Abstract
The membrane-spanning segments of integral membrane proteins often are flanked by aromatic or charged amino acid residues, which may “anchor” the transmembrane orientation. Single spanning transmembrane peptides such as those of the WALP family, acetyl-GWW(LA)nLWWA-amide, furthermore adopt a moderate average tilt within lipid bilayer membranes. To understand the anchor residue dependence of the tilt, we introduce Leu-Ala “spacers” between paired anchors and in some cases replace the outer tryptophans. The resulting peptides, acetyl-GX2ALW(LA)6LWLAX22A-amide, have Trp, Lys, Arg, or Gly in the two X positions. The apparent average orientations of the core helical sequences were determined in oriented phosphatidylcholine bilayer membranes of varying thickness using solid-state 2H NMR spectroscopy. When X is Lys, Arg, or Gly, the direction of the tilt is essentially constant in different lipids and presumably is dictated by the tryptophans (Trp5 and Trp19) that flank the inner helical core. The Leu-Ala spacers are no longer helical. The magnitude of the apparent helix tilt furthermore scales nicely with the bilayer thickness except when X is Trp. When X is Trp, the direction of tilt is less well defined in each phosphatidylcholine bilayer and varies up to 70° among 1,2-dioleoyl-sn-glycero-3-phosphocholine, 1,2-dimyristoyl-sn-glycero-3-phosphocholine, and 1,2-dilauroyl-sn-glycero-3-phosphocholine bilayer membranes. Indeed, the X = Trp case parallels earlier observations in which WALP family peptides having multiple Trp anchors show little dependence of the apparent tilt magnitude on bilayer thickness. The results shed new light on the interactions of arginine, lysine, tryptophan, and even glycine at lipid bilayer membrane interfaces.
Keywords: Membrane Bilayer; Membrane Structure; NMR; Peptide Chemical Synthesis; Peptide Interactions; Tryptophan; WALP Peptide; Glycine; Hydrophobic Matching; Lysine, Arginine
Introduction
The lipid bilayer environment has a profound influence on the properties of peptides and proteins found within it. It is significant that many membrane-spanning proteins have bands of aromatic and/or positively charged residues at the membrane interface, which could serve as anchors for the protein orientation and promote favorable protein-lipid interactions. This anchoring is a widespread characteristic that is observed for a variety of proteins having both α and β transmembrane folds (1–5). Furthermore, polar amino acids influence the topology of membrane proteins as the direction of insertion is driven by the asymmetric positioning of basic residues (Lys and Arg), giving rise to the “positive inside” rule for helical membrane proteins (6). Little is known, nevertheless, about the contributions of these residues in defining the orientations of the transmembrane segments within lipid bilayers (7).
Due to the inherent complexity of membrane proteins in the native biological membrane environment, model systems provide meaningful ways to address specific questions about protein/lipid interactions. In particular, model peptides of the WALP family, having the general sequence acetyl-GWW(LA)nLWWA-[ethanol]amide, have yielded valuable information about peptide orientations, dynamics, and lipid phase behavior (8–11). An original design of WALP and KALP peptides included four anchoring residues: two sequential Trp or Lys residues on each side of a core transmembrane α-helix (12). Comparisons of similar peptides revealed that the lysine-anchored peptides exhibit larger apparent tilt angles than their tryptophan counterparts (13). Furthermore, the nature of the anchoring residue influences the tilt direction. The detailed factors responsible for the apparent magnitude and direction of tilt are nonetheless still unclear because it is difficult to draw firm conclusions based upon four identical anchoring residues that are dispersed fairly evenly around a helical wheel projection.
The similar behavior of XALP23 (Leu/Ala core) and XLP23 (all-Leu core) peptides having the same X residue suggests the importance of the anchor residue identity (13). Magic angle spinning 1H NMR experiments with WALP and KALP peptides in DMPC2 bilayers furthermore indicate anchor-specific perturbations in lipid resonances (14). It was concluded that aromatic residues (such as Trp) are localized primarily to the carbonyl region of phospholipids, whereas charged residues (Lys, Arg) tend to be positioned farther outside of the membrane, potentially interacting with the lipid phosphate moieties (15) as well as with water. We seek now to address the different influence of aromatic versus charged residues upon the orientations of transmembrane domains, using model peptides as prototype examples.
Phosphorus (31P) NMR spectra of lipid bilayers incorporating WALP or KALP peptides show that the nature of the anchoring group also has a significant influence on the effective hydrophobic length. Both peptide series are capable of inducing isotropic and inverted hexagonal lipid phases when the peptide length is too short as compared with the membrane thickness. The detailed response is different, nevertheless; although KALP16 does not affect the organization of DOPC bilayer vesicles, the addition of WALP16 promotes the inverted hexagonal (HII) phase (15). Similar effects have been noted for longer members of the series; Lys-anchored peptides affect lipid phase behavior in a manner that suggests that their effective hydrophobic length is less than that of corresponding Trp-anchored peptides. Interestingly, the arginine-flanked RALP23 has different influence on the lipid phase behavior than comparable lysine and histidine analogues, suggesting that charge delocalization on the Arg guanidium group could play a role (16).
A related study of XALP23 peptides in lipids prone to the formation of non-bilayer phases indicated that the chemical nature of the anchoring residue plays a smaller role for the phase modulation (17). The response of mixed phosphatidylethanolamine-phosphatidylglycerol membranes to transmembrane peptides is similar for charged and polar un-ionized X anchors. Somewhat unexpectedly, similar responses have been observed for positively and negatively charged X residues.
