Abstract
Background
Previous studies have demonstrated individual differences in susceptibility to the detrimental effects of prenatal ethanol exposure. Many factors, including genetic differences, have been shown to play a role in susceptibility and resistance, but few studies have investigated the range of genetic variation in rodent models.
Methods
We examined ethanol teratogenesis in five inbred strains of mice: C57BL/6J (B6), Inbred Short-Sleep, C3H/Ibg, A/Ibg and 129S6/SvEvTac (129). Pregnant dams were intubated with either 5.8 g/kg ethanol (E) or an isocaloric amount of maltose-dextrin (MD) on day 9 of pregnancy. Dams were sacrificed on day 18 and fetuses were weighed, sexed and examined for gross morphological malformations. Every other fetus within a litter was then either placed in Bouin’s fixative for subsequent soft-tissue analyses or eviscerated and placed in ethanol for subsequent skeletal analyses.
Results
B6 mice exposed to ethanol in utero had fetal weight deficits and digit, kidney, brain ventricle and vertebral malformations. In contrast, 129 mice showed no teratogenesis. The remaining strains showed varying degrees of teratogenesis.
Conclusions
Differences among inbred strains demonstrates genetic variation in the teratogenic effects of ethanol. Identifying susceptible and resistant strains allows future studies to elucidate the genetic architecture underlying prenatal alcohol phenotypes.
Keywords: Prenatal Alcohol, Teratogenesis, Inbred Strains, Genetics
Introduction
Women who consume alcohol during pregnancy place their offspring at risk for a number of teratogenic outcomes. The most severe cases are diagnosed as Fetal Alcohol Syndrome (FAS), a disorder defined by prenatal and/or postnatal growth retardation, a characteristic pattern of craniofacial abnormalities and central nervous system dysfunction (Jones and Smith, 1973; Jones et al., 1973; Sokol et al., 2003). Because not all offspring exposed to alcohol prenatally display the full spectrum of FAS symptoms (particularly the facial dysmorphology), the term Fetal Alcohol Spectrum Disorders (FASD; Koren et al., 2003; Sokol et al., 2003) has been coined to describe varying degrees of ethanol teratogenesis, including FAS. The estimated incidence of FASD in the United States is 1% of live births (May and Gossage 2001; Sampson et al. 1997). Neurodevelopmental and behavioral deficits associated with prenatal ethanol exposure include developmental delay, attention deficits, hyperactivity, learning and memory impairments and diminished impulse control (Coles, 2001; Kelly et al., 1987; Kvigne et al., 2004; Sampson et al., 1997; Sokol et al., 2003).
A number of other physiological and morphological malformations have been reported following in utero ethanol exposure. These include renal anomalies (Assadi 1990; DeBeukelaer et al. 1977; Qazi et al. 1979; Taylor et al. 1994), limb and digit malformations (Froster and Baird 1992; Jones et al. 1973; Jones and Smith 1975; Pauli and Feldman 1986; Smith et al. 1981; van Rensburg 1981), dilated brain ventricles and other brain ventricle abnormalities (Clarren et al. 1978; Jones and Smith 1973, 1975; Konovalov et al. 1997) and skeletal malformations (axial and vertebral; Smith et al. 1981; Tredwell et al. 1982; Tsukahara and Kajii 1988). Interestingly, some of the skeletal defects reported following prenatal alcohol exposure are similar to anomalies seen in Klippel-Feil Syndrome (defects of segmentation or fusion of cervical and thoracic vertebra and rib anomalies; Lowry 1977; Neidengard et al. 1978; Schilgen and Loeser 1994).
Not all women who consume ethanol during pregnancy give birth to children with observable deficits, which demonstrates individual differences in susceptibility to ethanol teratogenesis. Many risk factors have been shown to contribute to the development of FASD, including the amount, timing and pattern of ethanol exposure, maternal age and parity, maternal ethnicity and socioeconomic status, cultural factors, maternal smoking and other drug abuse, maternal stress and psychological state, maternal diet/nutrition, maternal education, employment and marital status (Abel, 1995; Abel and Hannigan, 1995; Leonardson and Loudenberg, 2003).
