting cell proliferation and survival (60). In the present study, integrin αvβ3 was proven able to regulate rCYR61-induced BMP-2 expression, osteoblast cell proliferation and migration, and osteoblastic differentiation (Fig. 5). Furthermore, blockade of integrin αvβ3 inhibited ERK1/2 phosphorylation and BMP-2 protein expression. Thus, these results indicate that rCYR61-induced BMP-2-dependent osteoblastic differentiation is mediated through integrin αvβ3.
In addition to the BMP pathway that we have demonstrated here to be involved in CYR61 action on osteoblasts, the canonical Wnt pathway is also an important regulator of CYR61 activity in bone cells, as we described in the Introduction (37, 39). It has been reported that Wnt3a treatment significantly increased CYR61 mRNA levels in osteoblast-like cells (37), suggesting that CYR61 is potential downstream target of the Wnt signaling in controlling osteoblast maturation. Previously, we have found that the Wnt/β-catenin signaling pathway is a powerful enhancer of BMP2 gene expression in osteoblasts (23). However, we were not able to identify the functional binding site(s) in the BMP2 promoter responsible for β-catenin/TCF4 transactivation. Based on the results of current study, we reason that the stimulation of BMP2 expression by Wnt signaling in osteoblasts is likely mediated through CYR61. Not only regulating CYR61 gene expression, the recent report of Craig et al. (61) suggests that Wnt pathway may also directly bind to CYR61 and modulate CYR61 activity on osteoblasts. They found that Sclerostin, a proven endogenous antagonist that blocks both BMP and Wnt signaling pathways by binding to BMP ligands (62, 63) or Wnt receptor LRP5 (64, 65), can directly interact with CYR61 protein and affect proliferation of mature MC3T3/E1 osteoblast cells. The results from another study have suggested that Wnt induces osteoblast differentiation through BMPs, which is blocked by BMP antagonist Sclerostin, and the expression of BMP proteins in this autocrine loop is essential for Wnt-3A-induced osteoblast differentiation (66). Given the fact that both Wnt and BMP signaling pathways are extremely critical for osteoblast differentiation and bone formation, we think that, as an active coordinator in the cross-talk between these anabolic pathways, CYR61 and its signaling mechanism are a potential molecular target for identifying drugs that stimulate bone formation.
In summary, BMP-2-dependent osteoblastic differentiation may be regulated by CYR61 in pre-osteoblastic cells. The effects of rCYR61 on osteoblast cell maturation are strongly associated with BMP-2 production followed by ERK1/2 activation. Therefore, data suggest that rCYR61 may be beneficial in stimulating osteoblastic activity and promoting formation of bone tissue through integrin αvβ3/ILK/ERK signaling.
Supplementary Material
Acknowledgment
We thank RNAi Core (Academia Sinica, Taiwan) for providing specific siRNAs.
This work was supported in part by National Science Council Grants NSC 97-2314-B-002-060-MY3, NSC 98-3111-B-002-009 (to M.-L. Y.), National Science Council Grants NSC 96-2320-B-004-MY2, NSC 97-2320-B-039-039-MY3, and NSC 98-2815-C-039-082-B, Taiwan Merit Scholarship TMS-094-2-B-023 from the National Science Council of Taiwan, National Health Research Institutes Grant NHRI-EX97-9712BC from Taiwan, Department of Health Executive Yuan Grant DOH97-TD-G-111-024 from Taiwan, China Medical University Grants CMU96-220, CMU96-189, and CMU97-277, and an Odyssey Scholarship from M. D. Anderson Cancer Center (to J.-L. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–2.
- BMP
- bone morphogenetic protein
- CCN family
- connective tissue growth factor/cysteine-rich 61/nephroblastoma-overexpressed family
- CYR61
- cysteine-rich protein 61
- rCYR61
- recombinant CYR61
- ILK
- integrin-linked kinase
- GSK3β
- glycogen synthase kinase 3β
- qPCR
- quantitative real-time PCR
- αMEM
- α-minimal essential medium
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ALP
- alkaline phosphatase.
