Abstract
The phagocyte NADPH oxidase, dormant in resting cells, is activated during phagocytosis to produce superoxide, a precursor of microbicidal oxidants. The membrane-integrated protein gp91phox serves as the catalytic core, because it contains a complete electron-transporting apparatus from NADPH to molecular oxygen for superoxide production. Activation of gp91phox requires the cytosolic proteins p67phox, p47phox, and Rac (a small GTPase). p67phox, comprising 526 amino acids, moves upon cell stimulation to the membrane together with p47phox and there interacts with Rac; these processes are prerequisite for gp91phox activation. Here we show that a region of p67phox (amino acids 190–200) C-terminal to the Rac-binding domain is evolutionarily well conserved and participates in oxidase activation at a later stage in conjunction with an activation domain. Alanine substitution for Tyr-198, Leu-199, or Val-204 abrogates the ability of p67phox to support superoxide production by gp91phox-based oxidase as well as its related oxidases Nox1 and Nox3; the activation also involves other invariant residues such as Leu-193, Asp-197, and Gly-200. Intriguingly, replacement of Gln-192 by alanine or that of Tyr-198 by phenylalanine or tryptophan rather enhances superoxide production by gp91phox-based oxidase, suggesting a tuning role for these residues. Furthermore, the Y198A/V204A or L199A/V204A substitution leads to not only a complete loss of the activity of the reconstituted oxidase system but also a significant decrease in p67phox interaction with the gp91phox NADPH-binding domain, although these mutations affect neither the protein integrity nor the Rac binding activity. Thus the extended activation domain of p67phox (amino acids 190–210) containing the D(Y/F)LGK motif plays an essential role in oxidase activation probably by interacting with gp91phox.
Keywords: Evolution, Oxidase, Oxygen Radicals, Protein Domains, Superoxide Ion, Nox1, Nox3, Noxa1, gp91phox, p67phox
Introduction
The superoxide-producing NADPH oxidase in phagocytes such as neutrophils plays a crucial role in host defense against bacterial and fungal infections (1, 2). The phagocyte oxidase is dormant in resting cells but becomes activated during phagocytosis of pathogens to reduce molecular oxygen to superoxide, a precursor of powerful microbicidal oxidants, in conjunction with NADPH oxidation (3–8). The significance of the oxidase in host defense is evident from the fact that recurrent and life-threatening infections occur in patients with chronic granulomatous disease because of a hereditary defect of the superoxide-producing system in phagocytes (3–8). The catalytic core of the phagocyte oxidase is gp91phox, a membrane-spanning protein that forms a stable heterodimer with p22phox as flavocytochrome b558. gp91phox harbors six transmembrane fragments, bearing two distinct hemes, in the N-terminal half, and the FAD- and NADPH-binding domains in the C-terminal cytosolic region. Thus gp91phox contains a complete electron-transporting apparatus from NADPH via FAD and two hemes to molecular oxygen for superoxide production. Human genome encodes seven members of the Nox family NADPH oxidases with the same domain architecture as gp91phox (3–8). Among them, gp91phox, renamed Nox2 as a member of the family, is close to Nox1 and Nox3, both of which are expressed in nonphagocytic cells.
Activation of the phagocyte oxidase gp91phox/Nox2 requires the three proteins p47phox, p67phox, and the small GTPase Rac, all of which localize exclusively to the cytosol in resting cells. Indeed chronic granulomatous disease is caused by genetic deficiencies or mutations in p67phox, p47phox, or Rac2, in addition to those in gp91phox or p22phox (3–8). Upon cell stimulation, these cytosolic proteins move to the membrane to assemble with the gp91phox/Nox2-p22phox heterodimer, leading to superoxide production. In this process, p67phox translocates together with p47phox (9–11), whereas Rac is independently recruited to the membrane (12, 13). At the membrane, p67phox likely binds to Rac, and the p67phox-Rac complex is thought to induce a conformational change of gp91phox/Nox2, which may allow electrons to flow from NADPH to molecular oxygen (3–8).
The oxidase activator p67phox of 526 amino acid residues is composed of an N-terminal domain comprising four tetratricopeptide repeat (TPR)2 motifs, two SH3 domains, and a PB1 domain between the SH3 domains (see Fig. 1). The binding of p67phox to Rac is mediated via the N-terminal TPR domain of amino acids 1–186 (14, 15). On the other hand, the C-terminal SH3 domain of p67phox functions in membrane translocation by mediating a tail-to-tail interaction with p47phox (9–11), an organizer protein that directly interacts with both the membrane protein p22phox and phosphoinositides upon cell stimulation, leading to membrane translocation (16). The PB1 domain of p67phox is responsible for constitutive association with p40phox (17), an adaptor protein that is dispensable for oxidase activation but facilitates recruitment of p67phox to the membrane (13), especially the phagosomal membrane (18–21). Although the target for the N-terminal SH3 domain remains unidentified, it likely enhances oxidase activation, which may be mediated by facilitating interaction of p67phox with gp91phox/Nox2 (22). Such domain arrangement is crucial for p67phox to efficiently activate the phagocyte oxidase (23).
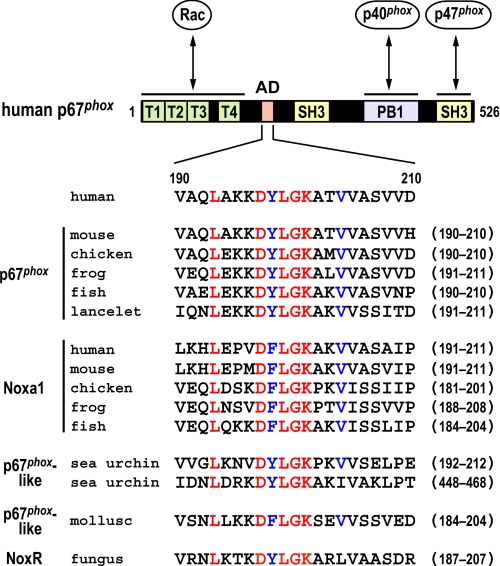
FIGURE 1.
Domain organization of human p67phox and comparison of the amino acid sequences of a region C-terminal to the TPR domain. The domain arrangement of human p67phox of 526 amino acids is schematically represented: the N-terminal domain comprising four TPR motifs, the N-terminal SH3 domain, the PB1 domain, and the C-terminal SH3 domain. The extended activation domain (AD; amino acids 190–210) described in the text is also indicated. The TPR domain binds upon cell stimulation to the small GTPase Rac, the PB1 domain mediates a constitutive PB1-PB1 interaction with p40phox, and the C-terminal SH3 domain is responsible for a tail-to-tail interaction with p47phox. The amino acid sequences of the extended activation domain of p67phox, Noxa1, and related proteins from various species are aligned: human, Homo sapiens; mouse, Mus musculus; chicken, Gallus gallus; frog, Xenopus tropicalis; fish, the Medaka fish Oryzias latipes; lancelet, B. floridae; sea urchin, Strongylocentrotus purpuratus; mollusk, L. gigantean; fungus, Epichloë festucae. The sea urchin p67phox-related protein contains two sets of the extended activation domain. Residues that are completely or well conserved are shown in red or blue, respectively.
In addition to these modular domains, a region C-terminal to the TPR domain of p67phox has been shown to be essential for oxidase activation; analyses using deletion mutant proteins of p67phox by us (24) and by Han et al. (25) have shown that a truncated p67phox of 212 or 210 amino acids is capable of fully activating the phagocyte NADPH oxidase in a cell-free system, but truncated forms of the protein shorter than 204 amino acids fail to support superoxide production. Furthermore, Han et al. (25) have demonstrated that amino acid replacement in the region of amino acids 201–210 (Lys-Ala-Thr-Val-Val-Ala-Ser-Val-Val-Asp; see Fig. 1) results in reduced activity of the phagocyte NADPH oxidase in a cell-free system; alanine substitution for Val-204 strongly attenuates oxidase activation, whereas the activation is slightly or moderately blocked by substitution of one of the other nine residues. Based on these findings, this region (amino acids 199–210) has been designated an activation domain (25), although the region adopts a flexible structure (26).
