Abstract
The extracellular protease plasmin cleaves mouse MCP1 (monocyte chemoattractant protein 1) at lysine 104, releasing a 50-amino acid C-terminal domain. The cleavage event increases the chemotactic activity of MCP1 and, by doing so, promotes the progression of excitotoxic injury in the central nervous system in pathological settings. The mechanism through which the cleavage event enhances MCP1-mediated chemoattraction is unknown; to investigate it, we use wild-type and mutant forms of recombinant MCP1. Full-length MCP1 (FL-MCP1) is secreted by cells as a dimer or multimer. We show that a mutant truncated at the C terminus, K104Stop-MCP1, does not dimerize, revealing that the C terminus mediates the interaction. MCP1 interacts with the monocyte/microglia receptor CCR2. The interaction is critical to the function of MCP1 because CCR2−/− microglia do not undergo chemotaxis in response to MCP1 stimulation. We show that stimulation of microglia with FL-MCP1 or K104Stop-MCP1 triggers CCR2 internalization, whereas a mutant form unable to be cleaved at lysine 104 (K104A-MCP1) is relatively ineffective in this assay, suggesting that the C-terminal region interferes with the MCP1-CCR2 interaction. Moreover, FL-MCP1 and K104Stop-MCP1 stimulation leads to activation of Rac1, a small GTPase involved in cell migration. Conversely, MCP1-stimulated microglial migration is blocked by the Rac1 inhibitor, NSC23766, demonstrating the requirement for Rac1 effector pathways in this response. Taken together, we propose a model for MCP1 localization, activation, and function based on the initial presence and then removal of its C terminus, coupled with a requisite downstream signaling pathway from CCR2 stimulation to Rac1 activation.
Keywords: Cell Migration, Chemokines, Mouse, Plasmin, Protease, Activation, Microglia
Introduction
Microglia, the immune-like cells normally present in the brain, derive from the bone marrow (1–5), enter the central nervous system (CNS) early during development, and reside in the parenchyma in a resting state characterized by ramified morphology. In the healthy brain, they continually extend and retract their processes to sense changes in the surrounding microenvironment (6). However, when there is an injury in the CNS, microglia switch to an activated state characterized by changes in gene expression, morphology, and proliferation (6–16). The activated microglia then migrate to the site of injury and modify the injury outcome.
The migration of microglia to the site of injury is stimulated by the local release of chemokines, a superfamily of structurally related small proteins that function as chemoattractants. A potent chemoattractant for monocytes/microglia (17), MCP1 (monocyte chemoattractant protein-1; also called CCL2), is up-regulated in many types of CNS injury, including ischemia, hemorrhage, trauma, infection, hypoxia, and peripheral nerve axotomy (18–24). The MCP1 protein is highly conserved in the N terminus among different species, whereas the C terminus is much more variable. The rodent MCP1 C terminus is decorated extensively with O-linked carbohydrates (25), which bind both soluble glycosaminoglycans (GAGs) and GAGs immobilized on the cell surface. Soluble GAGs inhibit the binding of MCP1 to its high affinity receptor CCR2 (30), which is expressed by microglia, astrocytes, and microvascular endothelial cells in the brain (26, 27). Cell surface GAGs, on the other hand, have been reported to concentrate MCP1 locally, promote MCP1 oligomerization, and thus facilitate the binding of MCP1 to CCR2 (28–31). Whether MCP1 functions as a monomer or homodimer, however, is still under debate. Although it is believed that MCP1 exerts its function as a homodimer (25, 32), it has also been suggested that MCP1 can bind to CCR2 and induce downstream signaling as a monomer (33, 34). It should be noted, however, that these experiments were conducted using human MCP1, not rodent MCP1, which contains a heavily glycosylated C-terminal extension.
Previous studies in our laboratory have shown that 1) the highly glycosylated C-terminal extension of mouse MCP1 is removed by plasmin, the active protease of plasminogen, and 2) the C terminus-truncated mouse MCP1 has a higher chemotactic potency than the full-length mouse MCP1 (35). Additionally, the fact that infusion of mouse MCP1 lacking the C terminus into plasminogen-deficient mice, which are more resistant to excitotoxic injury, restored microglial migration and neuronal loss, unlike the intact mouse MCP1, further suggests that the C-terminal extension negatively regulates the chemotactic ability of mouse MCP1 (35). How exactly the highly glycosylated C terminus regulates mouse MCP1 function, however, remains to be elucidated. Herein, we investigated several potential mechanisms: dimerization/oligomerization of mouse MCP1, interaction of MCP1 with CCR2, and intracellular signaling. We find that the C terminus of mouse MCP1 is required for MCP1 dimerization, attenuates the MCP1-CCR2 interaction, and suppresses MCP1-induced Rac activation and subsequent microglial migration.
EXPERIMENTAL PROCEDURES
Cell Culture
N9 cells were originally provided by Drs. S. Barger (University of Arkansas, Fayetteville, AR) and P. Ricciardi-Castagnoli (University of Milano-Bicocca, Milan, Italy). The cells were maintained in minimum Eagle's medium (MEM)2 supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C with 5% CO2.