To gain further insight into the molecular mechanisms that govern peptide-lipid interactions, several studies have addressed the apparent peptide orientations within lipid bilayers of varying thickness. WALP peptides in particular have been found to exhibit quite small apparent average tilt angles with relatively little dependence upon the lipid bilayer thickness, as observed by solid-state 2H and 15N NMR (18, 19). Importantly, the solid-state NMR methods are non-perturbing and allow investigations of the peptide orientations within the actual lipid environment (18, 19). The nature of WALP (or KALP) peptides, with four Trp (or Lys) residues occupying four radial locations, remains, however, less than ideal for assigning the effects of individual anchoring amino acid residues. To better understand the interplay between anchor residue identity and position, therefore, we have designed the X2,22W5,19ALP23 series of peptides, bearing the sequence acetyl-GX2ALW(LA)6LWLAX22A-[ethanol]amide (Table 1). These sequences share the (Leu-Ala)6.5 α-helical core of WALP19, yet the pairs of anchoring amino acids on either side of the core are separated by an additional short Leu-Ala spacer sequence. By keeping the inner anchor identity fixed (Trp5 and Trp19) while varying the outer anchor identity (X2 and X22), it becomes possible to examine the effects of different X residues on the peptide average orientation. As a control peptide, having just one obvious anchor on each side of the core helix, we employ the recently introduced GWALP23 sequence (19, 20) in which the X residues are glycine. The model in Fig. 1 illustrates the relative positions of the X2, Trp5, Trp19, and X22 residues.
TABLE 1.
Peptide sequences
FIGURE 1.
Ribbon model to represent XWALP23 peptides. The side chains of Trp5 and Trp19 (green) are roughly on the same side of the helix and are numbered in the lower view. The Cα atoms of residues Gly1 (black) and X2 and X22 (blue) are shown as spheres. The core α-helix is intact between the Trp residues but may unwind at the ends. The arrow shows the approximate direction of tilt when X is Gly, Arg, or Lys (see “Results” and Fig. 6).
The design of the XWALP23 peptides enables one to address important questions that have arisen in previous studies. Does the distance between the inner Trp anchors retain primary importance for the peptide behavior, as suggested by 31P NMR studies (14)? Do the outer X anchors make significant contributions to the apparent average peptide orientation? How do the properties vary with the identity of X? Is the charge on X a significant factor? When X is Trp, is it possible that the orientation of WWALP23 could be defined by the outer anchor positions? Finally, these peptides may help to promote understanding of a fundamental question. Which amino acid residues serve as primary determinants of the transmembrane helix tilt magnitude and direction?
EXPERIMENTAL PROCEDURES
N-Fmoc-protected amino acids, Rink amide resin, and Wang resin were purchased from Novabiochem and Advanced Chemtech (Louisville, KY). Commercial l-alanine, deuterated at Cα and Cβ carbons (Ala-d4), from Cambridge Isotope Laboratories (Andover, MA) was derivatized with an Fmoc-protecting group as described previously (21, 22). DLPC, DMPC, and DOPC were purchased from Avanti Polar Lipids (Alabaster, AL). Other reagents were from VWR Chemical (Irving, TX). All chemicals and reagents were of the highest grade available. Water was doubly deionized Milli-Q® water.
Peptides were synthesized on a model 433A solid-phase peptide synthesizer (Applied Biosystems; Foster City, CA) using modified FastMoc® chemistry, with extended deprotection and coupling times where needed. Two deuterium-labeled alanines at ∼60 and 100% isotope abundance levels were incorporated in a single peptide, allowing the NMR signals to be distinguished and assigned based upon the relative intensities (23). GWALP23 and WWALP23 were synthesized without protecting groups and were cleaved from Wang resin using 20% ethanolamine in dichloromethane. KWALP23 and RWALP23 were synthesized with protecting groups (tert-butyloxycarbonyl for Lys and Trp, and pentamethyl-2,3-dihydrobenzofuran-5-sulfonyl for Arg) (24) and were simultaneously deprotected and cleaved from Rink amide resin using TFA:phenol:triisopropylsilane:water in a 85:5:5:5 ratio. Crude peptides were purified on a 9.4 × 250-mm octyl-silica column (5 μm, 80 Å) using a 96–100% (X = Gly, Trp) or 92–96% (X = Lys, Arg) methanol gradient over 24 min at a flow rate of 1.7 ml/min (∼1100 p.s.i.). Peptides were lyophilized multiple times from acetonitrile:water (1:1) to ensure the complete removal of TFA. Purity of the peptides was verified by reversed-phase chromatography (see supplemental Fig. S1). Peptide identity was confirmed by means of MALDI mass spectrometry (supplemental Fig. S2). The absence of TFA in peptide samples was verified by 19F NMR spectroscopy (supplemental Fig. S3).
Circular dichroism measurements were performed on peptides incorporated into small unilamellar vesicles of DLPC at 1/40 (mol/mol) peptide/lipid (P/L), obtained by ultrasonic treatment. Spectra were recorded at 22 °C on a Jasco J-710 spectropolarimeter, using a 20 nm/min scan rate, 1.0-mm path length, 1.0 nm bandwidth, and 0.2 nm step resolution. Five scans were averaged to enhance the signal-to-noise ratio.
Solid-state NMR samples were prepared using macroscopically aligned lipid bilayers, as described previously (18). Briefly, peptide was dissolved in trifluoroethanol, and concentration was determined spectrophotometrically in methanol dilutions, using ϵ280 of 5600 m−1cm−1Trp−1. Peptide solution was added to 80 μmol of lipid in chloroform to achieve 1/40 P/L molar ratio. Solvent was removed under a stream of nitrogen, and sample was dried under vacuum. The peptide-lipid mixed film was redissolved in methanol:water 95:5 and distributed evenly among 40 glass slides (4.8 × 23 × 0.07 mm; Marienfeld; Lauda-Königshofen, Germany). Slides were dried under vacuum (<1.5 pascals) for at least 48 h and hydrated with 2H-depleted water (Cambridge) to achieve 45% hydration (w/w). Slides were sealed in a glass cuvette using epoxy and left to equilibrate at 40 °C for at least 48 h before measurement.
