Abstract
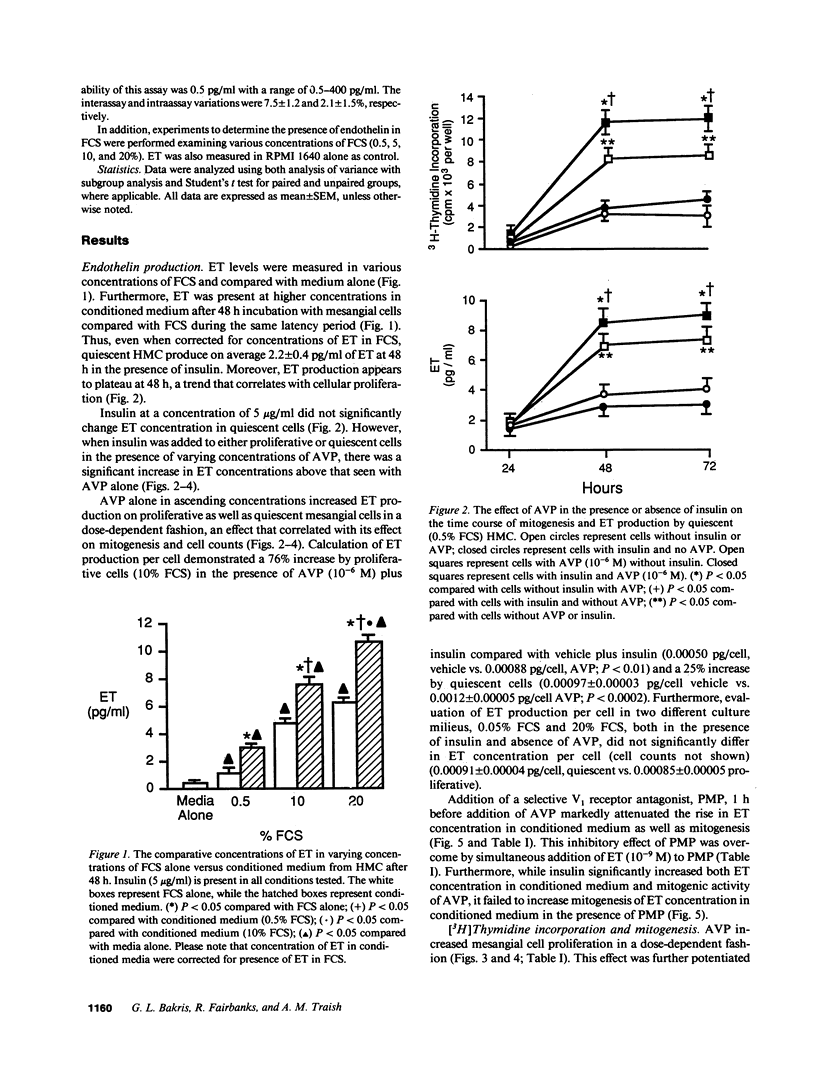
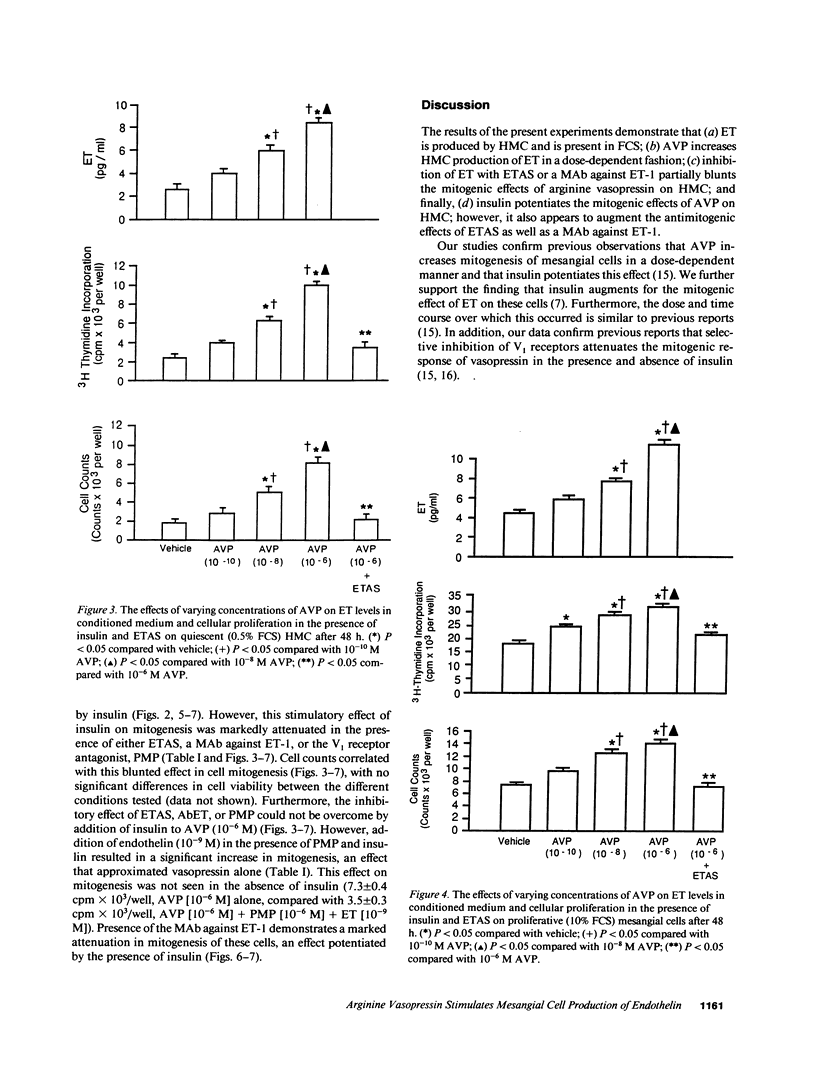
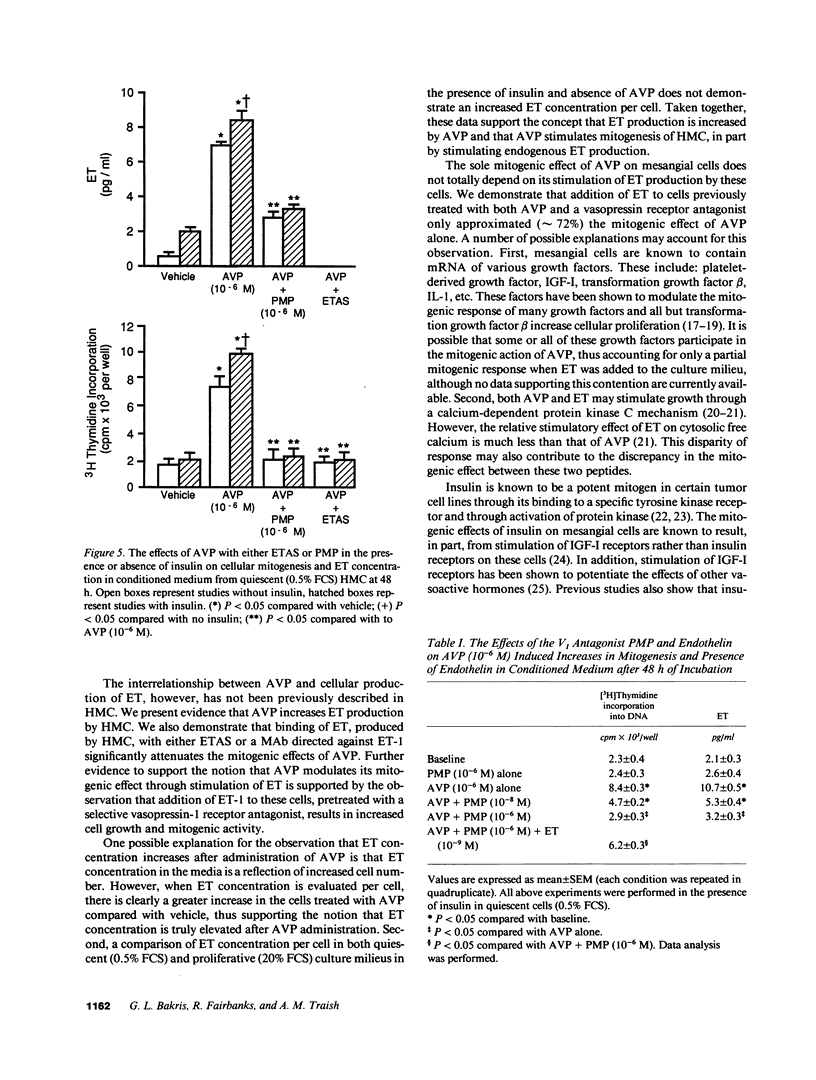
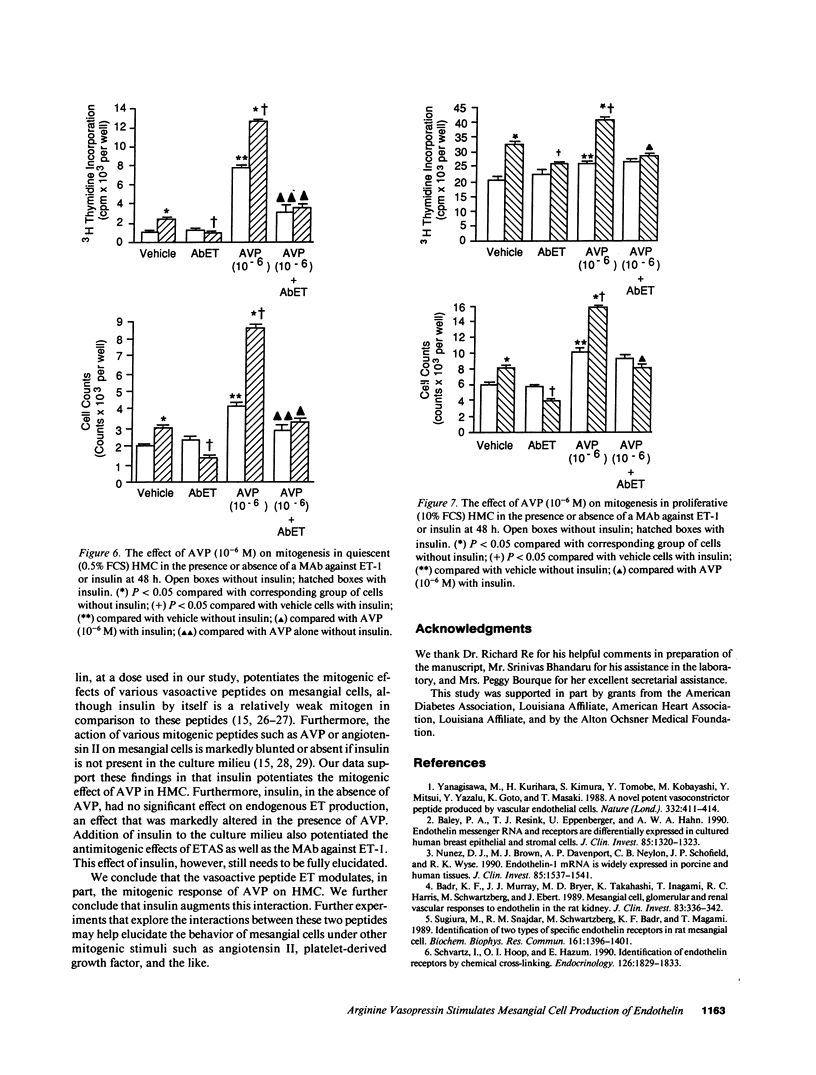
Endothelin (ET) is a vasoactive peptide produced by both endothelial epithelial cells with documented mitogenic action on mesangial cells. The present studies were designed to test the hypothesis that ET is also produced by human mesangial cells (HMC) and that other mitogens such as arginine vasopressin (AVP) and insulin stimulate cellular proliferation, in part, through modulation of endogenous production of this peptide. Studies were conducted on cultured normal HMC between the third and seventh passages. All mitogenesis experiments were carried out in 96-well plates and assessed by tritiated thymidine incorporation into DNA under various concentrations of AVP in the presence and absence of insulin, antiendothelin antisera (ETAS), a MAb against ET-1 (AbET), and a vasopressin-1 receptor antagonist. ET concentrations were measured daily from conditioned medium by a sensitive and specific RIA. ET was present in all concentrations of FCS as well as conditioned medium compared with medium alone. AVP (10(-6) M) in the presence of insulin increased ET production by quiescent HMC by 261% as well as cellular proliferation by 440% after 48 h incubation. In addition, cells cultured with ETAS or AbET demonstrated a blunted mitogenic response to AVP, a response not observed in cells cultured with ETAS where ET was added. Insulin significantly potentiated the