REFERENCES
- 1.Ducy P., Schinke T., Karsenty G. (2000) Science 289, 1501–1504 [DOI] [PubMed] [Google Scholar]
- 2.Harada S., Rodan G. A. (2003) Nature 423, 349–355 [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum S. L., Ross F. P. (2003) Nat. Rev. Genet. 4, 638–649 [DOI] [PubMed] [Google Scholar]
- 4.Manolagas S. C., Jilka R. L. (1995) N. Engl. J. Med. 332, 305–311 [DOI] [PubMed] [Google Scholar]
- 5.Boyle W. J., Simonet W. S., Lacey D. L. (2003) Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 6.Karsenty G. (2003) Nature 423, 316–318 [DOI] [PubMed] [Google Scholar]
- 7.Randell A., Sambrook P. N., Nguyen T. V., Lapsley H., Jones G., Kelly P. J., Eisman J. A. (1995) Osteoporos. Int. 5, 427–432 [DOI] [PubMed] [Google Scholar]
- 8.Center J. R., Nguyen T. V., Schneider D., Sambrook P. N., Eisman J. A. (1999) Lancet 353, 878–882 [DOI] [PubMed] [Google Scholar]
- 9.Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. (1988) Science 242, 1528–1534 [DOI] [PubMed] [Google Scholar]
- 10.Tsuji K., Bandyopadhyay A., Harfe B. D., Cox K., Kakar S., Gerstenfeld L., Einhorn T., Tabin C. J., Rosen V. (2006) Nat. Genet. 38, 1424–1429 [DOI] [PubMed] [Google Scholar]
- 11.Fleet J. C., Cashman K., Cox K., Rosen V. (1996) Endocrinology 137, 4605–4610 [DOI] [PubMed] [Google Scholar]
- 12.Meyer R. A., Jr., Meyer M. H., Tenholder M., Wondracek S., Wasserman R., Garges P. (2003) J Bone Joint Surg. Am. 85-A, 1243–1254 [DOI] [PubMed] [Google Scholar]
- 13.Styrkarsdottir U., Cazier J. B., Kong A., Rolfsson O., Larsen H., Bjarnadottir E., Johannsdottir V. D., Sigurdardottir M. S., Bagger Y., Christiansen C., Reynisdottir I., Grant S. F. A., Jonasson K., Frigge M. L., Gulcher J. R., Sigurdsson G., Stefansson K. (2003) PLoS Biol. 3, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mundy G., Garrett R., Harris S., Chan J., Chen D., Rossini G., Boyce B., Zhao M., Gutierrez G. (1999) Science 286, 1946–1949 [DOI] [PubMed] [Google Scholar]
- 15.Garrett I. R., Chen D., Gutierrez G., Zhao M., Escobedo A., Rossini G., Harris S. E., Gallwitz W., Kim K. B., Hu S., Crews C. M., Mundy G. R. (2003) J. Clin. Invest. 111, 1771–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su J. L., Yang C. Y., Zhao M., Kuo M. L., Yen M. L. (2007) J. Biol. Chem. 282, 19385–19398 [DOI] [PubMed] [Google Scholar]
- 17.Zhao M., Ko S. Y., Liu J. H., Chen D., Zhang J., Wang B., Harris S. E., Oyajobi B. O., Mundy G. R. (2009) Mol. Cell. Biol. 29, 1291–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou S., Turgeman G., Harris S. E., Leitman D. C., Komm B. S., Bodine P. V., Gazit D. (2003) Mol. Endocrinol. 17, 56–66 [DOI] [PubMed] [Google Scholar]
- 19.Virdi A. S., Cook L. J., Oreffo R. O., Triffitt J. T. (1998) Cell Mol. Biol. 44, 1237–1246 [PubMed] [Google Scholar]
- 20.Knosp W. M., Scott V., Bächinger H. P., Stadler H. S. (2004) Development 131, 4581–4592 [DOI] [PubMed] [Google Scholar]
- 21.Heller L. C., Li Y., Abrams K. L., Rogers M. B. (1999) J. Biol. Chem. 274, 1394–1400 [DOI] [PubMed] [Google Scholar]
- 22.Zhao M., Qiao M., Harris S. E., Chen D., Oyajobi B. O., Mundy G. R. (2006) Mol. Cell. Biol. 26, 6197–6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawadi G., Vayssière B., Dunn F., Baron R., Roman-Roman S. (2003) J. Bone Miner. Res. 18, 1842–1853 [DOI] [PubMed] [Google Scholar]