In the present study, we show that a short region between the TPR and activation domains in p67phox (amino acids 190–200) is evolutionarily well conserved and plays a crucial role in activation of the phagocyte NADPH oxidase. The region of amino acids 190–210 appears to function by interacting with gp91phox, because both the Y198A/V204A and L199A/V204A substitutions abrogate not only oxidase activity but also p67phox binding to the C-terminal NADPH-binding domain of gp91phox. On the other hand, these mutations affect neither the protein integrity, as estimated by NMR analysis, nor the Rac binding activity. These findings indicate that p67phox binds to Rac via the TPR domain, and thus its C-terminal region interacts directly with the NADPH-binding domain of gp91phox, which may induce a conformational change leading to superoxide production. Furthermore, we demonstrate that the region of amino acids 190–210 also contributes to p67phox-mediated activation of the nonphagocytic oxidase Nox3 and that the corresponding region of Noxa1, a homologue of p67phox, participates in activation of Nox1, another nonphagocytic oxidase.
EXPERIMENTAL PROCEDURES
Plasmid Construction
The DNA fragments encoding the following human proteins were prepared as described previously (14, 16, 22, 27): full-length p67phox (amino acid residues 1–526), p67phox-(1–212), full-length p47phox (amino acid residues 1–390), p47phox-(1–286), Noxo1, Noxa1, Rac1, gp91phox/Nox2, Nox1, Nox3, and p22phox. The DNA fragments encoding the gp91phox C-terminal NADPH-binding domain (gp91phox-C; amino acids 384–570) and p67phox-(1–301) were prepared by PCR using the cDNAs for full-length gp91phox and p67phox, respectively, as a template. Mutations leading to the indicated amino acid substitutions in p67phox and Noxa1 were introduced by PCR-mediated site-directed mutagenesis. The cDNAs were ligated to the following expression vectors: pGEX-6P (GE Healthcare Biosciences) for expression of proteins fused to GST in Escherichia coli; pcDNA3 (Invitrogen) for expression of Nox proteins in CHO cells; pEF-BOS for expression of HA-, FLAG-, or Myc-tagged proteins in CHO cells (22, 23, 27). All of the constructs were sequenced for confirmation of their identities.
Activation of the Phagocyte NADPH Oxidase gp91phox in a Whole Cell System
CHO cells endogenously expressing Rac1 were transfected using FuGENE 6 transfection reagent (Roche Applied Science) with the following plasmids: 0.5 μg of pEF-BOS-HA-p67phox or pEF-BOS-HA-Noxa1; 0.5 μg of pEF-BOS-FLAG-p47phox or pEF-BOS-FLAG-Noxo1; 1.0 μg of pcDNA3-gp91phox, pcDNA3-Nox1, or pcDNA3-Nox3; and 1.0 μg of pEF-BOS-p22phox. In the case of Nox1 activation by p67phox and Noxo1, the cells were also transfected with 0.5 μg of pEF-BOS-Myc-Rac1 (Q61L) or pEF-BOS vector.
The transfected cells were cultured for 24 h and harvested by incubation with trypsin/EDTA for 1 min at 37 °C. After being washed with Hepes-buffered saline (120 mm NaCl, 5 mm KCl, 5 mm glucose, 1 mm MgCl2, 1 mm CaCl2, and 17 mm Hepes, pH 7.4), the cells were resuspended at a concentration of 1 × 106 cells/ml in the buffer and tested for estimation of superoxide production. The superoxide-producing activity was determined by superoxide dismutase-inhibitable chemiluminescence with an enhancer-containing luminol-based detection system (Diogenes; National Diagnostics), as described previously (22, 23, 27). After the addition of the enhanced luminol-based substrate, the cells were preincubated for 5 min at 37 °C and subsequently stimulated at the same temperature with or without 200 ng/ml of phorbol 12-myristate 13-acetate (Research Biochemicals International). The chemiluminescence was monitored 37 °C using a luminometer (Auto Lumat LB953; EG&G Berthold). For estimation of protein levels of HA-p67phox, FLAG-p47phox, Myc-Rac1, and p22phox, proteins in cell lysates were subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Millipore), and probed with an anti-HA monoclonal antibody (Roche Applied Science), an anti-FLAG monoclonal antibody (Sigma-Aldrich), an anti-Myc monoclonal antibody (Roche Applied Science), and anti-p22phox polyclonal antibody (Santa Cruz Biotechnology), respectively. The blots were developed using ECL Plus (GE Healthcare) for visualization of the antibodies.
Cell-free Activation of the Phagocyte NADPH Oxidase
The membrane fraction of human neutrophils was prepared as described previously (13, 27, 28). GST-p47phox-(1–286) was purified using glutathione-Sepharose 4B (GE Healthcare) according to the manufacturer's protocols. Wild-type and mutant p67phox-(1–212) and Rac1 (Q61L) without a GST tag were prepared as follows: wild-type and mutant p67phox-(1–212) fused to GST and GST-Rac1 (Q61L) were bound to glutathione-Sepharose 4B and subsequently treated with PreScission Protease (GE Healthcare) in 100 mm KCl and 100 mm potassium phosphate, pH 7.0, which allowed GST-free proteins to be released. The proteins used in the present analysis were analyzed by SDS-PAGE, followed by protein staining with Coomassie Brilliant Blue.
The membranes (10 μg/ml) were mixed with wild-type or mutant p67phox-(1–212) at the indicated concentration, 100 nm GST-p47phox-(1–286), and 100 nm Rac1 (Q61L) in 100 mm potassium phosphate, pH 7.0, containing 75 μm cytochrome c, 1.0 mm FAD, 1.0 mm MgCl2, 1.0 mm EGTA, and 1.0 mm NaN3. After incubation with 100 μm SDS for 2 min at 25 °C, the reaction was initiated by the addition of 200 μm NADPH. The NADPH-dependent superoxide-producing activity was measured by determining the rate of superoxide dismutase-inhibitable ferricytochrome c reduction at 550–540 nm using a Hitachi 557 dual wavelength spectrophotometer (13, 27, 28). The superoxide-producing activity was represented as mol of superoxide produced/s/mol of cytochrome b558 heme; the heme content was calculated from the reduced-minus-oxidized absorption at 558 nm (27, 28).
Binding of Rac to p67phox
For in vitro pull-down assays for Rac binding to p67phox, 10 μg of GST alone or GST-p67phox-(1–301) with or without the indicated substitution was incubated for 15 min at 4 °C with 40 μg of Rac1 (Q61L) in 400 μl of 100 mm potassium phosphate, pH 7.0, containing 0.005% Triton X-100. A slurry of glutathione-Sepharose 4B beads was subsequently added, followed by further incubation for 30 min at 4 °C. After washing four times with the buffer above, the proteins were eluted from the beads with 20 mm glutathione in 200 mm NaCl and 200 mm Tris-HCl, pH 8.0, containing 0.1% Triton X-100. The eluate was subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Millipore), and probed with an anti-Rac monoclonal antibody.
NMR Measurements
The wild-type and mutant forms of p67phox-(1–212) were prepared by purification with glutathione-Sepharose 4B beads and subsequent cleavage with PreScission Protease. The proteins were further purified by size exclusion chromatography with a Superdex 200 HR 10/300 column (GE Healthcare) equilibrated with 100 mm KCl and 100 mm potassium phosphate, pH 7.0. The purified proteins were subsequently concentrated using Amicon Ultra (Millipore).
The wild-type and mutant forms of p67phox-(1–212) were dissolved at the concentration of 200 μm in 100 mm KCl and 100 mm potassium phosphate, pH 7.0, in 90% H2O, 10% D2O. The 1H NMR spectra of these proteins were recorded at 25 °C with an Avance 600 spectrometer equipped with a TXI CryoProbe (Bruker Biospin) with a spectral width of 8,389 Hz. The chemical shifts were referenced relative to the internal standard 2,2-dimethyl-2-silapentane-5-sulfonate.