mitogenic effects of AVP as well as media levels of ET, an effect significantly blunted by AbET. We conclude that ET is produced by HMC and its production is affected, in part, by both AVP and insulin. ET may thus serve to modulate the mitogenic effects of AVP on human mesangial cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud H. E., Poptic E., DiCorleto P. Production of platelet-derived growth factorlike protein by rat mesangial cells in culture. J Clin Invest. 1987 Sep;80(3):675–683. doi: 10.1172/JCI113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardaillou N., Nivez M. P., Striker G., Ardaillou R. Prostaglandin synthesis by human glomerular cells in culture. Prostaglandins. 1983 Nov;26(5):773–784. doi: 10.1016/0090-6980(83)90061-8. [DOI] [PubMed] [Google Scholar]
- Aron D. C., Rosenzweig J. L., Abboud H. E. Synthesis and binding of insulin-like growth factor I by human glomerular mesangial cells. J Clin Endocrinol Metab. 1989 Mar;68(3):585–591. doi: 10.1210/jcem-68-3-585. [DOI] [PubMed] [Google Scholar]
- Badr K. F., Murray J. J., Breyer M. D., Takahashi K., Inagami T., Harris R. C. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J Clin Invest. 1989 Jan;83(1):336–342. doi: 10.1172/JCI113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baley P. A., Resink T. J., Eppenberger U., Hahn A. W. Endothelin messenger RNA and receptors are differentially expressed in cultured human breast epithelial and stromal cells. J Clin Invest. 