Solid-state NMR spectra were recorded at 50 °C using two Bruker (Billerica, MA) Avance spectrometers, each operating at a proton frequency of 300 MHz. Spectra were obtained with the membrane normal either parallel (β = 0°) or perpendicular (β = 90°) to the applied magnetic field. For a peptide with fast averaging around the lipid bilayer normal (but not the peptide axis), the 2H quadrupolar splittings (Δνq) observed at β = 90° have absolute magnitude of one-half those at β = 0° (25). Spectra were recorded using a quadrupole echo pulse sequence (26), with pulse lengths of 3.2–4.5 μs, echo delays of 110–125 μs, and a recycle delay of 90 ms. Typically, 700,000 free induction decays were accumulated. Prior to Fourier transformation, an exponential weighting function resulting in a 100-Hz line broadening was applied. Proton-decoupled 31P NMR spectra were obtained with the Bruker zgpg pulse program, using a recycle delay of 5 s and pulse length of 6 μs. In 31P NMR spectra of aligned bilayers, peaks at β = 0° and β = 90° correspond to the 0° (σ//) and 90° (σ⊥) edges of a powder pattern (see supplemental Fig. S4).
Deuterium NMR signals from CβD3 groups of Ala-d4 residues in XWALP23 peptides were analyzed according to the geometric analysis of labeled alanines (GALA) method, implemented in Microsoft Excel (18, 27). The analysis is based on a relationship between the alanine CβD3 quadrupolar splitting (Δνq) and the angle θ between the alanine Cα–Cβ bond vector and the applied magnetic field
 |
The angle θ can be further expressed analytically in terms of peptide geometry and orientation, namely tilt magnitude (τ) and tilt direction (ρ, relative to Cα of Gly1), as well as the angle ϵ// between the helix axis and Cα–Cβ bond vector of a given alanine residue. Angle β represents the macroscopic sample orientation (defined above, with respect to Ho), and angle γ is the tetrahedral bond angle within a methyl group. Based upon earlier experience (18, 20), the value of ϵ// was set to 59.4°. The variables τ and ρ and a global order parameter, Szz, were treated as free parameters to minimize the r.m.s.d. between the observed Δνq values and those calculated using Equation 1 (18, 28). This analysis incorporates dynamics in a manner similar to “model 3” of Strandberg et al. (29). Further considerations were given to anisotropic molecular motions and to combined analyses of 15N-1H dipolar couplings and 2H quadrupolar splittings (see “Discussion”). Due to the symmetry considerations, GALA analysis returns four combinations of (τ, ρ) values. Here we report the (τ, ρ) data in a (90°, 360°) space, as in previous studies on WALP peptides (18, 19, 23, 28).
RESULTS
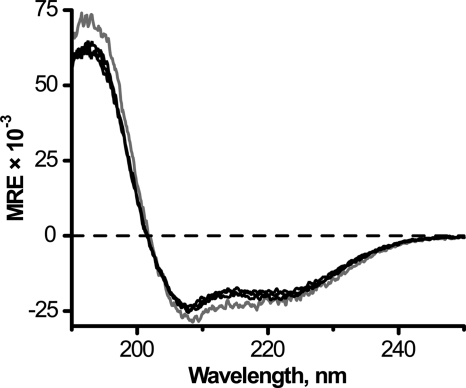
The hydrophobic core of XWALP23, composed of alternating Leu-Ala residues, is expected to adopt an α-helical conformation. To check the secondary structures, we recorded CD spectra for the peptides having variable X residues and incorporated them into hydrated DLPC bilayer membranes. All such peptides exhibited mean residue ellipticity profiles typical of an α-helix, characterized by minima at 208 and 222 nm and by the ratio ϵ222/ϵ208 between 0.74 and 0.86 (Fig. 2). When X is Gly, Lys, or Arg, the CD spectra exhibit high degree of overlap, whereas there is a change of ∼10% in ellipticity in the 205–225-nm region when X is Trp. The spectral difference when X is Trp may be indicative of a different extent of helix formation at the peptide termini but could also be caused by UV absorption by the side chains of the extra Trp residues (30, 31).
FIGURE 2.
CD spectra of X2,22W5,19ALP23 peptides in DLPC at 1/40 (P/L); 22 °C. The color code is gray when X is Trp or black when X is Gly, Lys, or Arg.
Having confirmed the overall α-helical character of the XWALP23 peptides, we next introduced Ala-d4 close to the center of the sequence, in positions 11 and 13. Deuterium NMR spectra of these peptides in lipid bilayers of varying thickness are illustrated in Fig. 3. If the peptides were not tilted in the membrane, the signals from all of the alanine CβD3 groups would show the same quadrupolar splittings (18); however, two pairs of peaks are readily identified in each spectrum in Fig. 3. The pattern displayed by variations in the magnitudes of the alanine CβD3 quadrupolar splittings is related to the apparent tilt angle τ, defined between the membrane normal and the helix axis of the peptide (18). It can be seen that the particular Ala11 and Ala13 Δνq magnitudes undergo changes for different X residue identities, being smallest for X = Trp, intermediate for X = Gly, and highest for X = Lys or Arg. There also exists a definite trend among the host lipids, with the largest Δνq values being observed in DLPC bilayer membranes, which suggests that the peptides may be more tilted when shorter lipids compose the bilayer.
FIGURE 3.
Deuterium NMR spectra for Ala13 (full deuteration) and Ala11 (partial deuteration) as a function of X residue identity in X2,22W5,19ALP23 peptides at β = 0° sample orientation; 50 °C. Peptides are incorporated at 1/40 (P/L) in DLPC, DMPC, or DOPC (left to right). The X residues are Gly, Trp, Lys, or Arg (top to bottom, as noted; depicted as panels G, W, K, and R).