Binding of p67phox to the C-terminal Domain of gp91phox
The C-terminal NADPH-binding domain of gp91phox fused to GST (GST-gp91phox-C) was purified using glutathione-Sepharose 4B. FLAG-tagged p67phox-(1–301) was expressed as a GST fusion protein in E. coli using the expression vector pGEX-6P and purified with glutathione-Sepharose 4B, followed by cleavage with PreScission Protease. For in vitro pull-down binding assays, 10 μg of GST-gp91phox-C or GST alone was incubated for 15 min at 4 °C with 300 μg of FLAG-p67phox-(1–301) and Rac1 (Q61L) in 400 μl of 100 mm potassium phosphate, pH 7.0. A slurry of glutathione-Sepharose 4B beads was subsequently added, followed by further incubation for 30 min at 4 °C. After washing four times with the buffer above, the proteins were eluted from the beads with 20 mm glutathione in 200 mm NaCl and 200 mm Tris-HCl, pH 8.0. The eluate was analyzed by immunoblot using an anti-FLAG monoclonal antibody for detection of FLAG-tagged p67phox.
RESULTS
Role of the Region of Amino Acids 190–205 in p67phox on Whole Cell Activation of the Phagocyte NADPH Oxidase
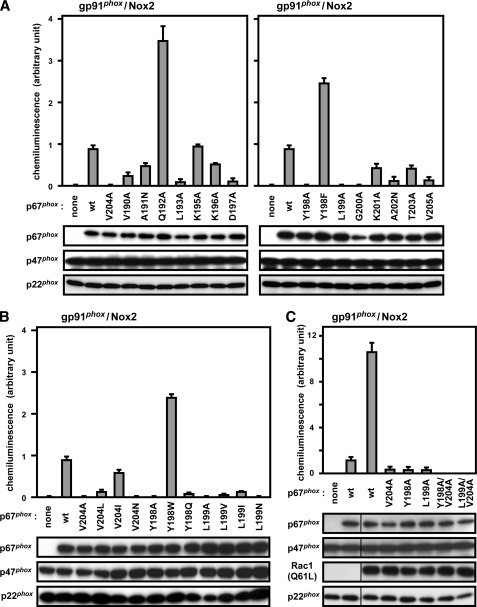
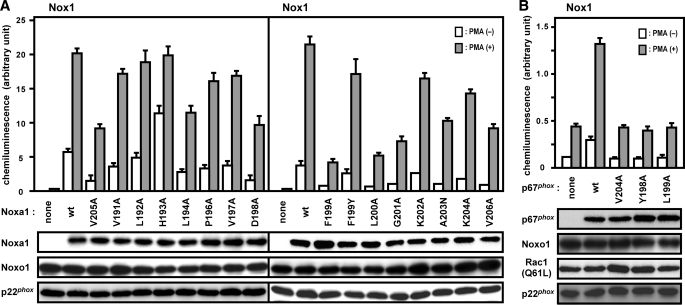
Although the region of amino acids 190–200, N-terminal to the activation domain, is well conserved among p67phox and its related proteins from various species (Fig. 1), the role of these residues has not been investigated. To test this, we expressed mutant p67phox proteins carrying an amino acid substitution in the region of residues 190–205 in CHO cells co-expressing gp91phox/Nox2 and p47phox as well as endogenous Rac; non-alanine residues were individually mutated to alanines, whereas Ala-191 and Ala-202 were converted to asparagine. As shown in Fig. 2A, a mutant protein carrying alanine substitution for Tyr-198 or Leu-199 failed to support superoxide production by gp91phox/Nox2, as did one with replacement of Val-204 by alanine; the V204A substitution leads to a defective activation of the phagocyte oxidase in a cell-free system, as originally shown by Han et al. (25). The replacement of the invariant residue Leu-193 or Asp-197 also led to a decrease in oxidase activity but to a lesser extent. Although alanine substitution for Gly-200 completely abrogated superoxide production by gp91phox/Nox2-based oxidase, the amount of the mutant protein was significantly reduced (Fig. 2A), indicating that the mutation results in an unstable protein. A mutant protein with alanine substitution for the invariant lysine (Lys-201) was half as active as the wild-type protein, which is consistent with the previous observation by Han et al. (25) using the mutant protein in a cell-free activation system for the phagocyte oxidase. In addition, alanine substitution for Val-205, an incompletely but well conserved residue, led to a significant loss of superoxide production in the whole cell system for gp91phox/Nox2, which also agrees with the previous finding using the cell-free system (25). Thus amino acid residues that are evolutionarily well conserved play a crucial role in gp91phox/Nox2 activation.
FIGURE 2.
Role for the Nox activation region of p67phox (amino acids 190–210) in gp91phox/Nox2 activation. A, role for a region C-terminal to the TPR domain of p67phox in a whole cell activation system of the phagocyte NADPH oxidase gp91phox/Nox2. HA-tagged p67phox (the wild-type (wt) or indicated mutant protein), FLAG-p47phox, gp91phox/Nox2, and p22phox were co-expressed in CHO cells. B, role for Tyr-198, Leu-199, and Val-204 in activation of gp91phox/Nox2. HA-tagged p67phox carrying substitution for Tyr-198, Leu-199, or Val-204 as well as FLAG-p47phox, gp91phox/Nox2, and p22phox were co-expressed in CHO cells. C, role for Tyr-198, Leu-199, and Val-204 in activation of gp91phox/Nox2 in the presence of Rac1 (Q61L). HA-tagged p67phox carrying the substitution for Tyr-198, Leu-199, or Val-204 as well as FLAG-p47phox, Myc-Rac1 (Q61L), gp91phox/Nox2, and p22phox were co-expressed in CHO cells. Note that although the cDNA for Rac1 (Q61L) was transfected in C, neither wild-type nor mutant Rac1 was ectopically expressed in A and B. The protein levels of HA-p67phox (the wild-type or indicated mutant protein), FLAG-p47phox, Myc-Rac1 (Q61L), and p22phox were analyzed by immunoblot with the anti-HA, anti-FLAG, anti-Myc, and anti-p22phox antibodies, respectively, as described under “Experimental Procedures.” The transfected cells were incubated for 5 min at 37 °C and then stimulated with phorbol 12-myristate 13-acetate (200 ng/ml). The chemiluminescence change by the superoxide produced was continuously monitored with DIOGENES, as described under “Experimental Procedures.” Each graph represents the means ± S.D. of the chemiluminescence values integrated for 10 min, which were obtained from three independent transfections.
We further investigated the role for Tyr-198, Leu-199, or Val-204, alanine substitution for which results in an almost complete loss of the activity of gp91phox/Nox2-based oxidase, using mutant proteins with replacement by a non-alanine residue. Tyr-198, an invariant residue in p67phox (Fig. 1), is replaced by phenylalanine in Noxa1. Because Noxa1 efficiently activates the nonphagocytic oxidase Nox1 but not gp91phox/Nox2 (30–33), it seemed possible that the replacement by phenylalanine in p67phox would abrogate superoxide production by gp91phox/Nox2-based oxidase. Unexpectedly, however, the Y198F substitution enhanced superoxide production by gp91phox/Nox2 (Fig. 2A). Furthermore, the production was also facilitated by the replacement by tryptophan (Fig. 2B). On the other hand, the Y198Q substitution led to a significant defect in superoxide production (Fig. 2B). Thus the aromaticity at position 198 is required for gp91phox/Nox2 activation. Leu-199 is completely conserved among p67phox and its related proteins in evolution (Fig. 1). A mutant p67phox carrying the replacement of the invariant leucine by asparagine, a hydrophilic residue, failed to activate gp91phox/Nox2 (Fig. 2B). The oxidase was only weakly but significantly activated by proteins with the replacement by valine or isoleucine (Fig. 2B), suggesting a role for the hydrophobic nature of the side chain. The hydrophobicity seems to be more important at position 204. This is because mutant p67phox proteins with the V204I or V204L substitution retained ∼60 and 10% of the activity, respectively, whereas the replacement by asparagine led to a loss of superoxide production by gp91phox/Nox2 (Fig. 2B).