1990 Apr;85(4):1320–1323. doi: 10.1172/JCI114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Cavero P. G., Miller W. L., Heublein D. M., Margulies K. B., Burnett J. C., Jr Endothelin in experimental congestive heart failure in the anesthetized dog. Am J Physiol. 1990 Aug;259(2 Pt 2):F312–F317. doi: 10.1152/ajprenal.1990.259.2.F312. [DOI] [PubMed] [Google Scholar]
- Clozel M., Fischli W., Guilly C. Specific binding of endothelin on human vascular smooth muscle cells in culture. J Clin Invest. 1989 May;83(5):1758–1761. doi: 10.1172/JCI114078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F. G., Striker L. J., Lesniak M. A., MacKay K., Roth J., Striker G. E. Studies on binding and mitogenic effect of insulin and insulin-like growth factor I in glomerular mesangial cells. Endocrinology. 1988 Jun;122(6):2788–2795. doi: 10.1210/endo-122-6-2788. [DOI] [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Ganz M. B., Pekar S. K., Perfetto M. C., Sterzel R. B. Arginine vasopressin promotes growth of rat glomerular mesangial cells in culture. Am J Physiol. 1988 Nov;255(5 Pt 2):F898–F906. doi: 10.1152/ajprenal.1988.255.5.F898. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Tsuda T., Alexander R. W. Endothelin stimulates diacylglycerol accumulation and activates protein kinase C in cultured vascular smooth muscle cells. J Biol Chem. 1989 May 15;264(14):8237–8240. [PubMed] [Google Scholar]
