Abstract
The signaling mechanisms by which neurotrophic receptors regulate neuronal survival and axonal growth are still incompletely understood. In the receptor tyrosine kinase RET, a receptor for GDNF (glial cell line-derived neurotrophic factor), the functions of the majority of tyrosine residues that become phosphorylated are still unknown. Here we have identified the protein-tyrosine phosphatase SHP2 as a novel direct interactor of RET and the first effector known to bind to phosphorylated Tyr687 in the juxtamembrane region of the receptor. We show that SHP2 is recruited to RET upon ligand binding in a cooperative fashion, such that both interaction with Tyr687 and association with components of the Tyr1062 signaling complex are required for stable recruitment of SHP2 to the receptor. SHP2 recruitment contributes to the ability of RET to activate the PI3K/AKT pathway and promote survival and neurite outgrowth in primary neurons. Furthermore, we find that activation of protein kinase A (PKA) by forskolin reduces the recruitment of SHP2 to RET and negatively affects ligand-mediated neurite outgrowth. In agreement with this, mutation of Ser696, a known PKA phosphorylation site in RET, enhances SHP2 binding to the receptor and eliminates the effect of forskolin on ligand-induced outgrowth. Together, these findings establish SHP2 as a novel positive regulator of the neurotrophic activities of RET and reveal Tyr687 as a critical platform for integration of RET and PKA signals. We anticipate that several other phosphotyrosines of unknown function in neuronal receptor tyrosine kinases will also support similar regulatory functions.
Keywords: Differentiation, Neurotrophic Factor, Protein Kinase A (PKA), Receptor Tyrosine Kinase, Signal Transduction, GDNF, GFRα1, RET, SHP2, Neurite Outgrowth
Introduction
Neurotrophic factors regulate neuronal survival, morphology, and function and are essential for the development and maintenance of the nervous system. Together with the neurotrophins, glial cell line-derived neurotrophic factor (GDNF)4 and related family members are among the most important neurotrophic factors in mammals. Because of the potent prosurvival and neuroprotective effects of GDNF on midbrain dopaminergic neurons, much of the initial interest in GDNF was focused on its possible therapeutic effects in Parkinson disease (1, 2). It has more recently been appreciated that GDNF and related factors affect a host of important processes in mature neurons and their precursors and also outside the nervous system. GDNF signals by binding to a receptor complex formed by the ligand-binding subunit GFRα1 and the signaling subunit RET, a member of the receptor tyrosine kinase (RTK) family (3). GFRα1 also associates with the neural cell adhesion molecule, NCAM, to mediate the effects of GDNF independently of RET (4–6). Some of the activities of GDNF can also be mediated by GFRα1 in the absence of either RET or NCAM, suggesting alternative mechanisms of transmembrane signaling (7, 8). A thorough understanding of the molecular mechanisms by which GDNF functions in different contexts will be essential to elucidating its contribution to nervous system development and exploiting its therapeutic potential.
Upon activation, RET undergoes autophosphorylation of intracellular tyrosine residues, which then serve as docking sites for downstream signaling effectors carrying Src homology 2 (SH2) or phosphotyrosine-binding domains. Previous studies have indicated that at least 14 of the 18 tyrosine residues present in the intracellular region of RET can become phosphorylated (9–11). Among those, Tyr900 and Tyr905 are present in the kinase activation loop and are known to be required for full activation (10). (The numbering of RET residues throughout this article corresponds to the human RET protein.) Of the remaining residues, however, only four (i.e. Tyr981, Tyr1015, Tyr1062, and Tyr1096) have actually been shown to directly bind a downstream effector in response to GDNF (12–15). The function of the majority of phosphorylated tyrosine residues in RET, including Tyr687, remains unknown.
A previous study showed that RET can be phosphorylated on Ser696 by PKA and that mutation of Ser696 affects the ability of RET to activate the small GTPase Rac1 and stimulate formation of cell lamellipodia (16). Interestingly, this deficit could be alleviated by simultaneous mutation of the nearby residue Tyr687. On the basis of these findings, it has been suggested that phosphorylation of Ser696 and Tyr687 may induce opposite effects on RET signaling (16, 17). However, the specific function of Tyr687 has not been established.
The location of Tyr687 in the juxtamembrane region of the receptor, a domain known to exhibit regulatory functions in other RTKs, suggests that it may be important for RET activation or some of its downstream effects. In the present study, we investigated the role of Tyr687 in RET signaling by screening for proteins capable of interacting with RET through this phosphorylated residue. We identified the protein-tyrosine phosphatase SHP2 as a direct and specific interactor of phospho-Tyr687. SHP2 contains two SH2 domains followed by a C-terminal phosphatase domain, and its most N-terminal SH2 domain has been shown to bind distinct phospho-Tyr residues in activated RTKs and some of their downstream phosphorylated adaptor proteins, such as Gab2 (18). SHP2 plays important roles in the regulation of the Ras/MAPK and PI3K/AKT pathways (18), and germ line mutations in the gene encoding SHP2 can lead to the Noonan and LEOPARD syndromes, characterized by cardiac abnormalities and dysmorphic facial features (18, 19). In an earlier study, we identified SHP2 as a component of a larger protein complex that is recruited to phospho-Tyr1062 in the tail of the activated RET receptor through a series of adaptor proteins, including Gab2, Grb2, and Shc (12). In that complex, Shc was shown to interact directly with phospho-Tyr1062, whereas Gab2 associated with Shc via Grb2, and SHP2 was bound to Gab2. In the present study, we reveal an unexpected role for the SHP2 phosphatase as a positive regulator of the neurotrophic effects of GDNF and identify Tyr687 as a novel site for signal integration in the RET receptor.
EXPERIMENTAL PROCEDURES
T7 Phage Display
A T7 phage display cDNA library was prepared from differentiated PC12 cells according to the manufacturer's instructions (Novagen). A synthetic phosphopeptide (sequence RRPAQAFPVS(pY)SSSGARRPS) carrying an N-terminal biotin followed by a double e-aminocaproic acid spacer was used as bait for library screening and phage micropanning as described previously (20).