Role for the p67phox Region of Amino Acids 190–200 in Cell-free Activation of the Phagocyte NADPH Oxidase
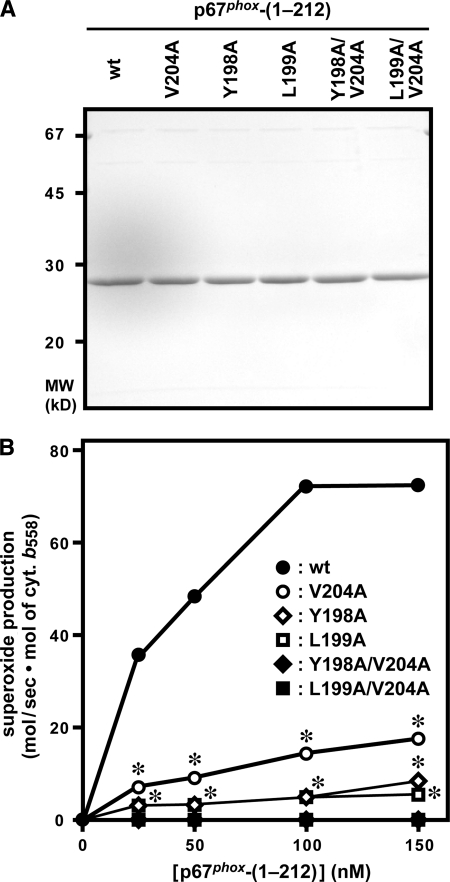
To confirm the role of Tyr-198, Leu-199, and Val-204 of p67phox, we prepared mutant proteins with alanine substitution for these residues (Fig. 3A) and tested their ability to support superoxide production under cell-free conditions for activation of the phagocyte NADPH oxidase. As shown in Fig. 3B, the single substitution Y198A, L199A, or V204A resulted in a significant, albeit incomplete, reduction in the activity of gp91phox/Nox2-based oxidase (Fig. 3B). Mutant p67phox proteins carrying the Y198A/V204A or L199A/V204A double substitution were completely inactive in cell-free activation of the phagocyte oxidase (Fig. 3B). The properties of these p67phox proteins in a cell-free system appear to be the same as those in a whole cell system in the presence of Rac1 (Q61L), a constitutively active form of this GTPase. Although endogenous Rac is sufficient to reconstitute a whole cell activation system for gp91phox/Nox2-based oxidase (29–33) (also shown in Fig. 2, A and B), ectopic expression of the constitutively active Rac1 (Q61L) is known to strongly enhance superoxide production by gp91phox/Nox2-based oxidase. Thus the whole cell system with ectoic expression of Rac1 (Q61L) enables us to accurately estimate whether a certain residue is absolutely required or not (34, 42). As shown in Fig. 2C, the double substitutions in p67phox, but not the single ones, resulted in a complete loss of the activity of gp91phox/Nox2-based oxidase when Rac1 (Q61L) was ectopically expressed (Fig. 2C). Thus Tyr-198, Leu-199, and Val-204 of p67phox play a crucial role in activation of the phagocyte oxidase reconstituted both in vivo and in vitro, and gp91phox/Nox2-based oxidase activity is completely lost by the Y198A/V204A or L199A/V204A double substitution.
FIGURE 3.
Role for Tyr-198, Leu-199, and Val-204 of p67phox in activation of the phagocyte NADPH oxidase under cell-free conditions. A, SDS-PAGE analysis of purified p67phox proteins that were used in a cell-free system for activation of the phagocyte NADPH oxidase. Wild-type (wt) p67phox-(1–212) or a mutant protein carrying the V204A, Y198A, L199A, Y198A/V204A, or L199A/V204A substitution was subjected to SDS-PAGE, followed by staining with Coomassie Brilliant Blue. The positions for marker proteins are indicated in kilodaltons. B, role for Tyr-198, Leu-199, and Val-204 of p67phox in a cell-free system for activation of the phagocyte NADPH oxidase. Human neutrophil membrane (10 μg/ml) as a source of the gp91phox-p22phox dimer was mixed with a mutant or wild-type p67phox protein at the indicated concentrations, 100 nm Rac1 (Q61L), and 100 nm GST-p47phox-(1–286). The mixture was incubated for 2 min at 25 °C with 100 μm SDS, and the reaction was initiated by the addition of 200 μm NADPH. The NADPH-dependent superoxide-producing activity was measured by the rate of superoxide dismutase-inhibitable cytochrome c reduction, as described under “Experimental Procedures.” Compared with the whole cell system, much higher amounts of superoxide were produced in the cell-free system, which enabled us to use the cytochrome c reduction method. Each graph represents the means ± S.D. of data from three independent experiments. *, p < 0.01 versus the mutant p67phox with the Y198A/V204A or L199A/V204A substitution.
Effects of Alanine Substitution for Tyr-198, Leu-199, and Val-204 of p67phox on Structural Integrity and Interaction with Rac
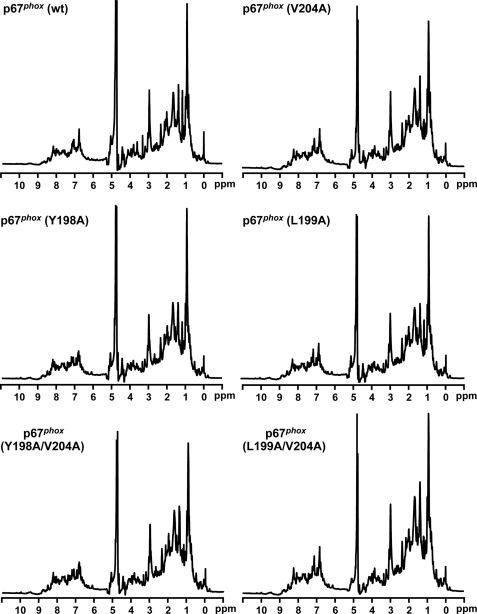
To rule out the possibility that alanine substitution for Tyr-198, Leu-199, and Val-204 of p67phox affects protein integrity, we measured 1H NMR spectra of the proteins with the mutations. As shown in Fig. 4, little difference was observed between 1H NMR spectra of the wild-type and mutant proteins carrying the Y198A, L199A, V204A, Y198A/V204A, and L199A/V204A substitutions (Fig. 4), indicating that these proteins are correctly folded. Thus the mutations at Tyr-198, Leu-199, and Val-204 appear not to affect the structural integrity of the protein.
FIGURE 4.
NMR analysis of wild-type p67phox and mutant p67phox carrying the Y198A, L198A, or V204A substitution. 1H NMR spectra of wild-type p67phox-(1–212) (wt) or a mutant protein carrying the V204A, Y198A, L199A, Y198A/V204A, or L199A/V204A substitution were measured in 100 mm KCl and 100 mm potassium phosphate, pH 7.0, in 90% H2O, 10% D2O at 25 °C as described under “Experimental Procedures.” The data are representative of results from four independent experiments.
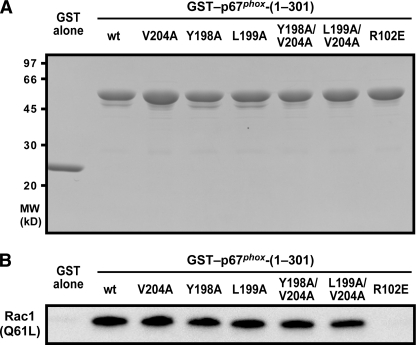
We next tested the effect of alanine substitution for Tyr-198, Leu-199, and Val-204 of p67phox on interaction with Rac. As shown in Fig. 5, Rac1 (Q61L) was efficiently precipitated by GST-fused p67phox but not by GST alone or GST-p67phox (R102E), a mutant protein defective in binding to Rac (14). Under these conditions, the GTPase was fully pulled down with mutant p67phox proteins carrying the Y198A, L199A, V204A, Y198A/V204A, or L199A/V204A substitution, indicating that they retain the ability of p67phox to interact with Rac. Taken together with the results obtained by the NMR measurements (Fig. 4), alanine substitution for Tyr-198, Leu-199, and Val-204 of p67phox affect neither the protein integrity nor the Rac binding activity.
FIGURE 5.