- 24.Ghosh-Choudhury N., Abboud S. L., Nishimura R., Celeste A., Mahimainathan L., Choudhury G. G. (2002) J. Biol. Chem. 277, 33361–33368 [DOI] [PubMed] [Google Scholar]
- 25.Brigstock D. R. (1999) Endocr. Rev. 20, 189–206 [DOI] [PubMed] [Google Scholar]
- 26.Croci S., Landuzzi L., Astolfi A., Nicoletti G., Rosolen A., Sartori F., Follo M. Y., Oliver N., De Giovanni C., Nanni P., Lollini P. L. (2004) Cancer Res. 64, 1730–1736 [DOI] [PubMed] [Google Scholar]
- 27.Lin M. T., Chang C. C., Chen S. T., Chang H. L., Su J. L., Chau Y. P., Kuo M. L. (2004) J. Biol. Chem. 279, 24015–24023 [DOI] [PubMed] [Google Scholar]
- 28.Menéndez J. A., Mehmi I., Griggs D. W., Lupu R. (2003) Endocr. Relat. Cancer. 10, 141–152 [DOI] [PubMed] [Google Scholar]
- 29.Babic A. M., Chen C. C., Lau L. F. (1999) Mol. Cell. Biol. 19, 2958–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.French D. M., Kaul R. J., D'Souza A. L., Crowley C. W., Bao M., Frantz G. D., Filvaroff E. H., Desnoyers L. (2004) Am. J. Pathol. 165, 855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leng E., Malcolm T., Tai G., Estable M., Sadowski I. (2002) J. Biomed. Sci. 9, 59–67 [DOI] [PubMed] [Google Scholar]
- 32.Hirasaki S., Koide N., Ujike K., Shinji T., Tsuji T. (2001) Hepatol. Res. 19, 294–305 [DOI] [PubMed] [Google Scholar]
- 33.O'Brien T. P., Lau L. F. (1992) Cell Growth Differ. 3, 645–654 [PubMed] [Google Scholar]
- 34.Wong M., Kireeva M. L., Kolesnikova T. V., Lau L. F. (1997) Dev. Biol. 192, 492–508 [DOI] [PubMed] [Google Scholar]
- 35.Lechner A., Schütze N., Siggelkow H., Seufert J., Jakob F. (2000) Bone 27, 53–60 [DOI] [PubMed] [Google Scholar]
- 36.Schütze N., Lechner A., Groll C., Siggelkow H., Hüfner M., Köhrle J., Jakob F. (1998) Endocrinology 139, 1761–1770 [DOI] [PubMed] [Google Scholar]
- 37.Si W., Kang Q., Luu H. H., Park J. K., Luo Q., Song W. X., Jiang W., Luo X., Li X., Yin H., Montag A. G., Haydon R. C., He T. C. (2006) Mol. Cell. Biol. 26, 2955–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schütze N., Kunzi-Rapp K., Wagemanns R., Nöth U., Jatzke S., Jakob F. (2005) Protein Expr. Purif. 42, 219–225 [DOI] [PubMed] [Google Scholar]
- 39.Schütze N., Schenk R., Fiedler J., Mattes T., Jakob F., Brenner R. E. (2007) BMC Cell Biol. 8, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang C. H., Hsu T. L., Lin W. W., Lai M. Z., Yang R. S., Hsieh S. L., Fu W. M. (2007) J. Biol. Chem. 282, 2346–2354 [DOI] [PubMed] [Google Scholar]
- 41.Lowry O. H., Roberts N. R., Wu M. L., Hixon W. S., Crawford E. J. (1954) J. Biol. Chem. 207, 19–37 [PubMed] [Google Scholar]
- 42.Tan C., Cruet-Hennequart S., Troussard A., Fazli L., Costello P., Sutton K., Wheeler J., Gleave M., Sanghera J., Dedhar S. (2004) Cancer Cell 5, 79–90 [DOI] [PubMed] [Google Scholar]
- 43.Cheng H., Jiang W., Phillips F. M., Haydon R. C., Peng Y., Zhou L., Luu H. H., An N., Breyer B., Vanichakarn P., Szatkowski J. P., Park J. Y., He T. C. (2003) J. Bone Joint Surg. Am. 85, 1544–1552 [DOI] [PubMed] [Google Scholar]
- 44.Kireeva M. L., Lam S. C., Lau L. F. (1998) J. Biol. Chem. 273, 3090–3096 [DOI] [PubMed] [Google Scholar]