Primary microglia were prepared from mixed cortical cultures, as described previously, with minor modifications (9). Briefly, brains of 1-day-old pups from wild-type or CCR2−/− mice were collected. After removing meninges and hippocampi, the cortical tissue was digested with trypsin (0.25% in Hanks' balanced saline solution) for 15 min at 37 °C. The tissue was mechanically dissociated by trituration and filtered through a 70-μm nylon mesh. The cell suspensions were plated onto poly-d-lysine-coated tissue culture dishes. Cultures were maintained in DMEM supplemented with 10% FBS. The medium was changed on day 3 and 14, and the microglia were collected through the addition of 15 mm lidocaine for 10 min at room temperature followed by centrifugation. The microglia were used for a migration assay immediately or maintained in DMEM with 1% FBS for 2 days before use in other experiments. Human embryonic kidney 293 (HEK293) cells were maintained in DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Cell Treatment
N9 cells or primary microglia were treated with 10 nm recombinant MCP1 proteins.
Generation of His6-tagged MCP1 Proteins
FL-, K104A-, and K104Stop-MCP1 were subcloned without the signal peptide into pET vectors with an N-terminal His6 tag. A C-terminal extension fragment (CT-MCP1) was subcloned into a pTYB1 vector with an N-terminal His6 tag. BL21 cells were transformed with these constructs, and expression of the target proteins was induced for 5 h using isopropyl-β-d-thiogalactopyranoside. Recombinant proteins were purified using cobalt affinity resins (Clontech) according to the manufacturer's instructions. The fractions were analyzed on 16% Tris-Tricine SDS-PAGE by Coomassie Blue staining. The purified proteins were confirmed by immunoblotting using 1:1000 anti-MCP1 antibody (Serotec and Cell Sciences) or 1:1000 anti-His6 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Transient Transfection
FL-, K104A-, and K104Stop-MCP1 subcloned into pcDNA3.1 vectors with N-terminal c-Myc or HA tags were transfected into HEK293 cells using Lipofectamine (Invitrogen) according to the manufacturer's protocol. Two days later, cell lysates were collected and used for co-immunoprecipitation (co-IP).
Co-immunoprecipitation
Mixtures of lysates of cells expressing c-Myc- and HA-tagged MCP1 proteins were precleared with protein A/G-agarose beads, incubated with rabbit anti-c-Myc (Sigma) or rat anti-HA (Roche Applied Science) antibody overnight at 4 °C, and the immunocomplexes were pulled down using protein A/G-agarose beads (Santa Cruz Biotechnology, Inc.) for 2 h at 4 °C and centrifugation. Pelleted proteins were subjected to immunoblot analysis with the anti-c-Myc and -HA antibodies to demonstrate equal expression and assess co-IP.
In Vitro Pull-down Assay
Purified recombinant His6-MCP1 and c-Myc-MCP1 proteins were mixed and co-IP as above, using anti-c-Myc and anti-His6 antibody (Roche Applied Science).
Western Blot Analysis and Immunoblotting
Cells were lysed in radioimmune precipitation buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mm PMSF, 1× protease inhibitor mixture, 1× Na3VO4). Protein concentrations were determined by a DC (Bio-Rad) protein assay. Lysates containing equal amounts of protein were resolved in 10 or 15% sodium SDS-PAGE, transferred to Immobilon-P transfer membranes (Millipore), blocked in 5% nonfat milk or Odyssey blocking buffer for 1 h at room temperature, incubated with primary antibodies overnight at 4 °C, washed, incubated with 1:5000 HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) or IRDye secondary antibodies (LI-COR) for 1 h at room temperature washed, and developed using ECL (Pierce) or scanned using Odyssey infrared imaging (LI-COR).
Rac Activity Assay
Activated Rac was quantified through specific interaction with its downstream effector p21-activated kinase (PAK) (36). PAK-binding domain purified as a GST fusion protein was immobilized to glutathione beads (generously provided by Dr. J. Prives, Stony Brook University). Microglia stimulated with 10 nm MCP proteins for varied times were lysed in radioimmune precipitation buffer. The activated Rac was pulled down using the GST-PAK-binding domain beads and detected by immunoblotting using mouse anti-Rac1 antibody. Total Rac1 was determined by immunoblotting samples of the cell lysates. Band intensities were quantified using Scion Image (Scion, Frederick, MD) or Odyssey Infrared Imaging. Rac activation in stimulated cells was normalized to the amount of activated Rac in control microglia.
Membrane Sheet Assay
This was performed as described previously with minor modifications (37). Briefly, primary microglia were plated on coverslips and activated with 100 ng/ml LPS overnight. The cells were then treated with 10 nm recombinant MCP1 for 1 h at 37 °C, followed by swelling in hypotonic buffer (25 mm KCl, 10 mm Hepes, 2 mm MgCl2, 1 mm EGTA, 1 mm PMSF, 1× protease inhibitor mixture, pH 7.5) for 20 min. After PBS washing, the cells were fixed with 4% paraformaldehyde, permeabilized, and stained with 1:1000 rat-anti-Mac2 antibody (BD Pharmingen) and 1:500 rabbit anti-CCR2 antibody (Epitomics), followed by 1:1000 fluorescence-conjugated secondary antibodies (Invitrogen). The cells were then imaged using Zeiss LSM510 confocal microscopy.
Immunofluorescent Imaging of the F-actin Cytoskeleton
To mimic the endogenous chemoattractant gradient, 12-well plates were spotted at the edges of the wells with heparin, which avidly binds MCP1. 15 μl of recombinant MCP1 proteins were then added directly onto the dried heparin spot to create an MCP1 point source. 1 h later, primary microglia attached to coverslips were placed in the well and cultured for varied periods of time, washed, and fixed with 4% paraformaldehyde for 15 min at room temperature. After PBS washing, the cells were incubated with 1:20 Alexa-647 phalloidin (Invitrogen) overnight at room temperature, washed, mounted using FluorMount with DAPI, and imaged using confocal microscopy.