Role for Tyr-198, Leu-199, and Val-204 of p67phox in interaction with Rac. A, SDS-PAGE analysis of purified GST fusion proteins that were used in a GST pull-down assay for interaction with Rac. GST alone and GST fusion protein of p67phox-(1–301) (the wild-type (wt) or a mutant protein carrying the V204A, Y198A, L199A, Y198A/V204A, L199A/V204A, or R102E substitution) was subjected to SDS-PAGE, followed by staining with Coomassie Brilliant Blue. The positions for marker proteins are indicated in kilodaltons. B, role for Tyr-198, Leu-199, and Val-204 of p67phox in interaction with Rac. GST-p67phox proteins (the wild-type and indicated mutant proteins) were incubated with Rac1 (Q61L) and pulled down with glutathione-Sepharose 4B beads. The precipitated proteins were analyzed by immunoblot with the anti-Rac antibody, as described under “Experimental Procedures.” The data are representative of results from four independent experiments.
Effects of Alanine Substitution for Tyr-198, Leu-199, and Val-204 of p67phox on Interaction with gp91phox
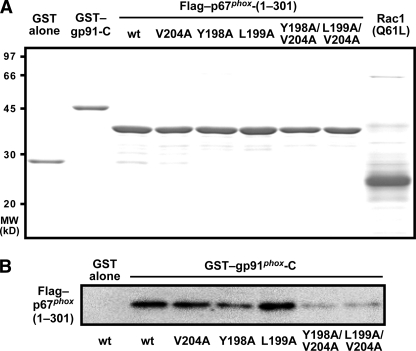
Activation of the phagocyte oxidase by p67phox is thought to involve its interaction with gp91phox (35–39). To test the role of the p67phox region of amino acids 190–210 in binding to the C-terminal NADPH-binding domain of gp91phox (amino acids 384–570), we performed a GST pull-down assay using purified p67phox proteins (Fig. 6A). As shown in Fig. 6B, a mutant p67phox carrying the single substitution Y198A, L199A, or V204A was co-precipitated with GST-fused gp91phox-C to an extent similar to the wild-type protein. On the other hand, the double substitution Y198A/V204A or L199A/V204A resulted in a significant loss of interaction with the gp91phox NADPH-binding domain (Fig. 6B). Thus the region of amino acids 190–210 appears to be involved in p67phox interaction with gp91phox.
FIGURE 6.
Role for Tyr-198, Leu-199, and Val-204 of p67phox in interaction with gp91phox. A, SDS-PAGE analysis of purified, GST-tagged, or FLAG-tagged proteins that were used for interaction of p67phox with gp91phox. GST alone, GST-fused protein of gp91phox-C, FLAG-tagged p67phox (the wild-type (wt) or a mutant protein carrying the V204A, Y198A, L199A, Y198A/V204A, or L199A/V204A substitution), and Rac1 (Q61L) without a tag were subjected to SDS-PAGE, followed by staining with Coomassie Brilliant Blue. The positions for marker proteins are indicated in kilodaltons. B, role for Tyr-198, Leu-199, and Val-204 of p67phox in interaction with gp91phox. GST-gp91phox-C was incubated with FLAG-p67phox proteins (the wild-type and indicated mutant proteins) in the presence of Rac1 (Q61L) and pulled down with glutathione-Sepharose 4B beads. The precipitated proteins were analyzed by immunoblot with the anti-FLAG antibody, as described under “Experimental Procedures.” The data are representative of results from four independent experiments.
Role for the TPR-flanking Region of p67phox and Noxa1 in Nox1 Activation
Although the nonphagocytic oxidase Nox1 is inactive on its own, it becomes activated to produce superoxide by Noxa1, a nonphagocytic protein homologous to p67phox, in conjunction with the p47phox-related protein Noxo1 (30–33). The Noxa1 region corresponding to the p67phox region of amino acids 190–210 is also evolutionarily conserved (Fig. 1), but even the role of the Noxa1 activation domain in Nox1 activation has not been studied. To know the function of the Noxa1 region, we expressed wild-type Noxa1 and mutant proteins carrying a substitution for a residue in the region of amino acids 191–206 in CHO cells. As shown in Fig. 7A, alanine substitution for Phe-199 or Leu-200 significantly but not completely abrogated superoxide production by Nox1; the L194A, D198A, G201A, V205A, or V206A substitution also impaired Nox1-based oxidase activity but to a lesser extent. It should be noted that the corresponding residues in human p67phox (Fig. 1) each play a crucial role in activation of the phagocyte oxidase gp91phox/Nox2. Interestingly, Noxa1 (F199Y) was much more effective than Noxa1 (F199A) in activating Nox1 (Fig. 7A). The result suggests a role for the aromaticity at position 199, which is the case for the corresponding position of p67phox (Tyr-198 in human p67phox). Thus the Noxa1 region corresponding to that of amino acids 190–210 in p67phox appears to participate in Nox1 activation.
FIGURE 7.
Role for the Nox activation region of p67phox and the corresponding region of Noxa1 in Nox1 activation. A, activation of Nox1 by wild-type (wt) or mutant Noxa1 and Noxo1. HA-Noxa1 (the wild-type or indicated mutant protein), FLAG-Noxo1, Nox1, and p22phox were co-expressed in CHO cells. B, activation of Nox1 by wild-type or mutant p67phox and Noxo1 in the presence of Rac1 (Q61L). HA-p67phox (the wild-type or indicated mutant protein), FLAG-Noxo1, Myc-Rac1 (Q61L), Nox1, and p22phox were co-expressed in CHO cells. Protein levels of HA-p67phox or HA-Noxa1, FLAG-Noxo1, Myc-Rac1 (Q61L), and p22phox were analyzed by immunoblot with the anti-HA, anti-FLAG, anti-Myc, and anti-p22phox antibodies, respectively, as described under “Experimental Procedures.” After preincubation for 5 min, the transfected cells were incubated at 37 °C with or without phorbol 12-myristate 13-acetate (PMA, 200 ng/ml). Chemiluminescence change by superoxide produced was continuously monitored with DIOGENES, as described under “Experimental Procedures.” Each graph represents the means ± S.D. of the chemiluminescence values integrated for 10 min, which were obtained from three independent transfections.
It is known that p67phox is also capable of activating Nox1 in the presence of Noxo1, but to approximately a hundred times lesser extent than Noxa1; co-expression of Rac1 (Q61L) enhances the p67phox-mediated activation of Nox1 several-fold (40). As illustrated in Fig. 7B, Nox1-based oxidase activity supported by p67phox in the presence of Noxo1 and Rac1 (Q61L) was abrogated by alanine substitution for Tyr-198, Leu-199, and Val-204. These findings suggest that the p67phox region of amino acids 190–210 is also involved in the activation of Nox1.
Role for the p67phox Region of Amino Acids 190–200 in Nox3 Activation
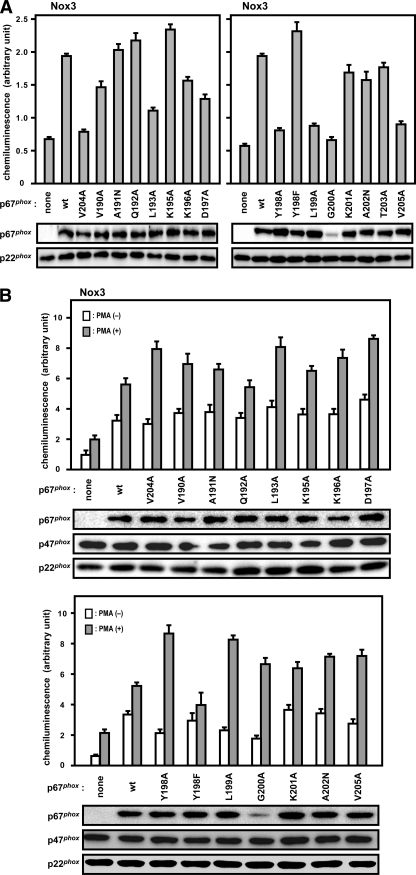
We finally tested the role for the p67phox region of amino acids 190–210 in activation of Nox3, another nonphagocytic oxidase. Unlike the closely related oxidases gp91phox/Nox2 and Nox1, Nox3 is capable of producing a substantial amount of superoxide even in the absence of regulatory proteins such as p67phox, Noxa1, p47phox, and Noxo1 (41, 42). Although the production is known to be enhanced several-fold in the presence of p67phox (41–44), the role of the activation domain in Nox3 activation by p67phox alone has not been investigated. As shown in Fig. 8A, the enhancement was significantly decreased by alanine substitution for Leu-193, Asp-197, Tyr-198, Leu-199, or Val-204. It should be noted that these residues all participate in the activation of gp91phox/Nox2 as well as Nox1. Thus the region of amino acids 190–210 likely plays a crucial role in p67phox-mediated enhancement of Nox3 activation.