- Jard S., Lombard C., Marie J., Devilliers G. Vasopressin receptors from cultured mesangial cells resemble V1a type. Am J Physiol. 1987 Jul;253(1 Pt 2):F41–F49. doi: 10.1152/ajprenal.1987.253.1.F41. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Crettaz M. Insulin receptors and the molecular mechanism of insulin action. Diabetes Metab Rev. 1985;1(1-2):5–32. doi: 10.1002/dmr.5610010103. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I. Insulin requirement for contraction of cultured rat glomerular mesangial cells in response to angiotensin II: possible role for insulin in modulating glomerular hemodynamics. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4190–4192. doi: 10.1073/pnas.79.13.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois D., Hinsch K. D., Saez J. M., Begeot M. Stimulatory effect of insulin and insulin-like growth factor I on Gi proteins and angiotensin-II-induced phosphoinositide breakdown in cultured bovine adrenal cells. Endocrinology. 1990 Apr;126(4):1867–1872. doi: 10.1210/endo-126-4-1867. [DOI] [PubMed] [Google Scholar]
- Nunez D. J., Brown M. J., Davenport A. P., Neylon C. B., Schofield J. P., Wyse R. K. Endothelin-1 mRNA is widely expressed in porcine and human tissues. J Clin Invest. 1990 May;85(5):1537–1541. doi: 10.1172/JCI114601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Schvartz I., Ittoop O., Hazum E. Identification of endothelin receptors by chemical cross-linking. Endocrinology. 1990 Apr;126(4):1829–1833. doi: 10.1210/endo-126-4-1829. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., DiCorleto P. E., Silver B. J., Abboud H. E. Mesangial cells express PDGF mRNAs and proliferate in response to PDGF. Am J Physiol. 1988 Oct;255(4 Pt 2):F674–F684. doi: 10.1152/ajprenal.1988.255.4.F674. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989 Feb;83(2):708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Killen P. D., Farin F. M. Human glomerular cells in vitro: isolation and characterization. Transplant Proc. 1980 Sep;12(3 Suppl 1):88–99. [PubMed] [Google Scholar]
- Sugiura M., Snajdar R. M., Schwartzberg M., Badr K. F., Inagami T. Identification of two types of specific endothelin receptors in rat mesangial cell. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1396–1401. doi: 10.1016/0006-291x(89)90829-2. [DOI] [PubMed] [Google Scholar]
- Traish A. M., Ettinger R., Kim N., Marshak-Rothstein A., Wotiz H. H. Development and characterization of monoclonal antibodies to a specific domain of human estrogen receptor. Steroids. 1990 May;55(5):196–208. doi: 10.1016/0039-128x(90)90017-6. [DOI] [PubMed] [Google Scholar]
- Traish A. M., Wotiz H. H. Monoclonal and polyclonal antibodies to human progesterone receptor peptide-(533-547) recognize a specific site in unactivated (8S) and activated (4S) progesterone receptor and distinguish between intact and proteolyzed receptors. Endocrinology. 1990 Sep;127(3):1167–1175. doi: 10.1210/endo-127-3-1167. [DOI] [PubMed] [Google Scholar]
- Wallnöfer A., Weir S., Rüegg U., Cauvin C. The mechanism of action of endothelin-1 as compared with other agonists in vascular smooth muscle. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S23–S45. doi: 10.1097/00005344-198900135-00008. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