Migration Assay
Migration assays were performed using chemotactic chambers (Boyden, NeuroProbe). Recombinant mouse MCP1 proteins or human MCP1 suspended in MEM with or without the Rac inhibitor, NSC23766, were added to the bottom wells of the chamber. Wild-type or CCR2−/− primary microglia suspended in MEM (5 × 105/ml) with or without the Rac inhibitor were added to the top wells, with a 5.0-μm filter inserted between the chemoattractants and microglial cells. Migration was allowed to proceed for 2 h at 37 °C. Cells that did not migrate into the membrane were wiped off, and cells that migrated into or through the membrane were fixed and stained with hematoxylin for 10 min at room temperature. The membranes were photographed at 100× magnification. Total migration was quantified by counting stained cells. The background for random cell movement (cells responding to MEM only) was subtracted.
Statistics
Statistics were performed using the two-tailed t test. *, p < 0.05 was considered significant. Error bars indicate the S.E.
RESULTS
The C Terminus of MCP1 Enables Multimerization
GAG modifications can enhance the local concentration of chemokines and facilitate their homodimerization/oligomerization (28–30). Mouse MCP1 has a highly GAG-glycosylated C-terminal extension (25). Because plasmin removes the C terminus of mouse MCP1 (35), this processing event could affect homodimerization/oligomerization for MCP1. To examine the role of MCP1 C-terminal extension in this context, co-IPs were performed using HEK293 cells transfected with HA- and c-Myc-tagged FL-MCP1, a full-length mutant that cannot be processed by plasmin (K104A) (supplemental Fig. 1), or a C-terminally truncated mutant that terminates at the plasmin cleavage site (K104Stop). We observed that the full-length protein and the plasmin-resistant mutant K104A readily form stable homodimers/oligomers (Fig. 1A). In contrast, however, homodimers/oligomers were not observed for the K104Stop.
FIGURE 1.
Homodimerization of recombinant mouse MCP1 proteins. A, HEK293 cells transfected with HA- and c-Myc-tagged FL-, K104A-, or K104Stop-MCP1 were lysed, immunoprecipitated with anti-c-Myc antibody, and immunoblotted (WB) using anti-HA antibody. Note that the 25-kDa IgG light chain runs just above the 23-kDa epitope-tagged FL- and K104A-MCP1 proteins but is easily distinguished from K104Stop MCP1, which is 6 kDa smaller than FL-MCP1. The lysate lane shows the equal levels of expression of the HA-tagged version of the three forms of MCP1. B, purified recombinant His6- and c-Myc-tagged MCP1 proteins were immunoprecipitated with anti-His6 antibody and immunoblotted with anti-c-Myc antibody. Shown is an experiment representative of four performed with similar results.
To determine whether MCP1 forms homodimers or whether individual MCP1 proteins associate indirectly by forming complexes with other proteins, the co-IP was performed using purified recombinant proteins. FL-MCP1 and K104A-MCP1 exhibited the ability to form stable homodimers/oligomers, and as expected, K104Stop-MCP1 failed to do so (Fig. 1B). Similar results were observed regardless of which anti-epitope antibody was used for the co-IP (data not shown). However, the isolated CT-MCP1 did not co-immunoprecipitate with FL-, K104A-, or K104Stop-MCP1 (data not shown), suggesting that the C-terminal extension is necessary but not sufficient for the interaction.
These data indicate that full-length MCP1 dimerizes/oligomerizes and that the plasmin cleavage event converts it to a monomeric form. A number of studies have reported that MCP1, like most other chemokines, binds to its receptor, CCR2, and exerts its function as a homodimer (25, 32). However, there is also evidence suggesting that it functions as a monomer (33, 34). To address whether MCP1 functions as a monomer or homodimer, we further investigated the MCP1-CCR2 interaction and intracellular signaling using these recombinant proteins.
N Terminus-containing MCP1 Proteins Reduce Membrane-bound CCR2
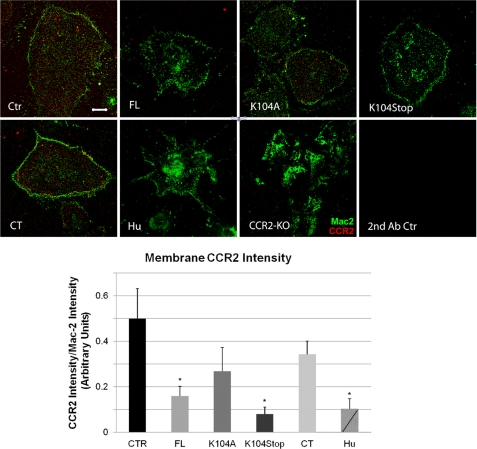
CCR2 is a critical receptor for MCP1 (34, 38) because CCR2-deficient microglia and macrophages fail to migrate in response to MCP1 stimulation (39, 40). CCR2 is expressed in the mouse CNS by microglia, astrocytes, and microvascular endothelial cells (26, 27). We used microglia to assess how modulation of the MCP1 protein structure affects CCR2 responses. Microglia were stimulated with human MCP1 or mouse wild-type and modified forms of MCP1 and assessed for changes in CCR2 amount and subcellular distribution. To undertake this study, we employed a commercial anti-CCR2 antiserum (Biovision), which detects an immunoreactive band present in brain lysates prepared from wild-type mice but not in ones prepared from CCR2−/− mice (supplemental Fig. 2). Treatment of microglia with wild-type and modified forms of MCP1 did not alter the total levels of CCR2 over a 2-h period, indicating that stimulation-induced receptor down-regulation and degradation does not occur within this time frame (supplemental Fig. 3). To examine potential changes in subcellular localization, the amount of CCR2 at the plasma membrane was assessed using a membrane sheet assay. In non-stimulated cells, CCR2 is observed in a punctate distribution on the plasma membrane (Fig. 2, Ctr). Stimulation of the microglia with full-length MCP1 (FL) greatly decreased the amount of plasma membrane-associated MCP1, presumably via receptor endocytosis. Similar findings were observed for K104Stop-MCP1, indicating the lack of requirement for the C-terminal extension for receptor engagement, and the C-terminal extension by itself (CT-MCP1) did not cause plasma membrane-associated CCR2 to decrease. Moreover, the decrease in plasma membrane-associated CCR2 was much less substantial for K104A-MCP1, suggesting that the presence of the C-terminal extension interferes with receptor engagement. Finally, incubation with the human MCP1, which naturally lacks the C-terminal extension of the murine protein, resulted in a significant decrease of the plasma-membrane associated CCR2 at levels comparable with those of K104Stop-MCP1. These findings are consistent with a previous report that MCP1 binds to CCR2 via its N terminus (41) and newly suggest that the C-terminal extension of mouse MCP1 interferes with the interaction between the MCP1 N terminus and CCR2.