FIGURE 8.
Role for the Nox activation region of p67phox in Nox3 activation. A, activation of Nox3 by wild-type (wt) or mutant p67phox. HA-p67phox (the wild-type or indicated mutant protein), Nox3, and p22phox were co-expressed in CHO cells. B, activation of Nox3 by wild-type or mutant p67phox and p47phox. HA-p67phox (the wild-type or indicated mutant protein), FLAG-p47phox, Nox3, and p22phox were co-expressed in CHO cells. Protein levels of HA-p67phox (the wild-type or indicated mutant protein), FLAG-p47phox, and p22phox were analyzed by immunoblot with the anti-HA, anti-FLAG, and anti-p22phox antibodies, respectively, as described under “Experimental Procedures.” After preincubation for 5 min, the transfected cells were incubated at 37 °C with or without phorbol 12-myristate 13-acetate (PMA, 200 ng/ml). Chemiluminescence change was continuously monitored with DIOGENES. Each graph represents the means ± S.D. of the chemiluminescence values integrated for 10 min, which were obtained from three independent transfections.
It is known that p47phox further facilitates Nox3 activation by p67phox (41–43). In contrast to the role of Val-204 in Nox3-based oxidase in the presence of p67phox alone (Fig. 8A), it is known that p67phox-enhanced activation of Nox3 in the presence of p47phox is not impaired by the V204A substitution (41, 43). Similarly, amino acid substitution at other positions in the region resulted in a similar or rather higher activity of Nox3-based oxidase, compared with wild-type p67phox (Fig. 8B). The mutant p67phox (Y198F), which is more effective than the wild-type protein in gp91phox/Nox2 activation (Fig. 2), supported superoxide production by Nox3 to a slightly lesser extent than wild-type p67phox (Fig. 8B). These findings suggest that, in the presence of p47phox, the p67phox region of amino acids 190–210 is not involved in Nox3 activation.
DISCUSSION
In the present study, we show that activation of the phagocyte NADPH oxidase (gp91phox/Nox2) requires a conserved region of human p67phox (amino acids 190–200), which is located between the N-terminal Rac-binding TPR domain and the previously identified activation domain (amino acids 199–210). Amino acid substitution for several residues in the region results in decreased superoxide production in both cell-free and whole cell systems for gp91phox/Nox2 activation (Fig. 2). In particular, alanine substitution for Tyr-198 or Leu-199, like that for Val-204, leads to an almost complete loss in oxidase activity (Figs. 2 and 3), and the Y198A/V204A or L199A/V204A double substitution completely abrogates the ability of p67phox to support superoxide production by gp91phox/Nox2-based oxidase (Figs. 2 and 3). The abrogation appears to occur partly by a decrease in interaction of p67phox with gp91phox (Fig. 6), although it is not due to a loss of protein integrity or Rac binding activity (Figs. 4 and 5).
The region of amino acids 190–210 in human p67phox seems to be largely in a flexible form in the absence of gp91phox. The N-terminal domain in p67phox comprises four TPR motifs, each folding into two antiparallel α-helices, and a succession of the motifs adopts an overall superhelical structure; the fourth TPR (amino acids 121–154) is followed by an α-helix of amino acids 155–166 and an extension (amino acids 168–186) that folds back into the internal hydrophobic groove of the superhelix, thereby stabilizing the overall structure (15, 26). Three residues next to the extension (amino acids 187–189) are not conserved among p67phox and its related proteins. On the other hand, its C-terminally flanking region, spanning amino acids 190–210, is evolutionarily well conserved (Fig. 1); the consensus sequence is ØXXLXX(K/Ø)D(Y/F)LGK(A/P)X(V/I/L)(V/I)(A/S)(S/A)ØX(Ω/P) (where Ø and Ω indicate residues with an aliphatic side chain and a charged residue, respectively, and X denotes any residue; completely conserved residues are in bold). The extended activation domain (amino acids 190–210) does not adopt an ordered structure in a truncated p67phox (amino acids 1–203) complexed with GTP-bound Rac (15), whereas an α-helix of amino acids 187–193 precedes an unstructured region downstream from Leu-193 in a noncomplexed p67phox (amino acids 1–213) (26). The flexibility may enable the extended activation domain to fit into a certain portion of gp91phox, likely inducing a conformational change for superoxide production. This module is not only required for oxidase activation but also seems to contribute to the tuning of oxidase activity. This is because the Q192A, Y198F, or Y198W substitution culminates in much greater production of superoxide compared with wild-type p67phox (Fig. 2).
The Asp-Tyr-Leu-Gly-Lys fragment in the extended activation domain of human p67phox (amino acids 197–201) is strictly conserved among p67phox and its related proteins except the residue at position 2 (Fig. 1). Although the tyrosine residue at position 2 in this motif is common to p67phox proteins, the position is occupied by phenylalanine in Noxa1. This raises the possibility that the residue at position 2 of the D(Y/F)LGK motif is responsible for the difference between p67phox and Noxa1 in oxidase activation; gp91phox/Nox2 are activated preferentially by p67phox, whereas Nox1 activation prefers Noxa1 to a much larger extent (30–33). As shown in the present study, p67phox (Y198F) is more effective than wild-type p67phox in gp91phox/Nox2 activation (Fig. 2), and wild-type Noxa1 activates Nox1 to a slightly greater extent than Noxa1 (F199Y) (Fig. 7). Thus gp91phox/Nox2 and Nox1 both prefer phenylalanine to tyrosine at position 2 of the D(Y/F)LGK motif in the activator proteins. In addition, although p67phox enhances Nox3 activation much more effectively than Noxa1 (40–43), wild-type p67phox facilitates superoxide production by Nox3 to a slightly lesser extent than p67phox (Y198F) (Fig. 8); Nox3 also prefers phenylalanine to tyrosine at position 2 of the D(Y/F)LGK motif. These findings indicate that the selectivity for Nox oxidases is not determined by the second residue (tyrosine or phenylalanine) of the D(Y/F)LGK motif in activator proteins, although this motif likely serves as an identifier that discriminates p67phox from Noxa1.
The D(Y/F)LGK motif is conserved not only in p67phox and Noxa1, but also in other p67phox-related proteins. The oxidase activator p67phox can be defined as a protein containing the Rac-binding TPR, extended activation, N-terminal SH3, PB1, and C-terminal SH3 domains (Fig. 1). The p67phox orthologues that satisfy this criteria are found only in the phylum Chordata of the animal kingdom, which comprises the subphyla Vertebrata, Urochordata, and Cephalochordata. p67phox indeed exists in the lancelet Branchiostoma floridae of the Cephalochordata, i.e. the basal group of the Chordata, although a homologue has not been identified in the Urochordata (8). Noxa1, a p67phox paralogue that lacks the N-terminal SH3 or functional PB1 domains (or both) is found solely in the subphylum Vertebrata containing fishes, amphibians, birds, and mammals (8, 45). On the other hand, p67phox-like proteins with a cassette comprising the N-terminal Rac-binding TPR domain and the extended activation domain are also present in other species of the animal kingdom (such as sea urchin in the phylum Echinodermata and snail in the phylum Mollusca) as well as those of the fungal kingdom. This cassette is thought to be minimally essential for oxidase activation, because a truncated p67phox of 212 or 210 amino acids, but not proteins shorter than 204 amino acids, is capable of fully activating the phagocyte NADPH oxidase under cell-free conditions (24, 25). In a p67phox-like protein of the mollusk Lottia gigantean (8, 45), which has the essential oxidase cassette but lacks SH3 and PB1 domains, the tyrosine residue in the D(Y/F)LGK motif is replaced by phenylalanine as is in Noxa1 (Fig. 1). On the other hand, the tyrosine residue is retained in the sea urchin p67phox-related protein, which harbors two sets of the essential oxidase cassette and a single C-terminal SH3 domain (8, 45), and also in the fungal oxidase regulator NoxR, which does not contain SH3 domains (46–48). The evolutionary conservation of tyrosine or phenylalanine at position 2 of the D(Y/F)LGK motif is consistent with its functional significance as highlighted by the present observation that alanine substitution for Tyr-198 in human p67phox or that for Phe-199 in human Noxa1 results in a defective superoxide-producing activity of the three distinct Nox oxidases: gp91phox/Nox2, Nox1, and Nox3 (Figs. 2, 3, 7, and 8). Intriguingly, replacement of Tyr-198 in human p67phox by tryptophan as well as that by phenylalanine results in a more robust superoxide production by the phagocyte oxidase than the wild-type protein (Fig. 2). Thus the aromaticity at position 2 of the D(Y/F)LGK motif appears to play a crucial role in oxidase activation. The reason for the requirement for the aromaticity is presently unknown. It should be clarified in future structural studies using a crystal of p67phox complexed with gp91phox/Nox2.