Studies have also shown that genetic differences partially explain why some offspring of women who consume alcohol during pregnancy are severely affected while others are not (Chasnoff, 1985; Christoffel and Salafsky, 1975; Palmer et al., 1974; Riikonen, 1994). These studies indicate that monozygotic twins are more similarly affected than dizygotic twins. A more comprehensive study examined ethanol exposure in utero in both monozygotic and dizygotic twins. The rate of concordance for diagnosis was 5/5 for monozygotic twins and 7/11 for dizygotic twins and the authors concluded that genes had a modulating influence on the teratogenic effects of alcohol (Streissguth and Dehaene, 1993). More recently, several studies have shown that different alleles of the alcohol dehydrogenase gene (ADH), an enzyme involved in ethanol metabolism, can influence the severity of teratogenesis in different ethnic populations (Das et al., 2004; McCarver et al., 1997; Stoler et al., 2002; Viljoen et al., 2001).
Mice are an excellent model organism for investigating genetic effects on many phenotypes, and are well suited for studying the effects of prenatal ethanol exposure (Driscoll et al., 1990). Several studies used inbred strains and selectively bred mice to demonstrate genetic variation in many teratogenic outcomes, including embryolethality, development of various brain structures, fetal weight gain, and digit, skeletal, ocular, renal and heart anomalies (Boehm et al., 1997; Cassells et al., 1987; Chernoff, 1977, 1980; Downing and Gilliam, 1999; Giknis et al., 1980; Gilliam and Irtenkauf, 1990; Gilliam et al., 1997; Persaud and Sam, 1992; Wainwright and Gagnon, 1985; Webster et al., 1980). Nevertheless, only a few inbred strains have been examined and the range of genetic variation is unknown for any prenatal ethanol phenotypes.
In the present study, we examined the effects of prenatal ethanol exposure on morphological malformations in five inbred strains of mice: Inbred Short-Sleep (ISS), C57BL/6J (B6), C3H/Ibg (C3H), A/Ibg (A) and 129S6/SvEvTac (129). The Inbred Long-Sleep (ILS) and ISS strains were derived by inbreeding Long-Sleep (LS) and Short-Sleep (SS) mice, selectively bred for differential sensitivity to a hypnotic dose of alcohol (McClearn and Kakihana, 1981). Previous research has shown that LS mice are more susceptible to some of the teratogenic properties of ethanol than SS mice (Gilliam and Kotch 1990, 1996; Gilliam et al., 1989a; Gilliam et al, 1989b). Because LS mice are also more susceptible to the hypnotic effects of ethanol, it suggests that one or more genes may mediate both the soporific and teratogenic properties of ethanol in these two lines. ILS and ISS have not been examined for ethanol teratogenesis. Due to poor reproduction, ILS mice were unavailable during the course of this study.
B6 is one of the most widely used inbred strains of mice in all of biomedical research. They are susceptible to fetal weight deficits and kidney, limb and skeletal malformations following prenatal ethanol exposure (Boehm et al., 1997; Downing and Gilliam, 1999; Gilliam and Irtenkauf, 1990; Gilliam et al., 1997; Webster et al., 1980). In this study they serve as a positive control for known teratogenic effects. One study examined the A strain (A/J from the Jackson Laboratory) and showed them to be more susceptible to skeletal malformations than even B6 following prenatal ethanol exposure (Boehm et al. 1997). We wanted to examine the A/Ibg substrain for similar effects. Three studies have looked at teratogenesis in C3H following prenatal ethanol exposure and found them to be susceptible to fetal weight deficits and brain and skeletal malformations (Chernoff 1977, 1980) and resistant to prenatal mortality (PNM) and soft-tissue malformations (Lochry et al. 1981). We wanted to confirm these effects in the C3H/Ibg substrain. To the best of our knowledge, no 129 strains of mice have been examined for morphological malformations following prenatal ethanol exposure. Results from this study will further characterize the range of genetic variation in ethanol teratogenesis in mus musculus.
Methods
Animals
Male and female B6, ISS, C3H, A and 129 mice were generated and housed in the specific pathogen-free (SPF) facility at the Institute for Behavioral Genetics, Boulder, CO. ISS mice were created at IBG and thus have the /IBG substrain designation. A mice were originally purchased from the Jackson Laboratory, but have been maintained for many years at IBG separate from the Jackson colony and genetic drift may have occurred, so they too have the /IBG designation. Likewise, the C3H mice were originally brought to IBG by Dr. Gerald McClearn from the University of California at Berkley in the 1960s and also have the /IBG designation. The 129 mice were recently obtained by IBG from Taconic and therefore still have the /Tac designation, while B6 mice were recently re-derived from Jackson Laboratory stock and have the /J designation. Males were individually housed while females were housed three to five per cage; mice were maintained on a 12-hr light/dark cycle (lights on at 0700) and were given food and water ad libitum. The temperature was kept at a constant 22° (± 2 °) C. All procedures were approved by the University of Colorado Institutional Animal Care and Use Committee, in accordance with National Institute of Health guidelines.