- 45.Pfaff M., Tangemann K., Müller B., Gurrath M., Müller G., Kessler H., Timpl R., Engel J. (1994) J. Biol. Chem. 269, 20233–20238 [PubMed] [Google Scholar]
- 46.McDonald P. C., Fielding A. B., Dedhar S. (2008) J. Cell Sci. 121, 3121–3132 [DOI] [PubMed] [Google Scholar]
- 47.Tang C. H., Lu D. Y., Tan T. W., Fu W. M., Yang R. S. (2007) J. Biol. Chem. 282, 25406–25415 [DOI] [PubMed] [Google Scholar]
- 48.Tang C. H., Yang R. S., Chien M. Y., Chen C. C., Fu W. M. (2008) Eur. J Pharmacol. 579, 40–49 [DOI] [PubMed] [Google Scholar]
- 49.Wu J. B., Fong Y. C., Tsai H. Y., Chen Y. F., Tsuzuki M., Tang C. H. (2008) Eur. J. Pharmacol. 588, 333–341 [DOI] [PubMed] [Google Scholar]
- 50.Parisi M. S., Gazzerro E., Rydziel S., Canalis E. (2006) Bone 38, 671–677 [DOI] [PubMed] [Google Scholar]
- 51.Sykaras N., Opperman L. A. (2003) J. Oral. Sci. 45, 57–73 [DOI] [PubMed] [Google Scholar]
- 52.Chen D., Zhao M., Mundy G. R. (2004) Growth Factors 22, 233–241 [DOI] [PubMed] [Google Scholar]
- 53.Viñals F., López-Rovira T., Rosa J. L., Ventura F. (2002) FEBS Lett. 510, 99–104 [DOI] [PubMed] [Google Scholar]
- 54.Maier S., Lutz R., Gelman L., Sarasa-Renedo A., Schenk S., Grashoff C., Chiquet M. (2008) Biochim. Biophys. Acta 1783, 1150–1162 [DOI] [PubMed] [Google Scholar]
- 55.Filipenko N. R., Attwell S., Roskelley C., Dedhars S. (2005) Oncogene 24, 5837–5849 [DOI] [PubMed] [Google Scholar]
- 56.Naor Z., Benard O., Seger R. (2000) Trends Endocrinol. Metab. 11, 91–99 [DOI] [PubMed] [Google Scholar]
- 57.Jaiswal R. K., Jaiswal N., Bruder S. P., Mbalaviele G., Marshak D. R., Pittenger M. F. (2000) J. Biol. Chem. 275, 9645–9652 [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez J. P., Rios S., Fernandez M., Santibanez J. F. (2004) J. Cell. Biochem. 92, 745–754 [DOI] [PubMed] [Google Scholar]
- 59.Cortizo A. M., Lettieri M. G., Barrio D. A., Mercer N., Etcheverry S. B., McCarthy A. D. (2003) Mol. Cell. Biochem. 250, 1–10 [DOI] [PubMed] [Google Scholar]
- 60.Chen Y., Du X. Y. (2007) J. Cell. Biochem. 100, 1337–1345 [DOI] [PubMed] [Google Scholar]
- 61.Craig T. A., Bhattacharya R., Mukhopadhyay D., Kumar R. (2010) Biochem. Biophys. Res. Commun. 392, 36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kusu N., Laurikkala J., Imanishi M., Usui H., Konishi M., Miyake A., Thesleff I., Itoh N. (2003) J. Biol. Chem. 278, 24113–24117 [DOI] [PubMed] [Google Scholar]
- 63.Winkler D. G., Sutherland M. K., Geoghegan J. C., Yu C., Hayes T., Skonier J. E., Shpektor D., Jonas M., Kovacevich B. R., Staehling-Hampton K., Appleby M., Brunkow M. E., Latham J. A. (2003) EMBO J. 22, 6267–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Semënov M., Tamai K., He X. (2005) J. Biol. Chem. 280, 26770–26775 [DOI] [PubMed] [Google Scholar]
- 65.Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., Harris S. E., Wu D. (2005) J. Biol. Chem. 280, 19883–19887 [DOI] [PubMed] [Google Scholar]
- 66.Winkler D. G., Sutherland M. S., Ojala E., Turcott E., Geoghegan J. C., Shpektor D., Skonier J. E., Yu C., Latham J. A. (2005) J. Biol. Chem. 280, 2498–2502 [DOI] [PubMed] [Google Scholar]
- 67.Suzuki A., Palmer G., Bonjour J. P., Caverzasio J. (1999) Endocrinology 140, 3177–3182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.