Like Tyr-198 and Val-204, Leu-199 in human p67phox, corresponding to the invariant leucine residue in the D(Y/F)LGK motif, is one of the most crucial residues for activation of gp91phox/Nox2, as well as Nox1 and Nox3 (Figs. 2, 3, 7, and 8). Alanine substitution for the equivalent residue Leu-200 in Noxa1 also results in an incomplete but significant loss of Nox1 activity (Fig. 7). Activation of gp91phox/Nox2 by p67phox is almost completely abrogated by replacement of Leu-199 with asparagine as well as alanine, whereas it is restored only slightly by isoleucine and marginally by valine (Fig. 2). The strict requirement for leucine explains well its evolutionary conservation among p67phox and its related proteins.
The glycine residue in the D(Y/F)LGK motif (Gly-200 in human p67phox) also plays a crucial role. Replacement of Gly-200 by alanine leads not only to a loss of superoxide production but also to a decrease in the amount of p67phox under the conditions where alanine substitution for other residues does not affect the protein level (Figs. 2 and 8). Thus it seems likely that Gly-200 is involved in protein stability. However, as described above, this glycine residue is located in a region that probably adopts a flexible or disordered structure (15, 26), and thus a protein with the G200A substitution is expected to retain protein integrity. Although the reason for this discrepancy is presently unknown, protein stability might require the flexibility of the adjacent bulky residues (Leu-199 and Lys-201 in p67phox), which can be provided only by the side chain-free residue glycine.
In contrast to the crucial role of the central three residues in the D(Y/F)LGK motif, the significance of the first residue aspartate and the last residue lysine is relatively low; alanine substitution for Asp-197 or Lys-201 in p67phox results in a moderate or slight defect in oxidase activation, respectively (Figs. 2 and 8), and Nox1-based oxidase activity supported by Noxa1 is also moderately or slightly abrogated by replacement of Asp-198 or Lys-202, respectively (Fig. 7). The leucine residue that is located four amino acids N-terminal to the D(Y/F)LGK motif (Leu-193 in human p67phox) is also completely conserved in evolution among p67phox and its related proteins (Fig. 1). Alanine substitution for Leu-193, as well as that for Asp-197, leads to a moderate decrease in superoxide production by gp91phox/Nox2- and Nox3-based oxidases (Figs. 2 and 8); similarly, replacement with alanine of the equivalent residue Leu-194 in Noxa1 moderately abrogates Nox1-based oxidase activity (Fig. 7). Thus Leu-193, Asp-197, and Lys-201 contribute to oxidase activation but to a relatively small extent, although the three residues are strictly conserved in evolution.
The valine residue that is located three amino acids C-terminal to the D(Y/F)LGK motif (Val-204 in human p67phox) is invariant among p67phox and its related proteins in animals but replaced by leucine in the fungal protein NoxR (Fig. 1). Replacement of Val-204 with alanine or asparagine results in an almost complete loss of the superoxide-producing activity of the phagocyte oxidase reconstituted both in vivo and in vitro (Figs. 2 and 3). The effect of alanine or asparagine is restored largely by isoleucine and partly by leucine. This indicates the importance of the hydrophobicity at this position and explains at least partially the reason for the substitution of leucine in NoxR. The reason for the requirement for the hydrophobicity should be clarified in future structural studies.
Little was known about the role of the activation domain of p67phox and its related proteins in activation of Nox1 and Nox3. The present study demonstrates that the extended activation domain of p67phox (amino acids 190–210) contributes to enhancement of Nox3 activation by p67phox in the absence of p47phox (Fig. 8A). The mechanism by which the p67phox domain activates Nox3 appears to be similar to that for gp91phox/Nox2, because the order of the effect of amino acid substitutions on gp91phox/Nox2 activation is the same as that on p67phox-dependent enhancement of Nox3-based oxidase activity; the most crucial residues are Tyr-198, Leu-199, and Val-204, and the next ones are Leu-193 and Asp-197 in both cases (Fig. 8A). On the other hand, the extended activation domain of p67phox does not participate in enhancement of Nox3-based oxidase activity by both p67phox and p47phox (Fig. 8B). This is consistent with previous observations that p67phox-dependent activation of Nox3 in the presence of p47phox is not impaired by the V204A substitution (41, 43). It should be noted that Rac is involved in Nox3 activation by p67phox alone, but not in that by p67phox in the presence of p47phox (40–42). The extended activation domain of Noxa1 appears to participate in Nox1 activation but in a less dependent manner than in gp91phox/Nox2 activation by p67phox; alanine substitution for Tyr-198, Leu-199, and Val-205 in Noxa1 leads to a substantial but partial loss of Nox1 activation (Fig. 7). Intriguingly, activation of Nox1 is less dependent on Rac than that of gp91phox (34, 42, 49). On the other hand, Rac and the extended activation domain are both strictly required for gp91phox activation (3–8, 34). Thus dependence of activation of the three oxidases on Rac is parallel to that on the extended activation domain, which is consistent with the hypothesis that binding of Rac is a prerequisite for action of the extended activation domain of p67phox.
The extended activation domain of p67phox likely interacts with the C-terminal NADPH-binding domain of gp91phox (Fig. 6). This suggests that the extended activation domain affects the conformation of the gp91phox NADPH-binding domain, which seems to be consistent with the proposal that the originally identified activation domain, i.e. the C-terminal half of the extended version, regulates electron transfer from NADPH to FAD in gp91phox (35). Although the mutations in the extended activation domain of p67phox lead to a complete loss of gp91phox/Nox2-based oxidase activity (Figs. 2 and 3), they result in a significant but incomplete loss of binding to gp91phox (Fig. 6). This may imply that the extended activation domain also functions via interacting with a region distinct from the NADPH-binding domain in gp91phox. The second region in gp91phox, which interacts with the extended activation domain, should be clarified in future studies.
In summary, here we have identified a region C-terminal to the Rac-binding TPR domain of p67phox (from the amino acid residue 190) as a novel functional moiety that plays a crucial role in oxidase activation together with the previously identified activation domain (amino acids 199–210). The extended activation domain of p67phox (amino acids 190–210) is evolutionarily well conserved and appears to serve by interacting with gp91phox, which probably induces a conformational change in this catalytic core, thereby facilitating electron flow from NADPH to molecular oxygen for superoxide production.
Acknowledgments
We thank Natsuko Morinaga (Kyushu University), Yohko Kage (Kyushu University), and Namiko Kubo (Kyushu University) for technical assistance and Minako Nishino (Kyushu University) for secretarial assistance.
This work was supported in part by grants-in-aid for scientific research and Targeted Proteins Research Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by CREST (Core Research for Evolutional Science and Technology) of the Japan Science and Technology Agency.
- TPR
- tetratricopeptide repeat
- SH
- Src homology
- PB
- Phox and Bem.