For a detailed study of the orientations of the XWALP23 peptides in lipid bilayers, we incorporated Ala-d4 residues throughout the hydrophobic core (residues 7, 9, 11, 13, 15, and 17) of each peptide, labeling two positions in each synthetic peptide. All of the respective 2H NMR spectra are presented as supplemental data (supplemental Figs. S5–S8), and the observed quadrupolar splitting magnitudes are reported in Table 2. Errors in the observed values were estimated from duplicate samples and also by performing the experiments at β angles of both 0 and 90° for the sample orientation because Δνq for a given peptide-lipid system follows the relationship
 |
The standard deviation was found generally to be within ±0.5 kHz, although larger deviations up to ±1.2 kHz were observed for a few cases where the peaks are relatively broad and exhibit large |Δνq| values.
TABLE 2.
Alanine CβD3 quadrupolar splittings (kHz) for XWALP23 peptides incorporated in different lipids
| Peptide | Lipida | Alanine position |
|||||
|---|---|---|---|---|---|---|---|
| 7 | 9 | 11 | 13 | 15 | 17 | ||
| GWALP23 | DLPC | 26.4 | 25.5 | 26.9 | 14.6 | 20.7 | 3.4 |
| DMPCb | 21.9 | 8.9 | 20.9 | 3.8 | 17.6 | 2.9 | |
| DMPCc | 22.6 | 12.4 | 21.7 | 7.4 | 19.0 | 2.4 | |
| DMPCd | 18.7 | 6.0 | 18.7 | 2.8 | 14.9 | 2.8 | |
| DOPC | 16.6 | 1.7 | 16.7 | 1.5 | 15.4 | 2.6 | |
| WWALP23 | DLPC | 1.2 | 13.9 | 4.1 | 13.8 | 2.2 | 10.8 |
| DMPC | 1.9 | 11.8 | 1.4 | 11.8 | 5.0 | 7.6 | |
| DOPC | 5.4 | 14.2 | 1.9 | 11.4 | 7.9 | 2.3 | |
| KWALP23 | DLPC | 28.6 | 22.2 | 26.2 | 13.7 | 20.2 | 5.0 |
| DMPC | 24.6 | 14.7 | 23.6 | 8.6 | 18.5 | 4.0 | |
| DOPC | 19.1 | 4.9 | 18.6 | 2.4 | 15.3 | 3.7 | |
| RWALP23 | DLPC | 25.7 | 28.9 | 29.0 | 17.2 | 22.4 | 4.0 |
| DMPC | 25.7 | 16.9 | 24.8 | 10.4 | 19.3 | 3.0 | |
| DOPC | 18.7 | 4.7 | 18.3 | 3.0 | 16.2 | 2.4 | |
a The peptide/lipid ratio was 1/40, unless noted otherwise.
b Signal from Ala3 is 21.1 kHz. Signal from Ala21 is 6.0 kHz.
c Peptide/lipid ratio of 1/80.
d Peptide/lipid ratio of 1/20.
For the KWALP23 peptide, we have revised a previous incorrect assignment of a Δνq value. Due to peak overlap, the Δνq for Ala9 in KWALP23, incorporated in DLPC, was interpreted as 13.6 kHz (23), whereas the actual value is 22.2 kHz (Table 2 and supplemental Fig. S7). We were able to clarify the situation using singly labeled peptides. With this newly revised assignment, the earlier suggestion of a kink (23) disappears from the GALA analysis of KWALP23 (see “Discussion”). Also, for GWALP23, small revisions to the Δνq values (Table 2) and to the fitted value of Szz led to a moderately larger apparent magnitude for the tilt angle τ in DLPC than previously reported (19).
The observed quadrupolar splittings were subjected to GALA analysis, at first incorporating dynamics by means of a straightforward isotropic variable Szz parameter, according to Equation 1. The quality of the fits was assessed by r.m.s.d. values, which typically are close to 1 kHz or lower. Because overly high or low values of ϵ// are characteristic of poor fits (18), the values of ϵ// representing the alanine side chain geometry was kept at 59.4°. The τ, ρ, Szz, and corresponding r.m.s.d. values are provided in supplemental Table S1, whereas the theoretical Δνq curves corresponding to the best fit τ, ρ, and Szz values are presented in Fig. 4, overlaid with the experimental data. The nearly constant phase of the sine wave amplitude curves, except when X is Trp (Fig. 4), illustrates that the screw rotation ρ is essentially constant when X is Lys, Arg, or Gly. Furthermore, the magnitude of the apparent tilt τ scales with the lipid bilayer thickness when X is Lys, Arg, or Gly (see “Discussion”). Although the GALA analysis often is not especially sensitive to Szz (18), the fits for the XWALP23 peptides nevertheless show trends toward lower Szz values (supplemental Table S1) when the host bilayer is DLPC or when the identity of X is Trp in all of the lipid bilayers, suggesting increased molecular motion in these cases. The incorporation of more complex dynamics models did not substantially alter the results or conclusions (see “Discussion”).
FIGURE 4.
GALA quadrupolar wave plots for X2,22W5,19ALP23 peptides in DLPC (red squares), DMPC (green circles), and DOPC (blue diamonds). The X residue identities are noted in each panel (Gly, Trp, Lys, or Arg, depicted as panels G, W, K, and R). The quadrupolar splittings of Ala3 and Ala21 for G2,22W5,19WALP23 in DMPC (from Fig. 5) were not used in the fitting but are shown as filled circles, far off the curve that fits the core α-helix.
To probe the effect of the peptide/lipid ratio on the peptide orientation, we have examined GWALP23 in DMPC at different values of P/L. When P/L is decreased from 1/20 to 1/40 and then to 1/80, there are small yet consistent increases in the quadrupolar splittings throughout the hydrophobic core (Table 2). These changes lead to only minor effects on the apparent tilt angle τ (namely, a change of <1° when the peptide is diluted from 1/20 to 1/40 and about 1° upon further dilution to 1/80).