Mating and Dosing
Two females were placed with a male for two hours each morning (7:00 am – 9:00 am) and examined for a seminal plug as evidence of mating. The morning of plug detection was designated gestational day 0 (GD 0). Because A females did not plug well when mated for two hours in the morning, we began to mate them overnight; 15 of the 21 A litters were generated by mating overnight, while 6 A litters were generated by mating for 2 hours in the morning. Plugged females were weighed and single-housed. At noon on GD 9, females were weighed to ascertain a 2 gram minimum weight gain as evidence of pregnancy. Females were then intragastrically intubated with either 5.8 g/kg ethanol (E) or an isocaloric amount of maltose-dextrin (MD). We chose the 5.8 g/kg dose on GD 9 because this is a sensitive period during organogenesis and we can reliably reproduce a pattern of malformations in the susceptible B6 strain. On GD 18, females were sacrificed at 2:00 pm; uterine horns were exposed and a count made of live, dead and resorbed fetuses. Live fetuses were weighed, sexed and examined for gross morphological malformations. Every other fetus within a litter was placed in Bouin’s fixative a minimum of four weeks for subsequent soft-tissue analyses using Wilson’s (1965) freehand slicing method. The remaining fetuses were placed in ethanol a minimum of 2 weeks. They were then macerated in a 1% KOH solution for 72 hours. Fetuses were then placed in a 1% KOH solution containing alizarin red for 6–9 hours. Stained fetuses/skeletons were then placed in 25% glycerin for 24 hours and then stored in 75% glycerin for subsequent skeletal analyses.
Malformations
Upon c-section, all fetuses were examined for gross morphological malformations, which consisted primarily of forepaw adactyly (missing digits) and syndactyly (fused digits). Soft-tissue malformations consisted of dilated brain ventricles and kidneys (hydronephrosis). Skeletal malformations included missing and fused ribs and asymmetrical, fused or missing vertebral arches and centra. All teratological exams were done blind, without knowledge of strain or treatment.
Data Analysis
Maternal data were examined using analysis of variance (ANOVA) with strain (ISS, B6, A, C3H and 129) and treatment (E or MD) as between group factors. Offspring data were analyzed with strain, treatment and sex as between group factors All analyses were performed using Statistical Package for the Social Sciences (SPSS, v. 15.0). Maternal variables included weight gain and prenatal mortality. Offspring variables included weight at c-section and morphological malformations. Litters with only one pup were not used for analyses. For all offspring variables, litter means (percent litter malformed for malformations) were the unit of analysis; male and female means were analyzed separately. Because we found no effect of sex on any measure of teratogenesis, we collapsed across sex for analyses and all figures depict combined male and female litter means. Post hoc analyses consisted of Bonferoni corrected t-tests within strain. An alpha level of .05 was used to assess significant results. Sample sizes were as follows: ISS-E 15 litters, ISS-MD 13; B6-E 13, B6-MD 12; 129-E 10, 129-MD 13; C3H-E 13, C3H-MD 13; A–E 11, A-MD 10.
Results
Maternal Data
Maternal Weight Gain
Percent maternal weight gain was calculated as: [(weight day 18 – weight day 9)/weight day 9]. There was a significant main effect of strain (F(4, 112) = 5.56, p < .001), but no main effect of treatment (F(1, 112) = 3.59, p < .062) and no strain by treatment interaction. As can be seen in Table 1, ISS dams put on less weight than all the other strains except C3H (p’s < .01). Even though ANOVA showed no main effect of treatment and there was no significant strain by treatment interaction, a t-test showed that B6 MD-treated dams put on more weight than E-treated B6 dams (p < .05), which is clearly evident in Table 1. Maternal weight gain was also calculated with fetal weight subtracted out [(weight day 18 – weight day 9 – fetal weight)/weight day 9] to examine whether differences in fetal weight could account for differences in maternal weight gain. With fetal weight subtracted out (adjusted percent maternal weight gain or APMWG), ANOVA showed a significant main effect of strain (F(4,112 = 6.124, p < .01) but no significant effect of treatment and no significant strain by treatment interaction. There were no differences in APMWG in B6 E- and MD-treated dams (Table 1) which shows that the difference found in PMWG in B6 dams was due to differences in fetal weight.