FIGURE 2.
Stimulation of microglia by N terminus-containing MCP1 proteins triggers CCR2 internalization. Primary microglia from wild-type mice activated overnight with 100 ng/ml LPS were treated with saline (Ctr) or 10 nm recombinant MCP1 proteins, rodent (FL, K104A, K104Stop, and CT) or human (Hu), for 1 h and then swelled in hypotonic buffer for 20 min to cause cell lysis. The plasma membrane sheets that remained adhered to the coverslips were stained for Mac-2 (green) to visualize the membrane sheets and CCR2 (red). CCR2−/− microglia (CCR2 KO) served as a control for the CCR2 antibody. Microglia were also immunostained with secondary antibodies (2nd Ab Ctr) only. Scale bar, 10 μm. The fluorescence intensity was quantified in 20 cells/condition in three separate experiments. The results were analyzed with one-way analysis of variance plus Dunn's test (*, p < 0.05). Error bars, S.D.
Plasmin-truncated MCP1 Exhibits Increased Chemotactic Activity
Migration of microglia in response to stimulation by the modified MCP1 proteins was next examined using a chemotaxis Boyden chamber assay. Wild-type MCP1 (FL) elicited a modest migratory response (140 ± 20 cells; Fig. 3). In contrast, K104A provoked a weaker response (60 ± 15 cells), again suggesting that the presence of the C-terminal extension interferes with receptor engagement/stimulation. Conversely, K104Stop triggered a much stronger response (460 ± 28 cells) than wild-type MCP-1, and the C-terminal extension by itself (CT-MCP1) caused no response, both confirming the lack of requirement for the C-terminal extension and suggesting that processing to remove the C-terminal extension is a key step in efficient CCR2 stimulation. Of note, the chemotactic activity of mouse K104Stop-MCP1 was comparable with that of human MCP1. CCR2−/− primary microglia failed to migrate in response to MCP1 recombinant proteins in this assay, confirming the indispensable role of the CCR2 receptor in MCP1-induced microglial migration.
FIGURE 3.
C-terminal truncation of MCP1 has much higher chemotactic potency and the chemotactic activity depends on Rac1. Primary microglia from wild-type or CCR2−/− mice were plated and evaluated for chemotaxis in response to recombinant MCP1 proteins, in the presence or absence of the Rac1 inhibitor NSC23766. Each experimental condition was assayed in triplicate. Data are expressed as mean ± S.D. **, p < 0.01; n.s., not significant; error bars, S.D.
Plasmin-mediated Cleavage of MCP1 Promotes Rac1 Activation
MCP1 engagement of CCR2 activates intracellular signaling cascades that differ depending on the cell type (38, 42). In microglia, one of these signaling pathways involves activation (GTP loading) of Rac, a member of the Rho family of small GTPases (38, 43). The activated Rac then promotes protrusion of lamellipodia and cell migration (38, 44, 45). As a molecular switch, Rac1 needs to be activated transiently and then deactivated. We assessed over time the activation of Rac using N9 microglial cells. FL-MCP1 induced a moderate level of Rac1 activation over the 90-min time course of the assay (Fig. 4A), and this increase was dependent on CCR2 stimulation because no Rac activation was seen in CCR2−/− primary microglia. In contrast, K104Stop-MCP1 provoked a rapid and dramatic increase in Rac1 activation in 5 min that was also extinguished rapidly, whereas K104A-MCP1 prompted a very weak response. These data again support the earlier findings that the C-terminal extension inhibits receptor engagement and mobilization.
FIGURE 4.
C-terminal truncation of MCP1 enhances the activation of Rac1. N9 microglial cells were treated with 10 nm MCP1 over time. Activated Rac1 was immunoprecipitated using GST-PAK-binding domain beads. At each time point, the activated and total Rac1 were detected by Western blotting. A, Western blots of activated and total Rac1 for human MCP1 and mouse FL-, K104A-, and K104Stop-MCP1 (both wild-type and CCR2−/− cells) and CT-MCP1. B, quantification of the blots in A. The blots were normalized to the value at zero time. The CT-MCP1 blot was also quantified but not shown on the graph because the values were negligible at all time points. Each experimental condition was assayed in triplicate, and the data were expressed as mean ± S.D. *, p < 0.05. Error bars, S.D.
As expected, the CT-MCP1 by itself did not cause Rac activation (Fig. 4). Surprisingly, unlike K104Stop-MCP1, human MCP1 induced a much lower and delayed Rac1 activation; it induced a 2-fold change 15 min after treatment. This effect, however, dissipated rapidly. These data suggest that the transient activation of Rac1 plays an important role in cell migration.