The side chains of lysine and arginine have high pKa values in aqueous solution and are expected to be charged at the peptide-lipid interface. It is considered that the anchoring properties of lysine and arginine arise in part from electrostatic interactions with the phosphate groups of lipid molecules, in addition to the propensity of charged polar groups to be in or near the aqueous phase. To test whether the microenvironment of the XWALP23 peptides with ionizable X residues would influence the average peptide orientation, we prepared oriented samples hydrated with HEPES buffer (pH 7.4) in 2H-depleted water containing 0.1 m NaCl. Deuterium NMR spectra of KWALP23 and RWALP23 incorporated in DMPC bilayers under these conditions were nearly identical to those where only water (unbuffered) was used for hydration (supplemental Fig. S9). The only notable effect is a small decrease in the signal-to-noise ratio for samples containing NaCl, which can be attributed to radio frequency power dissipation (32).
The transmembrane segments of proteins strongly favor secondary structures that maximize hydrogen bonding to help to satisfy the energetic requirements associated with partitioning the peptide backbone polar carbonyl groups into the lipid acyl chain environment (11, 33). Nevertheless, the requirements are less stringent for residues that are located near the lipid head group interfacial regions. With respect to the XWALP23 peptides, it is of interest therefore to know whether the α-helical conformation is retained throughout the peptide, including the Leu-Ala spacer segments between the inner and outer anchor residues, or whether the helical region might encompass only the core segment between Trp5 and Trp19. To address this question, we synthesized GWALP23 peptides having a single Ala-d4 label incorporated at either Ala3 or Ala21. The Δνq values from these peptides theoretically will indicate whether or not the secondary structure remains an essentially unbroken α-helix from Ala3 through Ala21.
The 2H NMR spectra of GWALP23 with Ala3 or Ala21 labeled are shown in Fig. 5. These residues are located 18 amino acids apart, exactly five helical turns in an ideal α-helix model with a 100° increment per amino acid, and could therefore give identical 2H quadrupolar splittings. However, the Δνq values exhibited by alanines at these two positions are very different, with neither of the signals compatible with the quadrupolar wave plot for the core transmembrane helix of GWALP23 (Fig. 4, panel G). These results reveal that the transmembrane α-helix in GWALP23, and presumably in XWALP23 peptides generally, is terminated at or near the innermost Trp residues, resulting in frayed edges outside of the core helix that is both flanked and defined by Trp5 and Trp19. This finding that the core helix terminates within or near each of the Leu-Ala spacers is consistent with an earlier statistical survey, which indicated that approximately two-thirds of the folded transmembrane structures were disordered in the interface region (7).
FIGURE 5.
Deuterium NMR spectra of G2,22W5,19ALP23, in DMPC (β = 0° sample orientation), with Ala-d4 incorporated outside of the Trp-flanked core sequence. A, Ala-d4 at position 3. B, Ala-d4 at position 21.
DISCUSSION
Time-honored experiments with model systems having multiple tryptophans per peptide terminal have demonstrated well the aggregate anchoring properties of Trp residues for gramicidin channels (34, 35) and WALP peptides (8, 15) at membrane/water interfaces. The present studies with the XWALP23 series of peptides enable initial assessments of the anchoring properties of individual Trp, Lys, and Arg residues near the ends of transmembrane peptide domains and segments. The present results concerning helix tilt furthermore complement earlier findings about interfacial side chain locations, namely that Lys side chains prefer anchoring positions that are about 3–4 Å farther from the lipid bilayer center than those of the Trp side chains (15). Indeed, a noteworthy feature for the XWALP23 design is that the X residue α-carbons should be separated from the Trp residue α-carbons by about 4 Å along the bilayer normal (depending upon the secondary structure of each Leu-Ala spacer). Remarkably, the properties of XWALP23 peptides having Gly residues in the X2 and X22 positions are quite similar to those having Lys or Arg. When X2 and X22 are Trp, effectively giving rise to extra Trp residues, the direction of peptide tilt becomes less well defined, and the magnitude of tilt loses its tendency to scale with the lipid bilayer thickness. Indeed, the peptide properties when extra Trp residues are present parallel earlier observations with WALP19 and WALP23 (18, 28, 36). These particular issues will be discussed in turn.
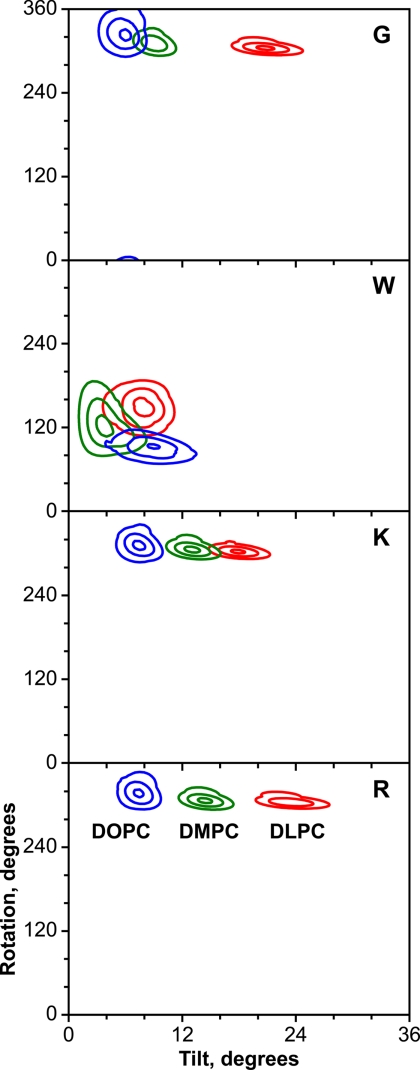
To visualize the anchor residue dependence of the apparent tilt, r.m.s.d. contour plots were constructed for the (τ, ρ) coordinates (Fig. 6). When fitted using an isotropic principal order parameter, the global minima for each of the XWALP23 peptides are well defined and generally encompass only a small range of apparent τ and ρ values. Reminiscent of WALP23, the largest allowed ranges for both τ and ρ are observed when X is Trp. Comparing the results in different lipids reveals that the apparent peptide tilt generally increases with decreasing hydrophobic thickness of the lipid bilayer; again the exception occurs when X = Trp (Fig. 6). GWALP23 and the peptides with positively charged Lys or Arg outer anchors undergo apparent tilt changes of ∼4–6° as the lipid acyl length increases by two methylene groups in each leaflet. The changes in the apparent tilt are presumably a consequence of hydrophobic (mis)matching, as was predicted for WALP family peptides. It is in this regard notable that the variation of apparent helix tilt angle with lipid bilayer thickness was much smaller for the original WALP series peptides: essentially nil for WALP19 (18) and a difference of only ∼4° for WALP23 between DLPC and DOPC (28). By contrast, for the XWALP23 series, the mismatch-induced changes in the apparent peptide tilt can be as large as 16° between DLPC and DOPC (Fig. 6). These changes are likely not due to altered lipid packing or acyl chain order because the influence of WALP family peptides on bilayer properties is noticeably small (12, 37), even at somewhat higher P/L ratios than implemented here.