Cell Culture and Transfection
MN1 is a motoneuron cell line that endogenously expresses RET and GFRα1 and responds to GDNF (21, 22). M23 cells were derived from MG87 fibroblasts (in turn, a subclone of NIH3T3 cells) by stable transfection of GFRα1 (12). COS, MN1, and M23 cells were grown in DMEM, 10% fetal bovine serum (FBS), and 1 mm glutamine. PC12 cells were grown in DMEM, 10% horse serum, 5% FBS, and glutamine on collagen-coated tissue culture plates. COS cells were transiently transfected with polyethylenimine (PEI, 2 mg/ml) and PC12 with Lipofectamine 2000 (Invitrogen). For assays of cell morphology, plasmids encoding either EGFP or dsRED (Clontech) were co-transfected with RET and control constructs. M23 cells were stably transfected with different RET constructs using PEI and grown in the presence of neomycin. All experiments were performed using at least two different cell clones carrying each construct. Transfection of sympathetic neurons from the superior cervical ganglion (SCG) was performed using a MicroPorator MP-100 (Digital Bio Technology) with two 30-ms pulses of 1100 V in resuspension buffer according to the manufacturer's instructions.
Immunoprecipitation, GST Pulldown, and Phosphatase Activity
Total cell lysates were obtained by extracting cell monolayers with buffer containing 1% Triton X-100 as described previously (23). One-tenth of the sample was saved aside and the rest used for overnight immunoprecipitation at 4 °C. The collection of immunoprecipitates and Western blotting followed standard techniques (23). The antibodies used were as follows: SHP2 C18 (sc-280), RET9 C-19 (sc-167), and RET51 C-29 (sc-1290) from Santa Cruz Biotechnology; phosphotyrosine 4G10 (05-321) from Chemicon; and phospho-ERK (9101), phospho-Src (2101), phospho-JNK (9251), phospho-AKT (9271), and total AKT (9272) from Cell Signaling. panRET monoclonal was provided by Anatoly Sharipo (University of Latvia, Riga). GST fusion proteins were produced in bacteria, coupled to glutathione-conjugated agarose beads (GE Healthcare), and used for pulldowns from cell lysates as described previously (24). All blots were developed using alkaline phosphatase-conjugated secondary antibodies and an enhanced chemifluorescence development reagent (GE Healthcare) and visualized in a Storm scanner (GE Healthcare). Phosphatase activity was assayed using a kit from Promega.
PC12 Cell Neurite Outgrowth
Twelve hours after transfection, PC12 cells were switched to differentiation medium (DMEM, 0.5% horse serum, 0.25% FBS, and glutamine) in the presence or absence of 50 ng/ml GDNF (R&D Systems) together with 150 ng/ml GFRα1-Fc (R&D Systems). Cells were left to differentiate for 3 or 6 days as indicated and subsequently imaged with an Axiovert 200 microscope (Zeiss). The number of EGFP-expressing cells carrying neurites at least twice the size of the cell soma was counted and then normalized to the total number of transfected cells. At least 300 cells from four different fields/well were counted for each construct.
SCG Primary Culture, Neurite Outgrowth, and Cell Survival
SCG were dissected from E21.5 rat embryos, dissociated by trypsin treatment, and transfected by microporation as described above. For neurite outgrowth assays, SCG neurons transfected with RET constructs were incubated in Neurobasal medium supplemented with B27 and glutamine in the presence of 1 ng/ml nerve growth factor (NGF; Alomone). A positive control was also set up with 100 ng/ml NGF on mock-transfected cells. After day 1 in vitro (DIV1), the cultures were photographed and the longest primary neurite was measured using Openlab software (Improvision). For cell survival assays, transfected SCG neurons were first cultured overnight in Neurobasal medium supplemented with B27, glutamine, and 10 ng/ml NGF. The following day, the medium was replaced with fresh medium containing an NGF-blocking antibody (Sigma). NGF at 10 ng/ml was used in mock-transfected cells as positive control. Cultures were photographed daily from DIV1 (always the same fields) on an Axiovert 200 microscope (Zeiss). Axonal fragmentation followed by cell soma collapse was assessed to quantify cell death.
RESULTS
Identification of SHP2 as a Direct and Specific Interactor of Phospho-Tyr687 in RET
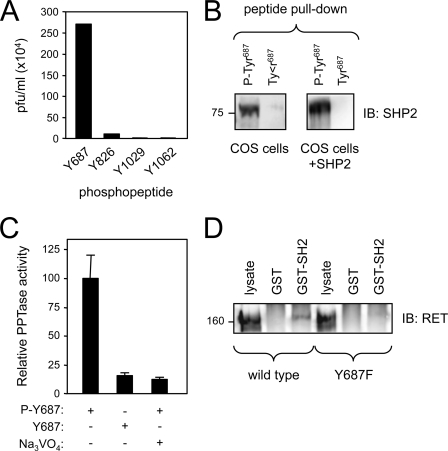
A phosphopeptide derived from human RET encompassing phospho-Tyr687 was used as bait to screen a T7 phage display cDNA library. The screen retrieved a phage clone encoding the N-terminal SH2 domain of the protein-tyrosine phosphatase SHP2. Micropanning experiments revealed that purified particles from this phage interacted specifically with the phospho-Tyr687 peptide but not with other phosphopeptides derived from other phospho-Tyr residues in RET (Fig. 1A). We note that the N-terminal SH2 domain of SHP2 has a loose consensus target sequence with Leu or Val at the −2 position, which conforms to the sequence surrounding Tyr687 in RET. The specific binding of full-length SHP2 to the phospho-Tyr687 peptide was tested in pulldown assays using agarose beads coupled to either phosphorylated or non-phosphorylated peptides encompassing Tyr687. SHP2 could only be recovered from COS cell total lysates by beads containing the phosphopeptide, indicating that the interaction was phosphorylation-dependent (Fig. 1B). Binding of phosphopeptides to the N-terminal SH2 domain of SHP2 is thought to relieve an intrinsic inhibitory conformation that blocks phosphatase activity (18). We therefore tested the effects of Tyr687 peptides on SHP2 activity. We found that the in vitro phosphatase activity of SHP2 recovered in immunoprecipitates from transfected COS cells was strongly enhanced by the addition of the phospho-Tyr687 peptide but not by the corresponding non-phosphorylated peptide and could be blocked by the phosphatase inhibitor Na3VO4 (Fig. 1C). Finally, in a complementary experiment, we tested the ability of the N-terminal SH2 domain of SHP2 to interact with full-length RET in a GST pulldown assay. A GST fusion protein containing SHP2 N-terminal SH2 domain, but not GST alone, was able to precipitate wild type RET from total lysates of transfected COS cells (Fig. 1D). We and others have shown previously that wild type RET can become activated upon overexpression in COS cells even in the absence of ligand (25). Importantly, a mutant RET carrying Phe instead of Tyr at position 687 (denoted as Y687F) failed to be recovered by the SH2 domain of SHP2 (Fig. 1D), underscoring the specificity of the interaction.