To assess whether activation of Rac1 is necessary for the stimulation of microglial migration, NSC23766, a Rac1-specific inhibitor, was employed. NSC23766 fully blocked microglial migration in response to MCP1 (Fig. 3).
Plasmin-mediated Cleavage of MCP1 Promotes the Formation of Lamellipodia
Lamellipodia, which are cytoskeletal actin projections at the leading edges of cells observed during cell migration, are induced by the activation of Rac1 (38, 43, 46). We thus investigated whether MCP1 truncation by plasmin induced lamellipodia formation. After incubation of primary microglia in the presence of a localized source of MCP1 to create a chemotactic gradient for different periods of time, the microglia were fixed and stained with phalloidin. Most of the control cells cultured in the absence of MCP1 exhibited actin cytoskeletal projections but did so in a uniform, non-polarized manner (Fig. 5, Ctr). Only 5–8% of the control cells exhibited lamellipodia (polarized actin cytoskeletal projections). In contrast, 20% of cells stimulated with a gradient of FL-MCP1 exhibited polarized actin cytoskeletal reorganization, and 50% of cells stimulated with K104Stop-MCP1 displayed lamellipodia, which were exaggerated in appearance. Similar to K104Stop-MCP1, 40% of human MCP1-treated cells showed unipolar lamellipodia. K104A-MCP1 and CT-MCP1, however, did not promote cellular polarization, suggesting that plasmin-mediated cleavage of MCP1 is critical for cell migration and the formation of lamellipodia.
FIGURE 5.
C-terminal truncation of MCP1 promotes the formation of lamellipodia. Primary microglia plated on coverslips were incubated with a point source of recombinant MCP1 protein (mouse or human (Hu)) for different periods of time. At each time point, the cells were washed, fixed, and stained for actin cytoskeleton using Alexa-Phalloidin. Scale bar, 20 μm. The percentage of unipolar cells (migrating toward the focal point of MCP1 protein; arrows) was quantified. Data are expressed as mean ± S.D. (error bars). n = 8. *, p < 0.05.
The Cleavage of MCP1 by Plasmin Does Not Affect the MAPK Cascade
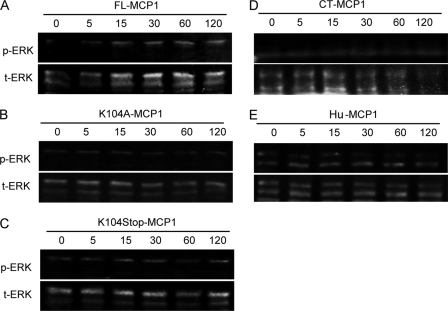
During microglial activation and migration, changes in gene expression and morphology also occur (6, 7, 16). To investigate whether MCP1 may affect characteristics of microglia activation, such as proliferation and differentiation, we explored other signaling pathways that could be involved. MAPK has been implicated in many cellular processes, including proliferation, differentiation, and apoptosis (47). Many factors acting through G protein-coupled receptors lead to the activation of MAPK cascades and finally activate ERK1/2 (48).
N9 cells were cultured with or without human MCP1 and recombinant mouse MCP1 proteins, and the levels of phosphorylated and total ERK1/2 were analyzed by Western blotting (Fig. 6). FL- and K104A-MCP1 promoted low level and prolonged ERK1/2 activation (1.2–1.4-fold change over the course of 120 min). Although K104Stop-MCP1 and human MCP1 also induced ERK1/2 phosphorylation, the activation was detectable as early as in 5 min and returned to base line in 30 min, indicating a higher potency. CT-MCP1, on the other hand, failed to activate ERK1/2. As a positive control, LPS dramatically enhanced phosphorylated ERK1/2. These data suggest that K104Stop-MCP1 is more active than the FL- or K104A-MCP1 in activation of the MAPK cascade, and its effect was comparable with human MCP1, but the major and more dramatic effect was evident on Rac activation.
FIGURE 6.
C-terminal truncation of MCP1 activates MAPK pathways more potently. Microglial cells were exposed to human MCP1 or recombinant mouse MCP1 proteins. At the indicated time points, the cells were lysed and analyzed for phosphorylated and total ERK1/2 by Western blotting. Hu, human.
DISCUSSION
MCP1 is a potent chemoattractant for monocytes and microglia. It has been reported to be involved in many diseases, including asthma (49, 50), rheumatoid arthritis (51, 52), and atherosclerosis (53, 54). In addition, MCP1 plays a very important role in excitotoxic injury. It has been shown that kainate injection induces excitotoxic injury, leading to microglial migration and neuronal death in wild-type mice. Previous studies in our laboratory reported that the kainate-induced microglial activation/migration and neuronal death were attenuated in mice deficient in MCP1 or plasminogen, the inactive precursor of plasmin (35, 55, 56). Further studies in our laboratory show that plasmin removes the highly glycosylated C terminus of mouse MCP1, and it is the truncated MCP1, not the intact MCP1, that is responsible for microglial migration and neuronal loss (35), thus identifying plasmin as an activator of MCP1 and offering an explanation for the diminished microglial recruitment in plasminogen−/− mice. However, the mechanisms underlying the enhanced biological functions of plasmin-cleaved MCP1 are not known. The data presented in this study argue that 1) plasmin-mediated cleavage of mouse MCP1 abrogates its potential for dimerization/oligomerization, 2) the C terminus of mouse MCP1 alone cannot bind to CCR2, 3) plasmin cleavage is required to engage the CCR2 receptor, 4) the monomer form is needed to cause engaged receptor to initiate signaling to Rac, 5) the Rac pathway is activated and critical, and 6) ERK is also activated but less dramatically.