FIGURE 6.
r.m.s.d. contour plots for X2,22W5,19ALP23 peptides in DLPC (red), DMPC (green), and DOPC (blue). Residue X is Gly, Trp, Lys, or Arg, depicted as panels G, W, K, and R. The identities of the bilayer lipids are also indicated by the labels in panel R. Contours are drawn at levels of 1, 2, and 3 kHz.
It has been noted that the fits to the Ala 2H quadrupolar splittings of WALP23 in DMPC differ when Szz is decomposed into explicit motion parameters (Δτ and Δρ), which are included in the analysis (29, 36, 38–40). We now surmise that this feature may be attributed to having “too many” interfacial indole rings, possibly competing with each other when the extra Trp residues are present in the interface region, as observed also in striking fashion with WWALP23 (in Fig. 6, compare panel W with the other panels). With WALP23, the best combined fits to chemical shift anisotropies (13C and 15N), dipolar couplings (13C-15N), and quadrupolar splitting (2H) involve Δρ motions of ± ∼80° about the helix axis, in addition to rapid rotational averaging about the bilayer normal (36). Such large amplitude Δρ motions would seem unrealistic when Arg and Lys side chains are present together with Trp (see also Ref. 20), yet the question of Δρ motion remains pertinent for GWALP23 itself.
The direction and magnitude of the apparent tilt for GWALP23 as well as KWALP23 have been verified by independent 15N polarization inversion with spin exchange at magic angle (PISEMA) experimental methods (19, 41). For both peptides, the GALA and PISEMA methods show excellent agreement when using a semistatic analysis with a (variable) principal order parameter Szz. Furthermore, combined analysis using 11 data points (2H quadrupolar splitting and 15N-1H dipolar couplings) also gives an excellent fit for GWALP23 in DLPC (see supplemental Table S2), whether or not a Gaussian approach is employed to treat Δρ and Δτ motions. (As noted, refinements of the Δνq values (Table 2) and of Szz, indicating increased motion, led to a modest increase in the apparent τ for GWALP23 in DLPC, as compared with a previous report (19).) Again, we infer that the presence of only one Trp at each terminal of the GWALP23 core helix may limit Δρ motions.
Among these XWALP23 peptides, WWALP23 is the outlier. When outer tryptophans Trp2 and Trp22 are present, the response of WWALP23 toward changes in the lipid environment again resembles that of the original WALP peptides. Indeed, there is considerable overlap of (τ, ρ) solutions for WWALP23 in the three lipids investigated (Fig. 6, panel W). Conceivably the important common feature among WALP19, WALP23, and WWALP23 is the presence of multiple, possibly competing, tryptophan anchors on both ends of the transmembrane peptide core helical sequence. The r.m.s.d. minima for WWALP23 also are broader as compared with the other XWALP23 peptides, which reflects an increasing uncertainty in ρ as the apparent τ value diminishes, probably reflecting increased motion, including Δρ motion (36). It is of further interest that the variation of ρ with lipid identity appears to be larger for WWALP23 (Fig. 6) than for WALP19 (18), WALP23 (28), or the other XWALP23 peptides (Fig. 6). Although the uncertainty in ρ is indeed quite large for WWALP23 in each of the lipid bilayers, the detailed influence of the short two-residue Leu-Ala spacers between the inner/outer Trps in WWALP23 may merit further investigation. It is in this regard noteworthy that the labels at spacer residues Ala3 and Ala21 (Fig. 4) indicate that the Leu-Ala spacer sequences in GWALP23 are not helical, although the spacer conformation remains unknown for WWALP23. The CD spectra (Fig. 2) suggest marginally increased helicity for WWALP23 as compared with the other XWALP23 peptides, which may or may not pertain to the spacer sequences. (Interestingly, a longer helical segment with more rigid ends would be expected to show greater sensitivity to lipid hydrophobic mismatch, whereas the opposite is observed for WWALP23.)
For the case of KWALP23 in DLPC, the quadrupolar splitting magnitudes are closely similar for the alanine 7, 9, and 11 side chains; namely 28.6, 22.2, and 26.2 kHz, respectively (Table 2). Also, in DMPC and DOPC, the Δνq values for the Ala7 and Ala11 side chains remain nearly indistinguishable (Table 2). These similarities cause peak overlap in the NMR spectra for multiply labeled peptides, which in turn led us to assign incorrectly the splittings for Ala9 in an earlier study (23). With the corrected assignments, the GALA fits for KWALP23 now are excellent (Fig. 6), and we dismiss an earlier suggestion of a kink in the transmembrane helix of KWALP23 (23). The similar fits for RWALP23 and KWALP23 (Figs. 4 and 6) further make the case that these peptides are not kinked.