FIGURE 1.
Identification of SHP2 as a direct interactor of phospho-Tyr687 in RET. A, micropanning of phage particles expressing the N-terminal SH2 domain of SHP2 against different phosphopeptides derived from RET. Results are expressed as particle-forming units (pfu)/ml. B, peptide pulldown of COS cell lysates analyzed by SHP2 immunoblotting (IB). Endogenous (left) as well as overexpressed SHP2 (right) could be recovered by pulldown with the phospho-Tyr687 peptide but not with its unphosphorylated form. C, stimulation of in vitro SHP2 phosphatase activity by phospho-Tyr687 peptide. Phosphatase activity is expressed in arbitrary units after normalization. The non-phosphorylated peptide had no effect. The phosphatase inhibitor Na3VO4 reduced SHP2 phosphatase activity to background levels. D, pulldown of RET constructs from COS cell lysates with a GST fusion protein containing the N-terminal SH2 domain of SHP2. The blot was probed with anti-RET antibodies. Wild type, but not Y687F mutant, interacted with the N-SH2 domain of SHP2. GST was used as negative control.
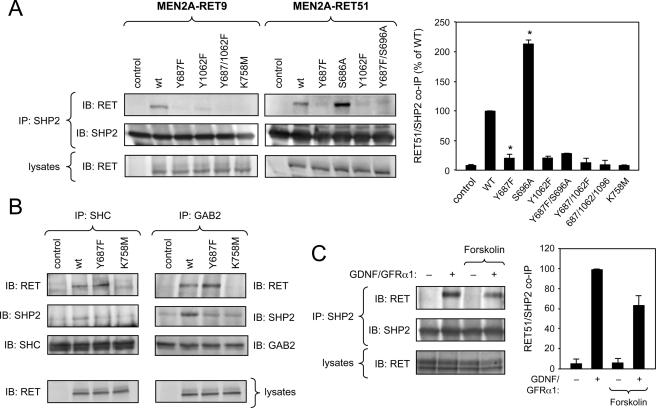
The association between full-length SHP2 and RET was investigated in COS cells transfected with plasmid constructs expressing a constitutively activated form of RET found in type 2A multiple endocrine neoplasia (MEN2A). RET MEN2A isoforms carry a C634R substitution in the extracellular domain that results in stabilization of RET dimers by an aberrant disulfide bridge leading to robust activation in the absence of ligand (26). Because of its mechanism of activation, the MEN2A mutation recapitulates all of the major features of ligand-mediated RET activity. Immunoprecipitation of endogenous SHP2 from lysates of transfected COS cells recovered the constitutively active form of RET carrying the MEN2A mutation in an otherwise wild type receptor (Fig. 2A, WT). SHP2 interacted with both RET9 and RET51 isoforms to a comparable degree. (In Fig. 2 and subsequent figures, RET9 and RET51 refer to two RET isoforms generated by the alternative splicing of two short exons located at the end of the gene about 400 residues downstream of Tyr687, leading to a C-terminal tail of 9 or 51 residues, respectively (27).) As expected, mutation of Lys758 in the ATP binding site of the RET kinase also abolished RET binding to SHP2. Importantly, the amount of RET proteins recovered by co-immunoprecipitation with SHP2 was significantly reduced by the Y687F mutation (Fig. 2A), thereby validating Tyr687 as a novel site of SHP2 binding in RET. Given that SHP2 had also been shown to associate with RET through the Gab2-Grb2-Shc complex, which is recruited to phospho-Tyr1062 (12, 15), we were expecting that a significant amount of the Y687F RET mutant would remain bound to SHP2. However, mutation of Tyr687 reduced SHP2 binding to almost background levels. It remained possible that the interaction of SHP2 with Tyr687 was much stronger than its association with the Tyr1062 signaling complex. However, mutation of Tyr1062 also resulted in an almost complete elimination of RET/SHP2 co-immunoprecipitation, despite the fact that Tyr687 was intact in this mutant (Fig. 2A). As expected, simultaneous mutation of Tyr687 and Tyr1062 completely eliminated SHP2 binding to RET (Fig. 2A). The behavior of the individual Tyr mutants suggested that SHP2 may not be recruited to the two sites independently but perhaps as part of a single signaling complex. To test this idea, we investigated the impact of the Y687F mutation on the assembly of the Tyr1062 signaling complex by looking at the levels of RET and SHP2 recovered in Shc and Gab2 immunoprecipitates. RET51 MEN2A proteins carrying wild type, Y687F, or kinase-dead K758M intracellular domains were compared in these experiments. As expected, wild type (but not kinase-dead) MEN2A RET could be recovered in Shc and Gab2 immunoprecipitates (Fig. 2B). Mutation of Tyr687 did not affect the association of either adaptor with the receptor (Fig. 2B), indicating that it does not interfere with the recruitment of receptor-proximal components of the Tyr1062 signaling complex. This also confirms that the Y687F mutation does not affect phosphorylation of Tyr1062. As expected, activated RET enhanced the association of SHP2 with either Shc or Gab2 above basal levels. In contrast, the Y687F mutant was unable to stimulate the interaction of SHP2 with either protein (Fig. 2B), suggesting that SHP2 binding to Tyr687 is required for its stable association with the Tyr1062 signaling complex.
FIGURE 2.