GAGs have been reported to increase local chemokine concentration and promote the oligomerization of chemokines both in vitro and in vivo (28–31). The C terminus of MCP1 is heavily O-glycosylated and has many GAG modifications (25). Thus, the C terminus of MCP1 has been speculated to promote MCP1 dimerization/oligomerization or stabilize the dimerization/oligomerization (25, 31). Consistent with this speculation, K104Stop-MCP1, which does not have the highly glycosylated C-terminal extension, did not homodimerize/oligomerize; this finding supports the hypothesis that the C-terminal extension of mouse MCP1 is necessary for its dimerization/oligomerization. Because the C-terminal fragment alone failed to form a stable dimer/oligomer with FL-, K104A-, or K104Stop-MCP1, it appears that both the N terminus and C terminus contribute to the formation of stable homodimers.
Upon ligand binding, many receptors, such as the interleukin-8 receptor, the μ-opioid receptor, and the pattern recognition receptor, undergo internalization (57–59). Like these receptors, CCR2 is also expected to translocate from the plasma membrane to intracellular vesicles upon MCP1 binding (60). Recently, by using GFP-CCR2-transfected cells or GFP-CCR2 transgenic mice, Jung et al. (61) have shown that upon MCP1 binding, CCR2 undergoes endocytosis. Consistent with this report, we showed that those N terminus-containing MCP1 proteins (FL-, K104A-, and K104Stop-MCP1) induced a significant reduction of membrane CCR2. CT-MCP1, because it does not have the N region of the MCP1 protein, failed to decrease membrane-bound CCR2. A significant decrease of plasma CCR2 was also revealed after treatment with the human MCP1, supporting the idea that the C terminus of mouse MCP1 hinders MCP1-CCR2 interaction.
Our data indicated that the binding of monomeric N-terminal domains of MCP1 to CCR2-activated Rac and induced the protrusion of lamellipodia, leading to microglial migration. We therefore propose a model to describe how mouse MCP1 may interact with CCR2 in the CNS. Upon secretion, mouse MCP1 binds via its glycosylated C terminus to neuronal cell membranes, an event that increases local MCP1 concentration. This increase in local MCP1 promotes the formation of dimers or higher order aggregates, which thus generate a point source for a potential chemokine gradient. Upon neuronal injury or exaggerated stimulation, the neurons form and secrete plasmin, which then cleaves and removes the C-terminal, GAG-glycosylated extension of mouse MCP1, thus abrogating MCP1 dimers. These truncated MCP1 monomers are more easily diffusible and able to form potent chemotactic gradients, bind to CCR2 on microglia, and initiate microglial intracellular signaling pathways. The pathways include activation of Rac1, rearrangement of the actin cytoskeleton with protrusion of lamellipodia, and eventual promotion of microglial migration along the MCP1 chemokine gradient. The plasmin-regulated extracellular cleavage step is a functional characteristic for other chemokines, such as cCAF and the hemofiltrate CC chemokine 1 (62, 63). For human MCP-1, the gene appears to have evolved to lose this regulatory cleavage step, since the human primary translated protein lacks the C-terminal extension that is cleaved off of the mouse MCP-1 protein by plasmin. It will be interesting to determine whether other, novel regulatory mechanisms have been developed to compensate for the lack of the plasmin-regulated cleavage step.
It should be noted that 1) unlike the mouse MCP1, human MCP1 does not have a heavily glycosylated C terminus, and 2) human MCP1 and mouse MCP1 truncated at the C terminus (K104Stop-MCP1) are highly homologous. Then a few questions need to be answered. What is the function of mouse MCP1 C-terminal extension? Do humans have an unknown protein that functions like the C-terminal extension of mouse MCP1? Do human and mouse MCP1 use different mechanisms to activate CCR2? Understanding of the functions of carbohydrate on mouse MCP1 will provide important clues for these questions.
Supplementary Material
Acknowledgments
We thank Drs. Robert Watson and Dumaine Williams for suggestions in the membrane sheet assay as well as Drs. Miguel Garcia-Diaz and Elena Yakubovskaya for help with the purification of recombinant MCP1. We also thank Dr. Michael A. Frohman for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant NS42168.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- MEM
- minimum Eagle's medium
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- PAK
- p21-activated kinase
- IP
- immunoprecipitation
- FL-MCP1
- full-length MCP1
- CT-MCP1
- C-terminal extension fragment of MCP1.