Remarkably, considerations of Δρ motion do not in any known case influence conclusions about the mean peptide rotation ρ0, which defines the direction of tilt. The lack of influence on ρ0 is true in both molecular dynamics simulations (38, 39, 42) and fits to solid-state NMR data (20, 29, 36, 40). The established definite ρ0 values are well illustrated using polar plots (Fig. 7) to view the best fit apparent (τ, ρ) results. Indeed, Fig. 7 shows the remarkable consistency of the tilt direction ρ for XWALP23 peptides in each of the DLPC, DMPC, and DOPC lipid bilayer membranes and for each X residue identity except Trp. When X is Trp, by contrast, the preferred ρ values are altered and unpredictably varied in the different lipids. At the same time, the apparent τ values for WWALP23 become quite similar, unexpectedly small, and rather insensitive to the bilayer hydrophobic thickness (Fig. 7). To be sure, the situation for WWALP23 is reminiscent of earlier results for WALP19 (18) and WALP23 (28), each of which also contains multiple, and possibly competing, Trp residues near each peptide terminal. In these cases, it appears that the added outer tryptophans may preclude additional tilting and that the preferred rotation angles and significant Δρ motion may arise from a compromise among the different Trp indole side chains that occupy different radial positions. These complexities may relate also to the now numerous comparisons among experimental observations and computational predictions for WALP23 (28, 29, 36, 38, 39, 42, 43). It is further of note that tryptophans near the N and C termini of transmembrane peptides exhibit different geometric and motional properties (44).
FIGURE 7.
Polar plot of ρ and τ for X2,22W5,19ALP23 peptides in DLPC (circles), DMPC (squares), and DOPC (triangles). Residue X is Gly (black), Lys (green), Arg (blue), or Trp (red, open symbols). Note that ρ values are similar and that τ values scale with bilayer thickness, except when X is Trp. G indicates Gly, and W indicates Trp
We observe additionally that the positively charged Lys and Arg residues generally increase the apparent tilt angle by small amounts, as compared with the case when X is Gly (Figs. 6 and 7), although conspicuously without changing the direction of the tilt of XWALP23 peptides. Furthermore, Arg causes marginally larger tilts than does Lys in equivalent situations (Fig. 7).
We conclude with a return to the anchoring properties of the Trp indole rings. An overview of Figs. 6 and 7 highlights the importance of the inner tryptophans Trp5 and Trp19 for the direction of the tilt. The tilt direction then can change when a single arginine is inserted between Trp5 and Trp19 (20) and will change even more dramatically when there exist additional tryptophans more distant from the bilayer center (Fig. 7). The added outer tryptophans seem not only to introduce additional dynamics (36) but also to confuse the issue of the preferred tilt, namely in WWALP23, WALP19, and WALP23. Indeed, the extra outer tryptophans not only modify the direction of peptide tilt but also flatten the dependence of the apparent tilt magnitude upon lipid bilayer thickness. In summary, for those XWALP23 membrane-spanning peptides in which only one Trp anchor is present near each end, the residues Trp5 and Trp19 seem to determine the direction of the tilt; the magnitude of the apparent tilt away from the bilayer normal scales with the lipid bilayer thickness; and the identity of residues X2 and X22, whether Gly, Lys, or Arg, exerts subtle influence upon the magnitude of the apparent tilt. When additional tryptophans Trp2 and Trp22 are present, the patterns of peptide behavior and response to lipid environment become not only altered but also less systematic.
Supplementary Material
Acknowledgment
The NMR facility was supported by National Institutes of Health Grant RR 15569.
This work was supported in part by National Science Foundation Grant MCB-0841227 and by the Arkansas Biosciences Institute.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. S1–S9.
- DMPC
- 1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DLPC
- 1,2-dilauroyl-sn-glycero-3-phosphocholine
- DOPC
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
- Fmoc
- fluorenylmethoxycarbonyl
- GALA
- geometric analysis of labeled alanines
- P/L
- peptide to lipid ratio
- r.m.s.d.
- root mean squared deviation.
REFERENCES
- 1.Yau W. M., Wimley W. C., Gawrisch K., White S. H. (1998) Biochemistry 37, 14713–14718 [DOI] [PubMed] [Google Scholar]
- 2.Chiang C. S., Shirinian L., Sukharev S. (2005) Biochemistry 44, 12589–12597 [DOI] [PubMed] [Google Scholar]
- 3.Gibbons W. J., Jr., Karp E. S., Cellar N. A., Minto R. E., Lorigan G. A. (2006) Biophys. J. 90, 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stopar D., Spruijt R. B., Hemminga M. A. (2006) Chem. Phys. Lipids 141, 83–93 [DOI] [PubMed] [Google Scholar]
- 5.Page R. C., Lee S., Moore J. D., Opella S. J., Cross T. A. (2009) Protein Sci. 18, 134–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Heijne G. (1986) EMBO J. 5, 3021–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granseth E., von Heijne G., Elofsson A. (2005) J. Mol. Biol. 346, 377–385 [DOI] [PubMed] [Google Scholar]