SHP2 binding determinants in RET and regulation by PKA activity. A, analysis of co-immunoprecipitation (IP) of SHP2 with constitutively activated MEN2A constructs of RET9 and RET51 isoforms in COS cells. The blots (IB) show representative examples of a series of experiments. The histogram summarizes SHP2/RET interaction results for the RET51 isoform from three independent experiments. Results are expressed as the average ± S.D. of normalized RET levels in SHP2 immunoprecipitates. *, p < 0.05 (versus WT). B, analysis of RET and SHP2 co-immunoprecipitation with components of the Tyr1062 signaling complex, Shc and Gab2. Immunoblotting of total cell lysates (bottom) shows comparable levels of RET51 MEN2A proteins. C, interaction of endogenous SHP2 and RET in the motoneuron cell line MN1. Prior to SHP2 immunoprecipitation, cells had been serum-starved and stimulated with GDNF and soluble GFRα1 for 20 min as indicated. Pretreatment with forskolin for 2 h resulted in a reduction of SHP2/RET co-immunoprecipitation. The histogram summarizes the results of the average ± S.D. of four independent experiments.
Activation of PKA Interferes with SHP2 Binding to RET via Tyr687
As explained above, a previous study described an epistatic interaction between mutations in Tyr687 and Ser696 and proposed that phosphorylation of RET by PKA in Ser696 may affect the function of Tyr687 (16). Interestingly, we found that mutation of Ser696 into Ala enhanced the interaction of RET with SHP2 (Fig. 2A). This effect could be abolished by concomitant mutation of Tyr687, in agreement with a functional interaction between the two phosphorylation sites. We explored this relationship further in cells expressing endogenous RET and SHP2 proteins. MN1 is a motoneuron cell line derived from immortalized motoneuron precursors, which expresses RET and responds to GDNF (22). We found that treatment of MN1 cells with GDNF together with soluble GFRα1, which further enhances GDNF responses in these cells (28), strongly stimulated SHP2 binding to RET (Fig. 2C), showing that endogenous SHP2 and RET can interact in a ligand-dependent manner in intact cells. Importantly, this interaction was reduced by pretreatment with the adenylate cyclase activator forskolin, which resulted in rapid stimulation of PKA activity (Fig. 2C). Together, these data suggest that phosphorylation of RET by PKA in Ser696 interferes with the recruitment of SHP2 to Tyr687 in the activated receptor.
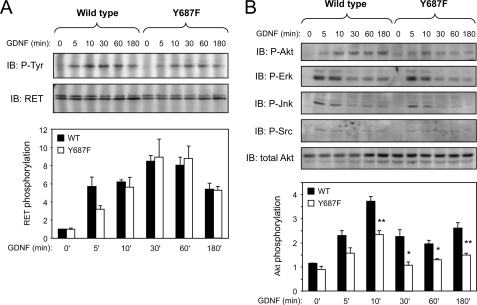
Mutation of Tyr687 in RET Impairs GDNF-dependent AKT Signaling
To investigate the signaling roles of Tyr687, we generated clonal fibroblast cell lines stably expressing GFRα1 together with either wild type RET51 or the Y687F mutant. The Y687F mutant was expressed at levels comparable with the wild type and displayed a similar maximal level of tyrosine phosphorylation upon GDNF treatment (Fig. 3A). Analysis of downstream targets of RET signaling revealed an impairment of the Y687F mutant to activate the AKT signaling pathway (Fig. 3B). At 5 min of stimulation, activation of AKT by the Y687F mutant was significantly lower than that observed with wild type RET, a deficit that continued through later time points of receptor activation. Other pathways, such as those involving activation of ERK, JNK, and Src kinases, were not affected by mutation of Tyr687 (Fig. 3B).
FIGURE 3.
Mutation of Tyr687 in RET impairs GDNF-dependent AKT signaling. A, analysis of RET tyrosine autophosphorylation in response to GDNF stimulation in stably transfected fibroblast cell lines expressing wild type or Y687F mutant RET51. RET immunoprecipitates were probed with anti-phosphotyrosine antibodies. The histogram shows the average results in arbitrary units normalized to total RET levels from three experiments using two different sets of wild type and mutant RET-expressing cell clones. No significant differences were observed between Y687F and wild type. B, activation of downstream signaling pathways by wild type and Y687F mutant RET51 following GDNF stimulation of stably transfected fibroblast cell lines. Total cell lysates were analyzed with the indicated phospho-specific antibodies. Reprobing against total Akt shows equal loading in all lanes. The blots (IB) show representative examples of several experiments. The histogram shows average results of Akt phosphorylation in arbitrary units normalized to total Akt levels from four experiments. *, p < 0.05, **, p < 0.005.
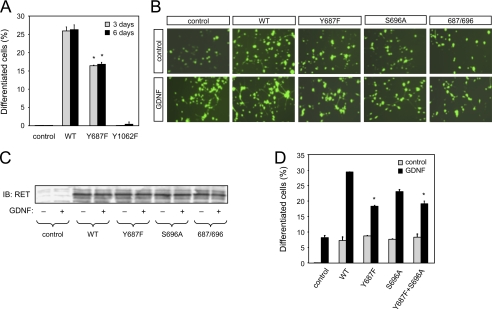
Mutation of Tyr687 or SHP2 Inhibition Affects the Ability of RET to Induce Neuronal Differentiation of PC12 Cells
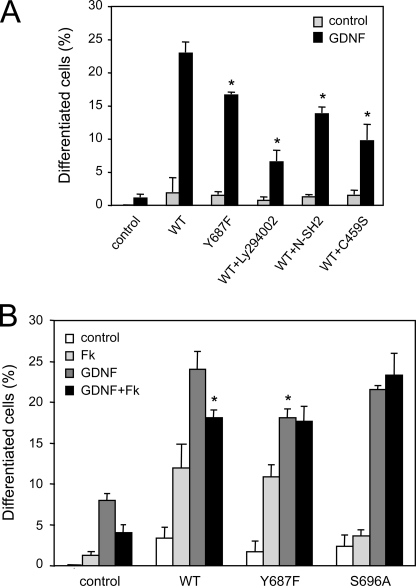
Activation of RET by the MEN2A mutation in the membrane-proximal region of its extracellular domain can drive neuronal differentiation of PC12 cells (29). We exploited this property to compare the ability of MEN2A wild type and Y687F mutant RET constructs to elicit neurite outgrowth in PC12 cells at 3 or 6 days after transfection and serum withdrawal. In the MEN2A background, the Y678F mutant was less efficient than wild type RET at inducing neuronal differentiation of PC12 cells at either time point (Fig. 4A). As expected from previous work (30), mutation of Tyr1062 completely abolished the ability of RET to induce neurite outgrowth (Fig. 4A). We then compared the ability of GDNF to induce neuronal differentiation of PC12 cells expressing different RET variants independently of the MEN2A mutation. PC12 cells normally express low levels of RET, so stimulation with GDNF and soluble GFRα1 induced some level of neurite outgrowth even in naive or mock-transfected cells (Fig. 4, B–D). Overexpression of wild type RET also elicited some level of differentiation by itself but conferred a strong ligand-dependent neurite induction effect. Under these conditions, a RET construct carrying the Y687F mutation was significantly less efficient than wild type in mediating ligand-dependent neurite extension in PC12 cells, confirming the deficit in neuronal differentiation of this mutant (Fig. 4, B–D). Mutation of Ser696 had only a modest effect on RET activity in this assay, and the double mutant Y687F/S696A was not different from Y687F on its own.