REFERENCES
- 1.Chan W. Y., Kohsaka S., Rezaie P. (2007) Brain Res. Rev. 53, 344–354 [DOI] [PubMed] [Google Scholar]
- 2.Ling E. A. (1979) J. Anat. 128, 847–858 [PMC free article] [PubMed] [Google Scholar]
- 3.Hailer N. P., Heppner F. L., Haas D., Nitsch R. (1997) Eur. J. Neurosci. 9, 863–866 [DOI] [PubMed] [Google Scholar]
- 4.Ono K., Takii T., Onozaki K., Ikawa M., Okabe M., Sawada M. (1999) Biochem. Biophys. Res. Commun. 262, 610–614 [DOI] [PubMed] [Google Scholar]
- 5.Wu Y. P., McMahon E., Kraine M. R., Tisch R., Meyers A., Frelinger J., Matsushima G. K., Suzuki K. (2000) Am. J. Pathol. 156, 1849–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nimmerjahn A., Kirchhoff F., Helmchen F. (2005) Science 308, 1314–1318 [DOI] [PubMed] [Google Scholar]
- 7.Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., Littman D. R., Dustin M. L., Gan W. B. (2005) Nat. Neurosci. 8, 752–758 [DOI] [PubMed] [Google Scholar]
- 8.Nakajima K., Kohsaka S. (2004) Curr. Drug Targets Cardiovasc. Haematol. Disord. 4, 65–84 [DOI] [PubMed] [Google Scholar]
- 9.Giulian D., Baker T. J. (1986) J. Neurosci. 6, 2163–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzumura A., Mezitis S. G., Gonatas N. K., Silberberg D. H. (1987) J. Neuroimmunol. 15, 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulvestad E., Williams K., Bjerkvig R., Tiekotter K., Antel J., Matre R. (1994) J. Leukoc. Biol. 56, 732–740 [DOI] [PubMed] [Google Scholar]
- 12.Abromson-Leeman S., Hayashi M., Martin C., Sobel R., al-Sabbagh A., Weiner H., Dorf M. E. (1993) J. Neuroimmunol. 45, 89–101 [DOI] [PubMed] [Google Scholar]
- 13.Aloisi F. (2001) Glia 36, 165–179 [DOI] [PubMed] [Google Scholar]
- 14.Kim S. U., de Vellis J. (2005) J. Neurosci. Res. 81, 302–313 [DOI] [PubMed] [Google Scholar]
- 15.Hanisch U. K., Kettenmann H. (2007) Nat. Neurosci. 10, 1387–1394 [DOI] [PubMed] [Google Scholar]
- 16.Andersson P. B., Perry V. H., Gordon S. (1991) Neuroscience 42, 201–214 [DOI] [PubMed] [Google Scholar]
- 17.Van Der Voorn P., Tekstra J., Beelen R. H., Tensen C. P., Van Der Valk P., De Groot C. J. (1999) Am. J. Pathol. 154, 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimitrijevic O. B., Stamatovic S. M., Keep R. F., Andjelkovic A. V. (2006) J. Cereb. Blood Flow Metab. 26, 797–810 [DOI] [PubMed] [Google Scholar]
- 19.Mahad D. J., Ransohoff R. M. (2003) Semin. Immunol. 15, 23–32 [DOI] [PubMed] [Google Scholar]
- 20.Capoccia B. J., Gregory A. D., Link D. C. (2008) J. Leukoc. Biol. 84, 760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frangogiannis N. G., Dewald O., Xia Y., Ren G., Haudek S., Leucker T., Kraemer D., Taffet G., Rollins B. J., Entman M. L. (2007) Circulation 115, 584–592 [DOI] [PubMed] [Google Scholar]
- 22.Yan Y. P., Sailor K. A., Lang B. T., Park S. W., Vemuganti R., Dempsey R. J. (2007) J. Cereb. Blood Flow Metab. 27, 1213–1224 [DOI] [PubMed] [Google Scholar]
- 23.Morimoto H., Hirose M., Takahashi M., Kawaguchi M., Ise H., Kolattukudy P. E., Yamada M., Ikeda U. (2008) Cardiovasc. Res. 78, 554–562 [DOI] [PubMed] [Google Scholar]
- 24.Kim G. H., Kellner C. P., Hahn D. K., Desantis B. M., Musabbir M., Starke R. M., Rynkowski M., Komotar R. J., Otten M. L., Sciacca R., Schmidt J. M., Mayer S. A., Connolly E. S., Jr. (2008) J. Neurosurg. 109, 38–43 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Ernst C. A., Rollins B. J. (1996) Methods 10, 93–103 [DOI] [PubMed] [Google Scholar]
- 26.Banisadr G., Quéraud-Lesaux F., Boutterin M. C., Pélaprat D., Zalc B., Rostène W., Haour F., Parsadaniantz S. M. (2002) J. Neurochem. 81, 257–269 [DOI] [PubMed] [Google Scholar]
- 27.Ge S., Song L., Serwanski D. R., Kuziel W. A., Pachter J. S. (2008) J. Neurochem. 104, 1219–1232 [DOI] [PubMed] [Google Scholar]
- 28.Hoogewerf A. J., Kuschert G. S., Proudfoot A. E., Borlat F., Clark-Lewis I., Power C. A., Wells T. N. (1997) Biochemistry 36, 13570–13578 [DOI] [PubMed] [Google Scholar]
- 29.Lau E. K., Paavola C. D., Johnson Z., Gaudry J. P., Geretti E., Borlat F., Kungl A. J., Proudfoot A. E., Handel T. M. (2004) J. Biol. Chem. 279, 22294–22305 [DOI] [PubMed] [Google Scholar]
- 30.Kuschert G. S., Coulin F., Power C. A., Proudfoot A. E., Hubbard R. E., Hoogewerf A. J., Wells T. N. (1999) Biochemistry 38, 12959–12968 [DOI] [PubMed] [Google Scholar]
- 31.Handel T. M., Johnson Z., Crown S. E., Lau E. K., Proudfoot A. E. (2005) Annu. Rev. Biochem. 74, 385–410 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Rollins B. J. (1995) Mol. Cell Biol. 15, 4851–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paolini J. F., Willard D., Consler T., Luther M., Krangel M. S. (1994) J. Immunol. 153, 2704–2717 [PubMed] [Google Scholar]