- 8.Killian J. A., Salemink I., de Planque M. R., Lindblom G., Koeppe R. E., 2nd, Greathouse D. V. (1996) Biochemistry 35, 1037–1045 [DOI] [PubMed] [Google Scholar]
- 9.Kovacs F. A., Denny J. K., Song Z., Quine J. R., Cross T. A. (2000) J. Mol. Biol. 295, 117–125 [DOI] [PubMed] [Google Scholar]
- 10.Nomura K., Ferrat G., Nakajima T., Darbon H., Iwashita T., Corzo G. (2005) Biophys. J. 89, 4067–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White S. H., von Heijne G. (2005) Curr. Opin. Struct. Biol. 15, 378–386 [DOI] [PubMed] [Google Scholar]
- 12.de Planque M. R., Greathouse D. V., Koeppe R. E., 2nd, Schäfer H., Marsh D., Killian J. A. (1998) Biochemistry 37, 9333–9345 [DOI] [PubMed] [Google Scholar]
- 13.Ozdirekcan S., Rijkers D. T., Liskamp R. M., Killian J. A. (2005) Biochemistry 44, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 14.de Planque M. R., Bonev B. B., Demmers J. A., Greathouse D. V., Koeppe R. E., 2nd, Separovic F., Watts A., Killian J. A. (2003) Biochemistry 42, 5341–5348 [DOI] [PubMed] [Google Scholar]
- 15.de Planque M. R., Kruijtzer J. A., Liskamp R. M., Marsh D., Greathouse D. V., Koeppe R. E., 2nd, de Kruijff B., Killian J. A. (1999) J. Biol. Chem. 274, 20839–20846 [DOI] [PubMed] [Google Scholar]
- 16.de Planque M. R., Boots J. W., Rijkers D. T., Liskamp R. M., Greathouse D. V., Killian J. A. (2002) Biochemistry 41, 8396–8404 [DOI] [PubMed] [Google Scholar]
- 17.Strandberg E., Morein S., Rijkers D. T., Liskamp R. M., van der Wel P. C., Killian J. A. (2002) Biochemistry 41, 7190–7198 [DOI] [PubMed] [Google Scholar]
- 18.van der Wel P. C., Strandberg E., Killian J. A., Koeppe R. E., 2nd (2002) Biophys. J. 83, 1479–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vostrikov V. V., Grant C. V., Daily A. E., Opella S. J., Koeppe R. E., 2nd (2008) J. Am. Chem. Soc. 130, 12584–12585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vostrikov V. V., Hall B. A., Greathouse D. V., Koeppe R. E., 2nd, Sansom M. S. P. (2010) J. Am. Chem. Soc. 132, 5803–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ten Kortenaar P. B. W., Van Dijk B. G., Peeters J. M., Raaben B. J., Adams P. J. H. M., Tesser G. I. (1986) Int. J. Pept. Protein Res. 27, 398–400 [Google Scholar]
- 22.Greathouse D. V., Koeppe R. E., 2nd, Providence L. L., Shobana S., Andersen O. S. (1999) Methods Enzymol. 294, 525–550 [DOI] [PubMed] [Google Scholar]
- 23.Daily A. E., Greathouse D. V., van der Wel P. C., Koeppe R. E., 2nd (2008) Biophys. J. 94, 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isidro-Llobet A., Alvarez M., Albericio F. (2009) Chem. Rev. 109, 2455–2504 [DOI] [PubMed] [Google Scholar]
- 25.Aisenbrey C., Bechinger B. (2004) J. Am. Chem. Soc. 126, 16676–16683 [DOI] [PubMed] [Google Scholar]
- 26.Davis J. H., Jeffrey K. R., Bloom M., Valic M. I., Higgs T. P. (1976) Chem. Phys. Lett. 42, 390–394 [Google Scholar]
- 27.Thomas R., Vostrikov V. V., Greathouse D. V., Koeppe R. E., 2nd (2009) Biochemistry 48, 11883–11891 [DOI] [PubMed] [Google Scholar]
- 28.Strandberg E., Ozdirekcan S., Rijkers D. T., van der Wel P. C., Koeppe R. E., 2nd, Liskamp R. M., Killian J. A. (2004) Biophys. J. 86, 3709–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strandberg E., Esteban-Martín S., Salgado J., Ulrich A. S. (2009) Biophys. J. 96, 3223–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakrabartty A., Kortemme T., Padmanabhan S., Baldwin R. L. (1993) Biochemistry 32, 5560–5565 [DOI] [PubMed] [Google Scholar]
- 31.Strömstedt A. A., Pasupuleti M., Schmidtchen A., Malmsten M. (2009) Biochim. Biophys. Acta. 1788, 1916–1923 [DOI] [PubMed] [Google Scholar]
- 32.Hautbergue G. M., Golovanov A. P. (2008) J. Magn. Res. 191, 335–339 [DOI] [PubMed] [Google Scholar]
- 33.Page R. C., Kim S., Cross T. A. (2008) Structure 16, 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell A. M., Koeppe R. E., 2nd, Andersen O. S. (1990) Science 250, 1256–1259 [DOI] [PubMed] [Google Scholar]
- 35.Separovic F., Gehrmann J., Milne T., Cornell B. A., Lin S. Y., Smith R. (1994) Biophys. J. 67, 1495–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt A., Rougier L., Réat V., Jolibois F., Saurel O., Czaplicki J., Killian J. A., Milon A. (2010) Biophys. J. 98, 1864–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morein S., Killian J. A., Sperotto M. M. (2002) Biophys. J. 82, 1405–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozdirekcan S., Etchebest C., Killian J. A., Fuchs P. F. J. (2007) J. Am. Chem. Soc. 129, 15174–15181 [DOI] [PubMed] [Google Scholar]
- 39.Esteban-Martín S., Salgado J. (2007) Biophys. J. 93, 4278–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteban-Martín S., Strandberg E., Fuertes G., Ulrich A. S., Salgado J. (2009) Biophys. J. 96, 3233–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vostrikov V. V., Grant C. V., Opella S. J., Koeppe R. E., 2nd (2009) Biophys. J. 96, 454a.(abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esteban-Martín S., Giménez D., Fuertes G., Salgado J. (2009) Biochemistry 48, 11441–11448 [DOI] [PubMed] [Google Scholar]
- 43.Holt A., Koehorst R. B., Rutters-Meijneke T., Gelb M. H., Rijkers D. T., Hemminga M. A., Killian J. A. (2009) Biophys. J. 97, 2258–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Wel P. C., Reed N. D., Greathouse D. V., Koeppe R. E., 2nd (2007) Biochemistry 46, 7514–7524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.