FIGURE 4.
Mutation of Tyr687 affects the ability of RET to induce neuronal differentiation of PC12 cells. A, neuronal differentiation of PC12 cells expressing constitutively activated MEN2A RET51 constructs. Cells displaying neuritic extensions longer than 2 cell diameters were counted at 3 and 6 days post-transfection. The results were normalized to the total number of transfected (i.e. GFP-expressing) PC12 cells in each field and are expressed as the average ± S.D. of triplicate determinations. *, p < 0.05 versus WT. Similar results were obtained in three additional experiments. B, ligand-mediated neuronal differentiation of PC12 cells expressing wild type and mutant RET51 constructs. Differentiation of cells transiently transfected with the indicated constructs was induced by stimulation with GDNF and soluble GFRα1 in serum-free medium. The micrographs show fields of GFP-expressing cells transfected with different RET constructs under control conditions and following ligand stimulation. C, immunoblot (IB) of lysates from transfected PC12 cells indicating similar amounts of RET for each construct and condition. D, percentage of differentiated PC12 cells for each condition and mutant construct after 3 days of treatment. Results are expressed as average ± S.D. of triplicate determinations. *, p < 0.05 versus WT + GDNF. Similar results were obtained in three additional experiments.
The detrimental effect of Y687F on neuronal differentiation could be due to an impairment in the ability of the mutant RET receptor to recruit and activate SHP2 or to defects in the activation of another, yet unidentified Tyr687 effector. We reasoned that if the former was the case, inhibition of SHP2 should result in a deficit at least comparable with that caused by the Y687F mutation. Inhibition of SHP2 activity was achieved by introducing SHP2 constructs expressing either the N-terminal SH2 domain of SHP2 or the catalytically inactive C459S point mutant, both of which can act as dominant negatives of SHP2 function (31). We found that transfection of either of these two SHP2 mutants reduced the ability of wild type RET to mediate GDNF-dependent differentiation of PC12 cells to a degree that was comparable to the effect caused by the Y687F mutation (Fig. 5A). In agreement with a role of the PI3K/AKT pathway in the neuronal differentiation of PC12 cells by the activities of RET and SHP2, the PI3K inhibitor Ly294002 also reduced GDNF-mediated neurite outgrowth (Fig. 5A). Together, these results suggest that recruitment of SHP2 to Tyr687 and activation of the PI3K/AKT pathway are important for the ability of GDNF to induce neuronal differentiation of PC12 cells via RET.
FIGURE 5.
SHP2 inhibition and PKA activation reduce neuronal differentiation of PC12 cells in response to GDNF-mediated RET activation. A, neuronal differentiation of PC12 cells expressing WT or mutant RET51 constructs co-transfected with dominant negative SHP2 constructs N-SH2 or C459S or treated with the PI3K inhibitor Ly294002. Under the conditions of this experiment, Ly294002 did not have any effect on cell survival. Results are expressed as the average ± S.D. of triplicate determinations. *, p < 0.05 versus WT + GDNF. Similar results were obtained in two additional experiments. B, neuronal differentiation of PC12 cells expressing the indicated wild type or mutant RET51 constructs following treatment with GDNF/GFRα1, forskolin, or their combination. Results are expressed as the average ± S.D. of triplicate determinations. *, p < 0.05 versus WT + GDNF. Similar results were obtained in two additional experiments.
Activation of PKA Diminishes the Ability of GDNF to Induce PC12 Cell Differentiation via RET
In view of the ability of PKA to affect the recruitment of SHP2 to RET and the importance of SHP2 and Tyr687 for RET-mediated neuronal differentiation, we investigated the effects of PKA activation by forskolin on the ability of GDNF to stimulate neurite outgrowth in PC12 cells expressing wild type and mutant RET molecules. In naive cells, forskolin had a small positive effect on neurite extension (Fig. 5B), in agreement with previous observations (32). As seen previously, RET overexpression promoted some neuronal differentiation even without ligand stimulation both in the absence or presence of forskolin. As expected, GDNF stimulated neurite outgrowth in both naive and RET-overexpressing cells. In this case, however, forskolin treatment significantly reduced GDNF-mediated differentiation of PC12 cells expressing the wild type RET receptor, phenocopying the effect of the Y687F mutation (Fig. 5B). Interestingly, GDNF-mediated differentiation of PC12 cells expressing the Y687F or S696A mutant was unaffected by forskolin treatment.
Signaling through Tyr687 Is Required for RET-mediated Neurite Outgrowth and Survival of Sympathetic Neurons
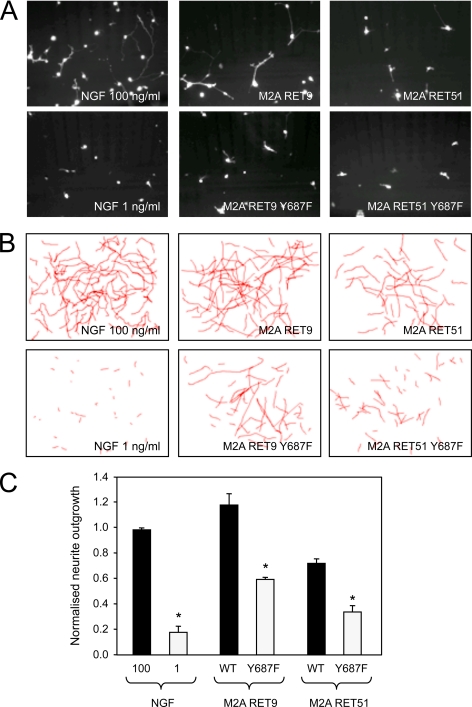
We tested the physiological significance of Tyr687 signaling in primary cells by introducing wild type and mutant RET constructs into sympathetic neurons isolated from the superior cervical ganglion of rat embryos. As these neurons express high levels of endogenous GDNF receptors, we used MEN2A-activated RET constructs in these experiments, thereby omitting the need for ligand addition. After transfection, neurons were plated in low NGF to allow cell survival prior to RET expression. By itself, this amount of NGF failed to induce any significant neurite outgrowth (Fig. 6). In contrast, cells that received RET MEN2A constructs developed neurites of a length comparable with those elicited by a high concentration of NGF. In this assay, RET51 was consistently less potent than RET9, in agreement with previous studies (27, 33). Importantly, mutation of Tyr687 reduced the neurite outgrowth evoked by either RET isoform by ∼50% (Fig. 6).