REFERENCES
- 1.Nathan C. (2006) Nat. Rev. Immunol. 6, 173–182 [DOI] [PubMed] [Google Scholar]
- 2.Nauseef W. M. (2007) Immunol. Rev. 219, 88–102 [DOI] [PubMed] [Google Scholar]
- 3.Geiszt M., Leto T. L. (2004) J. Biol. Chem. 279, 51715–51718 [DOI] [PubMed] [Google Scholar]
- 4.Quinn M. T., Gauss K. A. (2004) J. Leukocyte Biol. 76, 760–781 [DOI] [PubMed] [Google Scholar]
- 5.Sumimoto H., Miyano K., Takeya R. (2005) Biochem. Biophys. Res. Commun. 338, 677–686 [DOI] [PubMed] [Google Scholar]
- 6.Groemping Y., Rittinger K. (2005) Biochem. J. 386, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambeth J. D., Kawahara T., Diebold B. (2007) Free Radic. Biol. Med. 43, 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumimoto H. (2008) FEBS J. 275, 3249–3277 [DOI] [PubMed] [Google Scholar]
- 9.Finan P., Shimizu Y., Gout I., Hsuan J., Truong O., Butcher C., Bennett P., Waterfield M. D., Kellie S. (1994) J. Biol. Chem. 269, 13752–13755 [PubMed] [Google Scholar]
- 10.Leusen J. H., Fluiter K., Hilarius P. M., Roos D., Verhoeven A. J., Bolscher B. G. (1995) J. Biol. Chem. 270, 11216–11221 [DOI] [PubMed] [Google Scholar]
- 11.Mizuki K., Takeya R., Kuribayashi F., Nobuhisa I., Kohda D., Nunoi H., Takeshige K., Sumimoto H. (2005) Arch. Biochem. Biophys. 444, 185–194 [DOI] [PubMed] [Google Scholar]
- 12.Heyworth P. G., Bohl B. P., Bokoch G. M., Curnutte J. T. (1994) J. Biol. Chem. 269, 30749–30752 [PubMed] [Google Scholar]
- 13.Kuribayashi F., Nunoi H., Wakamatsu K., Tsunawaki S., Sato K., Ito T., Sumimoto H. (2002) EMBO J. 21, 6312–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koga H., Terasawa H., Nunoi H., Takeshige K., Inagaki F., Sumimoto H. (1999) J. Biol. Chem. 274, 25051–25060 [DOI] [PubMed] [Google Scholar]
- 15.Lapouge K., Smith S. J., Walker P. A., Gamblin S. J., Smerdon S. J., Rittinger K. (2000) Mol. Cell 6, 899–907 [DOI] [PubMed] [Google Scholar]
- 16.Ago T., Kuribayashi F., Hiroaki H., Takeya R., Ito T., Kohda D., Sumimoto H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4474–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumimoto H., Kamakura S., Ito T. (2007) Sci. STKE 2007, re6. [DOI] [PubMed] [Google Scholar]
- 18.Suh C. I., Stull N. D., Li X. J., Tian W., Price M. O., Grinstein S., Yaffe M. B., Atkinson S., Dinauer M. C. (2006) J. Exp. Med. 203, 1915–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellson C. D., Davidson K., Ferguson G. J., O'Connor R., Stephens L. R., Hawkins P. T. (2006) J. Exp. Med. 203, 1927–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueyama T., Kusakabe T., Karasawa S., Kawasaki T., Shimizu A., Son J., Leto T. L., Miyawaki A., Saito N. (2008) J. Immunol. 181, 629–640 [DOI] [PubMed] [Google Scholar]
- 21.Bissonnette S. A., Glazier C. M., Stewart M. Q., Brown G. E., Ellson C. D., Yaffe M. B. (2008) J. Biol. Chem. 283, 2108–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maehara Y., Miyano K., Sumimoto H. (2009) Biochem. Biophys. Res. Commun. 379, 589–593 [DOI] [PubMed] [Google Scholar]
- 23.Yuzawa S., Miyano K., Honbou K., Inagaki F., Sumimoto H. (2009) J. Innate Immun. 1, 543–555 [DOI] [PubMed] [Google Scholar]
- 24.Hata K., Takeshige K., Sumimoto H. (1997) Biochem. Biophys. Res. Commun. 241, 226–231 [DOI] [PubMed] [Google Scholar]
- 25.Han C. H., Freeman J. L., Lee T., Motalebi S. A., Lambeth J. D. (1998) J. Biol. Chem. 273, 16663–16668 [DOI] [PubMed] [Google Scholar]
- 26.Grizot S., Fieschi F., Dagher M. C., Pebay-Peyroula E. (2001) J. Biol. Chem. 276, 21627–21631 [DOI] [PubMed] [Google Scholar]
- 27.Miyano K., Koga H., Minakami R., Sumimoto H. (2009) Biochem. J. 422, 373–382 [DOI] [PubMed] [Google Scholar]
- 28.Taura M., Miyano K., Minakami R., Kamakura S., Takeya R., Sumimoto H. (2009) Biochem. J. 419, 329–338 [DOI] [PubMed] [Google Scholar]
- 29.Price M. O., McPhail L. C., Lambeth J. D., Han C. H., Knaus U. G., Dinauer M. C. (2002) Blood 99, 2653–2661 [DOI] [PubMed] [Google Scholar]
- 30.Bánfi B., Clark R. A., Steger K., Krause K. H. (2003) J. Biol. Chem. 278, 3510–3513 [DOI] [PubMed] [Google Scholar]
- 31.Geiszt M., Lekstrom K., Witta J., Leto T. L. (2003) J. Biol. Chem. 278, 20006–20012 [DOI] [PubMed] [Google Scholar]
- 32.Takeya R., Ueno N., Kami K., Taura M., Kohjima M., Izaki T., Nunoi H., Sumimoto H. (2003) J. Biol. Chem. 278, 25234–25246 [DOI] [PubMed] [Google Scholar]
- 33.Cheng G., Lambeth J. D. (2004) J. Biol. Chem. 279, 4737–4742 [DOI] [PubMed] [Google Scholar]
- 34.Miyano K., Ueno N., Takeya R., Sumimoto H. (2006) J. Biol. Chem. 281, 21857–21868 [DOI] [PubMed] [Google Scholar]
- 35.Nisimoto Y., Motalebi S., Han C. H., Lambeth J. D. (1999) J. Biol. Chem. 274, 22999–23005 [DOI] [PubMed] [Google Scholar]
- 36.Diebold B. A., Bokoch G. M. (2001) Nat. Immunol. 2, 211–215 [DOI] [PubMed] [Google Scholar]
- 37.Sarfstein R., Gorzalczany Y., Mizrahi A., Berdichevsky Y., Molshanski-Mor S., Weinbaum C., Hirshberg M., Dagher M. C., Pick E. (2004) J. Biol. Chem. 279, 16007–16016 [DOI] [PubMed] [Google Scholar]
- 38.Berdichevsky Y., Mizrahi A., Ugolev Y., Molshanski-Mor S., Pick E. (2007) J. Biol. Chem. 282, 22122–22139 [DOI] [PubMed] [Google Scholar]
- 39.Kao Y. Y., Gianni D., Bohl B., Taylor R. M., Bokoch G. M. (2008) J. Biol. Chem. 283, 12736–12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyano K., Sumimoto H. (2007) Biochimie 89, 1133–1144 [DOI] [PubMed] [Google Scholar]
- 41.Ueno N., Takeya R., Miyano K., Kikuchi H., Sumimoto H. (2005) J. Biol. Chem. 280, 23328–23339 [DOI] [PubMed] [Google Scholar]
- 42.Ueyama T., Geiszt M., Leto T. L. (2006) Mol. Cell. Biol. 26, 2160–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng G., Ritsick D., Lambeth J. D. (2004) J. Biol. Chem. 279, 34250–34255 [DOI] [PubMed] [Google Scholar]
- 44.Bánfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., Krause K. H. (2004) J. Biol. Chem. 279, 46065–46072 [DOI] [PubMed] [Google Scholar]
- 45.Kawahara T., Lambeth J. D. (2007) BMC Evol. Biol. 7, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takemoto D., Tanaka A., Scott B. (2006) Plant Cell 18, 2807–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takemoto D., Tanaka A., Scott B. (2007) Fungal Genet. Biol. 44, 1065–1076 [DOI] [PubMed] [Google Scholar]
- 48.Cano-Domínguez N., Alvarez-Delfín K., Hansberg W., Aguirre J. (2008) Eukaryot. Cell 7, 1352–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng G., Diebold B. A., Hughes Y., Lambeth J. D. (2006) J. Biol. Chem. 281, 17718–17726 [DOI] [PubMed] [Google Scholar]