- 34.Paavola C. D., Hemmerich S., Grunberger D., Polsky I., Bloom A., Freedman R., Mulkins M., Bhakta S., McCarley D., Wiesent L., Wong B., Jarnagin K., Handel T. M. (1998) J. Biol. Chem. 273, 33157–33165 [DOI] [PubMed] [Google Scholar]
- 35.Sheehan J. J., Zhou C., Gravanis I., Rogove A. D., Wu Y. P., Bogenhagen D. F., Tsirka S. E. (2007) J. Neurosci. 27, 1738–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Pozo M. A., Price L. S., Alderson N. B., Ren X. D., Schwartz M. A. (2000) EMBO J. 19, 2008–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson L. J., Pang S., Harris D. S., Heuser J., James D. E. (1992) J. Cell Biol. 117, 1181–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terashima Y., Onai N., Murai M., Enomoto M., Poonpiriya V., Hamada T., Motomura K., Suwa M., Ezaki T., Haga T., Kanegasaki S., Matsushima K. (2005) Nat. Immunol. 6, 827–835 [DOI] [PubMed] [Google Scholar]
- 39.El Khoury J., Toft M., Hickman S. E., Means T. K., Terada K., Geula C., Luster A. D. (2007) Nat. Med. 13, 432–438 [DOI] [PubMed] [Google Scholar]
- 40.Chen B. P., Kuziel W. A., Lane T. E. (2001) J. Immunol. 167, 4585–4592 [DOI] [PubMed] [Google Scholar]
- 41.Hemmerich S., Paavola C., Bloom A., Bhakta S., Freedman R., Grunberger D., Krstenansky J., Lee S., McCarley D., Mulkins M., Wong B., Pease J., Mizoue L., Mirzadegan T., Polsky I., Thompson K., Handel T. M., Jarnagin K. (1999) Biochemistry 38, 13013–13025 [DOI] [PubMed] [Google Scholar]
- 42.Stamatovic S. M., Dimitrijevic O. B., Keep R. F., Andjelkovic A. V. (2006) J. Biol. Chem. 281, 8379–8388 [DOI] [PubMed] [Google Scholar]
- 43.Maghazachi A. A. (2000) Int. J. Biochem. Cell Biol. 32, 931–943 [DOI] [PubMed] [Google Scholar]
- 44.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 45.Rickert P., Weiner O. D., Wang F., Bourne H. R., Servant G. (2000) Trends Cell Biol. 10, 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pankov R., Endo Y., Even-Ram S., Araki M., Clark K., Cukierman E., Matsumoto K., Yamada K. M. (2005) J. Cell Biol. 170, 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiménez-Sainz M. C., Fast B., Mayor F., Jr., Aragay A. M. (2003) Mol. Pharmacol. 64, 773–782 [DOI] [PubMed] [Google Scholar]
- 48.Marinissen M. J., Gutkind J. S. (2001) Trends Pharmacol. Sci. 22, 368–376 [DOI] [PubMed] [Google Scholar]
- 49.Lamkhioued B., Garcia-Zepeda E. A., Abi-Younes S., Nakamura H., Jedrzkiewicz S., Wagner L., Renzi P. M., Allakhverdi Z., Lilly C., Hamid Q., Luster A. D. (2000) Am. J. Respir. Crit. Care Med. 162, 723–732 [DOI] [PubMed] [Google Scholar]
- 50.Tillie-Leblond I., Hammad H., Desurmont S., Pugin J., Wallaert B., Tonnel A. B., Gosset P. (2000) Am. J. Respir. Crit. Care Med. 162, 586–592 [DOI] [PubMed] [Google Scholar]
- 51.Kunkel S. L., Lukacs N., Kasama T., Strieter R. M. (1996) J. Leukoc. Biol. 59, 6–12 [DOI] [PubMed] [Google Scholar]
- 52.Koch A. E., Kunkel S. L., Harlow L. A., Johnson B., Evanoff H. L., Haines G. K., Burdick M. D., Pope R. M., Strieter R. M. (1992) J. Clin. Invest. 90, 772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kowala M. C., Recce R., Beyer S., Gu C., Valentine M. (2000) Atherosclerosis 149, 323–330 [DOI] [PubMed] [Google Scholar]
- 54.Chen Y. L., Chang Y. J., Jiang M. J. (1999) Atherosclerosis 143, 115–123 [DOI] [PubMed] [Google Scholar]
- 55.Tsirka S. E., Rogove A. D., Bugge T. H., Degen J. L., Strickland S. (1997) J. Neurosci. 17, 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogove A. D., Siao C., Keyt B., Strickland S., Tsirka S. E. (1999) J. Cell Sci. 112, 4007–4016 [DOI] [PubMed] [Google Scholar]
- 57.Robatzek S., Chinchilla D., Boller T. (2006) Genes Dev. 20, 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ray E., Samanta A. K. (1996) FEBS Lett. 378, 235–239 [DOI] [PubMed] [Google Scholar]
- 59.Minnis J. G., Patierno S., Kohlmeier S. E., Brecha N. C., Tonini M., Sternini C. (2003) Neuroscience 119, 33–42 [DOI] [PubMed] [Google Scholar]
- 60.Favre N., Camps M., Arod C., Chabert C., Rommel C., Pasquali C. (2008) Proteomics 8, 4560–4576 [DOI] [PubMed] [Google Scholar]
- 61.Jung H., Bhangoo S., Banisadr G., Freitag C., Ren D., White F. A., Miller R. J. (2009) J. Neurosci. 29, 8051–8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vakili J., Ständker L., Detheux M., Vassart G., Forssmann W. G., Parmentier M. (2001) J. Immunol. 167, 3406–3413 [DOI] [PubMed] [Google Scholar]
- 63.Martins-Green M. (2001) Int. J. Biochem. Cell Biol. 33, 427–432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.