FIGURE 6.
Signaling through Tyr687 is required for RET-mediated neurite outgrowth in sympathetic neurons. A, representative micrographs of dsRed-expressing SCG neurons 30 h after microporation with the indicated constructs maintained in the presence of 1 ng/ml NGF. Examples from wells maintained in only 100 and 1 ng/ml NGF are also shown. B, camera lucida drawings depicting the longest neurites of transfected SCG neurons in 10 different fields superimposed onto each other. C, neurite outgrowth in SCG neurons transfected with wild type or Y687F mutant MEN2A RET constructs. Neurite outgrowth was measured as the length of the longest neurite in each transfected neuron and normalized to the average values obtained with 100 ng/ml NGF. Results are expressed as the average ± S.D. of three independent experiments each performed in triplicate. *, p < 0.05 versus WT (corresponding solid bars).
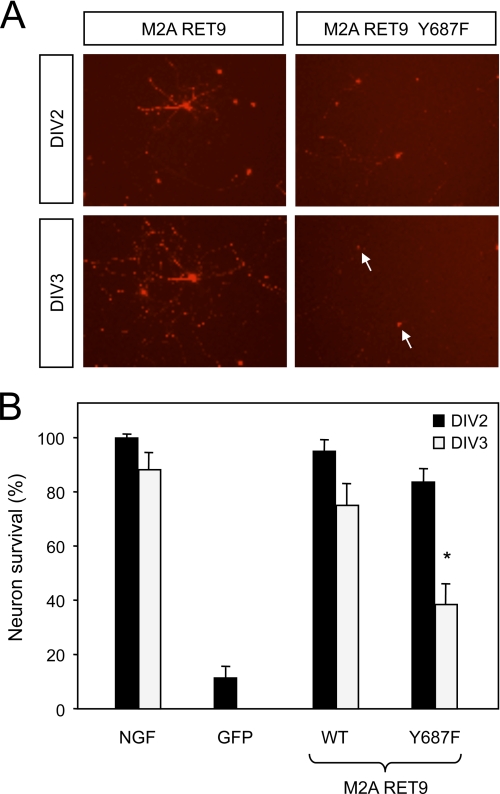
As the PI3K/AKT pathway has also been implicated in neuronal survival (34), we investigated the importance of Tyr687 signaling in RET-mediated survival of sympathetic neurons. As noted previously, a low amount of NGF was used immediately after transfection to allow cell recovery. On the following day (DIV1), NGF was depleted by the addition of NGF-blocking antibodies, whereas control cultures were left in the presence of NGF. Massive cell death was observed in mock-transfected neurons. In contrast, cells that received the RET MEN2A construct were rescued to levels comparable with those displayed by cultures that remained in NGF (Fig. 7). Significantly, reduced survival was observed in neurons that received the Y687F mutant construct, particularly at DIV3 (Fig. 7). Together, these data demonstrate the importance of Tyr687 signaling for the neurotrophic activity of RET in sympathetic neurons.
FIGURE 7.
Signaling through Tyr687 contributes to RET-mediated survival of sympathetic neurons. A, photomicrographs of SCG neurons co-transfected with dsRed and the indicated RET constructs. SCGs were grown in NGF overnight and then switched to medium containing NGF-blocking antibodies. Arrows denote dead cells. B, survival of SCG neurons transfected with wild type or Y687F mutant RET constructs or GFP as negative control. Survival is indicated as a percentage of cell number relative to DIV1 (which was arbitrarily set to 100%). Results are expressed as the average ± S.D. of three independent experiments each performed in quadruplicate. *, p < 0.05 versus WT at DIV3.
DISCUSSION
Although the signaling pathways activated by neurotrophic receptors have been studied extensively, the processes that fine-tune receptor output and integrate receptor signaling with the activity of other pathways remain to be elucidated. Our finding that Tyr687 coordinates the recruitment of SHP2 to RET and contributes to RET neurotrophic activity reveals the first known function of this autophosphorylation site and a novel role for SHP2 in RET signaling. Although the related protein-tyrosine phosphatase SHP1 functions primarily as a negative regulator of RTK signaling (35), SHP2 has been shown to contribute positively to the activation of downstream pathways in several contexts (18).
Although the juxtamembrane region of the RET intracellular domain was not revealed in a recent crystallographic study of the RET kinase (10), juxtamembrane domains are known to serve important regulatory functions in other RTKs. Recruitment of SHP2 to Tyr687 could establish a new signaling platform working in parallel with the signaling complex that assembles at Tyr1062, the main output site of RET activity (12). Alternatively, it could work in concert with Tyr1062 by regulating complex assembly or function. Intriguingly, although Tyr687 is a direct binding site for SHP2, mutation of Tyr1062 reduced the recruitment of the phosphatase to nearly background levels. Conversely, although SHP2 can be recruited to the Tyr1062 signaling complex, mutation of Tyr687 also reduced the association of SHP2 with RET almost completely. This mutation, however, did not affect the recruitment of either Shc or Gab2 to the receptor, indicating that phosphorylation of Tyr1062 is not perturbed by the Tyr687 mutation. Together, these observations suggest that SHP2 may not be recruited independently to Tyr687 and Tyr1062. In agreement with this idea, we found that the association of SHP2 with Shc and Gab2, two components of the Tyr1062 signaling complex, was diminished after mutation of Tyr687. SHP2 contains two SH2 domains in its N-terminal region and has been found to form homodimers through its C-terminal domain (36), so it is possible that SHP2 may be able to interact with at least two phospho-Tyr targets simultaneously. In this regard, it was observed previously that the two SH2 domains of the p85 subunit of PI3K can each bind a separate site on receptors such as P-Tyr741 and P-Tyr750 in PDGFR (platelet-derived growth factor receptor) (37). Moreover, it has also been shown that Gab1 requires P-Tyr1349 and P-Tyr1356 for c-Met binding (38), PTP1B (phosphotyrosine phosphatase 1B) requires P-Tyr1234 and P-Tyr1235 for c-Met binding (39), and RasGap requires P-Tyr604 and P-Tyr610 for EphB2 binding (40). In all of these cases, however, the two phosphorylated sites are in close proximity. Although Tyr687 and Tyr1062 are further apart in RET, it should be considered that the Shc-Gab2 complex bound to Tyr1062 could help to bridge the distance between the two residues for efficient SHP2 binding. On the basis our results, we propose that stable recruitment of SHP2 to activated RET requires its association with both Tyr687 and the Tyr1062 signaling complex in a cooperative fashion. In this way, SHP2 may contribute to the stabilization of a network of SH2, SH3, and phosphotyrosine-binding domain interactions that integrates the output of these two tyrosine residues and results in the efficient activation of PI3K. The molecular details of the mechanisms by which SHP2 contributes to RET-mediated PI3K signaling remain to be elucidated.
As cells are normally exposed to many different signals simultaneously, a full understanding of cell behavior under physiological conditions will require elucidation of the ways in which different signaling pathways interact with each other. cAMP is a crucial second messenger downstream of G protein-coupled receptors, and PKA is one of its major intracellular effectors. Although G protein-coupled receptors are ubiquitous in neurons, the effects of PKA on neurotrophic receptor signaling are poorly understood. Our findings indicate that activation of PKA reduces the interaction of SHP2 with RET and diminishes GDNF-mediated neurite outgrowth. The fact that the effect of forskolin on RET function was abolished by mutation of Ser696 is in agreement with this being a PKA phosphorylation site in the receptor, as proposed previously (16). Because of its proximity to Tyr687, phosphorylation of Ser696 could interfere with the phosphorylation of Tyr687 or with the binding of SHP2 to this residue either sterically or electrostatically.
Mutation of Tyr687 had a pronounced effect on the neurotrophic activities of RET in sympathetic neurons, particularly on its ability to induce neurite outgrowth. SHP2 can thus function as a positive regulator of the activities of neurotrophic receptors, an observation that is in agreement with the effects of dominant negative mutants of SHP2 in PC12 cell differentiation. Mutations in PTPN11, the human gene encoding SHP2, have been found in patients suffering from Noonan and Leopard syndromes, characterized by facial and cardiac abnormalities and often associated with mental retardation (18, 19). In mice, the disruption of SHP2 function in neural crest cells results in deficits in glial development that resemble defects in neuregulin signaling (41). SHP2 is thus prominently placed to contribute to several aspects of neural development through its ability to regulate neurotrophic factor signaling.
Acknowledgments
We thank Masahide Takahashi for the S696A mutant construct used in preliminary experiments. We also thank Drs. David Kaplan and Anthony Pawson for advice on cooperative binding of RTK effectors.
This work was supported by grants from the Swedish Research Council, the Swedish Cancer Society, and the European Union VIth and VIIth Framework Programs.
- GDNF
- glial cell line-derived neurotrophic factor
- RTK
- receptor tyrosine kinase
- SH2 and SH3
- Src homology 2 and 3, respectively
- PKA
- protein kinase A
- SCG
- superior cervical ganglion
- DIV
- day in vitro.
REFERENCES
- 1.Gash D. M., Zhang Z., Ovadia A., Cass W. A., Yi A., Simmerman L., Russell D., Martin D., Lapchak P. A., Collins F., Hoffer B. J., Gerhardt G. A. (1996) Nature 380, 252–255 [DOI] [PubMed] [Google Scholar]
- 2.Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. (1993) Science 260, 1130–1132 [DOI] [PubMed] [Google Scholar]
- 3.Airaksinen M. S., Saarma M. (2002) Nat. Rev. Neurosci. 3, 383–394 [DOI] [PubMed] [Google Scholar]
- 4.Iwase T., Jung C. G., Bae H., Zhang M., Soliven B. (2005) J. Neurochem. 94, 1488–1499 [DOI] [PubMed] [Google Scholar]
- 5.Nielsen J., Gotfryd K., Li S., Kulahin N., Soroka V., Rasmussen K. K., Bock E., Berezin V. (2009) J. Neurosci. 29, 11360–11376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paratcha G., Ledda F., Ibáñez C. F. (2003) Cell 113, 867–879 [DOI] [PubMed] [Google Scholar]
- 7.Canty A. J., Dietze J., Harvey M., Enomoto H., Milbrandt J., Ibáñez C. F. (2009) J. Neurosci. 29, 10695–10705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozas E., Ibáñez C. F. (2005) Neuron 45, 701–713 [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto Y., Takeda K., Okuno Y., Yamakawa Y., Ito Y., Taguchi R., Kato M., Suzuki H., Takahashi M., Nakashima I. (2004) J. Biol. Chem. 279, 14213–14224 [DOI] [PubMed] [Google Scholar]
- 10.Knowles P. P., Murray-Rust J., Kjaer S., Scott R. P., Hanrahan S., Santoro M., Ibáñez C. F., McDonald N. Q. (2006) J. Biol. Chem. 281, 33577–33587 [DOI] [PubMed] [Google Scholar]
- 11.Liu X., Vega Q. C., Decker R. A., Pandey A., Worby C. A., Dixon J. E. (1996) J. Biol. Chem. 271, 5309–5312 [DOI] [PubMed] [Google Scholar]
- 12.Besset V., Scott R. P., Ibáñez C. F. (2000) J. Biol. Chem. 275, 39159–39166 [DOI] [PubMed] [Google Scholar]
- 13.Coulpier M., Anders J., Ibáñez C. F. (2002) J. Biol. Chem. 277, 1991–1999 [DOI] [PubMed] [Google Scholar]
- 14.Encinas M., Crowder R. J., Milbrandt J., Johnson E. M., Jr. (2004) J. Biol. Chem. 279, 18262–18269 [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H., Ichihara M., Iwashita T., Murakami H., Shimono Y., Kawai K., Kurokawa K., Murakumo Y., Imai T., Funahashi H., Nakao A., Takahashi M. (2000) Oncogene 19, 4469–4475 [DOI] [PubMed] [Google Scholar]
- 16.Fukuda T., Kiuchi K., Takahashi M. (2002) J. Biol. Chem. 277, 19114–19121 [DOI] [PubMed] [Google Scholar]
- 17.Asai N., Fukuda T., Wu Z., Enomoto A., Pachnis V., Takahashi M., Costantini F. (2006) Development 133, 4507–4516 [DOI] [PubMed] [Google Scholar]
- 18.Chan G., Kalaitzidis D., Neel B. G. (2008) Cancer Metastasis Rev. 27, 179–192 [DOI] [PubMed] [Google Scholar]
- 19.Tartaglia M., Mehler E. L., Goldberg R., Zampino G., Brunner H. G., Kremer H., van der Burgt I., Crosby A. H., Ion A., Jeffery S., Kalidas K., Patton M. A., Kucherlapati R. S., Gelb B. D. (2001) Nat. Genet. 29, 465–468 [DOI] [PubMed] [Google Scholar]
- 20.Vilar M., Murillo-Carretero M., Mira H., Magnusson K., Besset V., Ibáñez C. F. (2006) EMBO J. 25, 1219–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paratcha G., Ledda F., Baars L., Coulpier M., Besset V., Anders J., Scott R., Ibáñez C. F. (2001) Neuron 29, 171–184 [DOI] [PubMed] [Google Scholar]
- 22.Trupp M., Arenas E., Fainzilber M., Nilsson A. S., Sieber B. A., Grigoriou M., Kilkenny C., Salazar-Grueso E., Pachnis V., Arumäe U., Sariola H., Saarma M., Ibáñez C. F. (1996) Nature 381, 785–789 [DOI] [PubMed] [Google Scholar]
- 23.Kjaer S., Kurokawa K., Perrinjaquet M., Abrescia C., Ibáñez C. F. (2006) Oncogene 25, 7086–7095 [DOI] [PubMed] [Google Scholar]
- 24.Blokzijl A., ten Dijke P., Ibáñez C. F. (2002) Curr. Biol. 12, 35–45 [DOI] [PubMed] [Google Scholar]
- 25.Trupp M., Raynoschek C., Belluardo N., Ibáñez C. F. (1998) Mol. Cell Neurosci. 11, 47–63 [DOI] [PubMed] [Google Scholar]
- 26.Santoro M., Carlomagno F., Romano A., Bottaro D. P., Dathan N. A., Grieco M., Fusco A., Vecchio G., Matoskova B., Kraus M. H., et al. (1995) Science 267, 381–383 [DOI] [PubMed] [Google Scholar]
- 27.Scott R. P., Eketjäll S., Aineskog H., Ibáñez C. F. (2005) J. Biol. Chem. 280, 13442–13449 [DOI] [PubMed] [Google Scholar]
- 28.Ledda F., Paratcha G., Ibáñez C. F. (2002) Neuron 36, 387–401 [DOI] [PubMed] [Google Scholar]
- 29.Rossel M., Pasini A., Chappuis S., Geneste O., Fournier L., Schuffenecker I., Takahashi M., van Grunsven L. A., Urdiales J. L., Rudkin B. B., Lenoir G. M., Billaud M. (1997) Oncogene 14, 265–275 [DOI] [PubMed] [Google Scholar]
- 30.De Vita G., Melillo R. M., Carlomagno F., Visconti R., Castellone M. D., Bellacosa A., Billaud M., Fusco A., Tsichlis P. N., Santoro M. (2000) Cancer Res. 60, 3727–3731 [PubMed] [Google Scholar]
- 31.Cai T., Nishida K., Hirano T., Khavari P. A. (2002) J. Cell Biol. 159, 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caillaud T., Opstal W. Y., Scarceriaux V., Billardon C., Rostene W. (1995) Mol. Neurobiol. 10, 105–114 [DOI] [PubMed] [Google Scholar]
- 33.de Graaff E., Srinivas S., Kilkenny C., D'Agati V., Mankoo B. S., Costantini F., Pachnis V. (2001) Genes Dev. 15, 2433–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunet A., Datta S. R., Greenberg M. E. (2001) Curr. Opin. Neurobiol. 11, 297–305 [DOI] [PubMed] [Google Scholar]
- 35.Marsh H. N., Dubreuil C. I., Quevedo C., Lee A., Majdan M., Walsh G. S., Hausdorff S., Said F. A., Zoueva O., Kozlowski M., Siminovitch K., Neel B. G., Miller F. D., Kaplan D. R. (2003) J. Cell Biol. 163, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Q., Lerner-Marmarosh N., Che W., Ohta S., Osawa M., Yoshizumi M., Glassman M., Yan C., Berk B. C., Abe J. (2002) J. Biol. Chem. 277, 29330–29341 [DOI] [PubMed] [Google Scholar]
- 37.Joly M., Kazlauskas A., Corvera S. (1995) J. Biol. Chem. 270, 13225–13230 [DOI] [PubMed] [Google Scholar]
- 38.Schaeper U., Gehring N. H., Fuchs K. P., Sachs M., Kempkes B., Birchmeier W. (2000) J. Cell Biol. 149, 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sangwan V., Paliouras G. N., Abella J. V., Dubé N., Monast A., Tremblay M. L., Park M. (2008) J. Biol. Chem. 283, 34374–34383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holland S. J., Gale N. W., Gish G. D., Roth R. A., Songyang Z., Cantley L. C., Henkemeyer M., Yancopoulos G. D., Pawson T. (1997) EMBO J. 16, 3877–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossmann K. S., Wende H., Paul F. E., Cheret C., Garratt A. N., Zurborg S., Feinberg K., Besser D., Schulz H., Peles E., Selbach M., Birchmeier W., Birchmeier C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 16704–16709 [DOI] [PMC free article] [PubMed] [Google Scholar]