Table 1.
Mean (± SEM) percent maternal weight gain, prenatal mortality and offspring weight at c-section. Sample size is shown in parentheses under treatment for each strain
| ISS | B6 | 129 | C3H | A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E | MD | E | MD | E | MD | E | MD | E | MD | |
| (15) | (13) | (13) | (12) | (10) | (13) | (13) | (13) | (11) | (10) | |
| PMWGA | 33(2) | 31(3) | 41(11) | 62(4) | 48(3) | 51(2) | 40(3) | 43(4) | 47(4) | 52(4) |
| APMWGB | 18(1) | 16(1) | 21(2) | 24(2) | 15(2) | 16(1) | 22(2) | 22(2) | 20(2) | 20(2) |
| PNMC | 23(5) | 16(5) | 11(3) | 3(1) | 16(5) | 4(2) | 38(6) | 34(6) | 23(6) | 19(5) |
| ♀ CSWTD | .90(.03) | .94(.03) | 1.04(.03) | 1.16(.08) | 1.18(.02) | 1.20(.02) | 1.06(.04) | 1.13(.06) | .82(.03) | .89(.06) |
| ♂ CSWT | .87(.03) | .95(.04) | 1.06(.03) | 1.19(.03) | 1.24(.02) | 1.20(.03) | 1.10(.04) | 1.15 (.02) | .86(.04) | .93(.03) |
Percent maternal weight gain = (weight day 18 – weight day 9)/weight day 9. ISS put on less weight than all strains except C3H (p’s < .01).
Adjusted percent maternal weight gain = (weight day 18 – weight day 9 – fetal weight)/weight day 8.
Prenatal mortality = [(resorptions + dead)/implantation sites] × 100. C3H mice had greater PNM than B6, 129 (p’s < .001) and ISS (p < .03); E-treated dams had greater PNM than MD-treated dams, p < .03.
Weight at c-section. A fetuses weighed less than all other strains except ISS, p’s < .001; E-exposed fetuses weighed less than MD-exposed fetuses, p < .001.
Prenatal Mortality
Prenatal mortality was calculated by dividing the number of resorptions plus dead fetuses by the number of implantation sites. There were significant main effects of strain (F(4, 112) = 9.24, p < .001) and treatment (F(1, 118) = 4.13, p < .05), but no significant interaction. C3H dams had greater prenatal mortality than B6, 129 (p’s < .001) and ISS (p < .03) dams; E-treated dams had greater prenatal mortality than MD-treated dams (p < .03; Table 1).
Offspring Data
Fetal Weight
Fetal weight was defined as pups’ weight at c-sectioning. There were significant main effects of strain (F(4, 209) = 67.46, p < .001) and treatment (F(1, 209) = 16.44, p < .001), but no significant interactions among variables. Post hoc analyses showed that A pups weighed significantly less than all strains except ISS (p’s < .001); E-treated pups weighed less than MD-treated pups (p < .001).
Gross Morphological Malformations
Upon c-section, pups were examined for gross morphological malformations, which consisted primarily of forepaw adactyly and syndactyly. These two measures were combined to give a measure of digit malformations. There were significant main effects of strain (F(4, 209 = 4.76, p < .001) and treatment (F(1, 209) = 10.77, p < .001) and a significant strain by treatment interaction (F(4, 209) = 5.87, p < .001). Post hoc analyses showed that compared to MD-controls, E-treated B6 (p < .01) and C3H (p < .03) fetuses had increased digit malformations (Table 2).
Table 2.
Mean (± SEM) litter percent for digit, kidney, brain ventricle, rib and vertebral malformations. Sample sizes are indicated in parentheses under strain and treatment.
| ISS | B6 | 129 | C3H | A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E | MD | E | MD | E | MD | E | MD | E | MD | |
| (15) | (13) | (13) | (12) | (10) | (13) | (13) | (13) | (11) | (10) | |
| DigitA | 0 | 0 | 34(10) | 0 | 0 | 6(2) | 29(6) | 0 | 7(4) | 7(5) |
| KidneyB | 0 | 0 | 36(19) | 3(2) | 19(4) | 0 | 0 | 0 | 22(9) | 8(5) |
| VentriclesC | 48(11) | 3(1) | 22(11) | 0 | 0 | 0 | 0 | 0 | 44(12) | 6(2) |
| RibD | 15(5) | 0 | 3(1) | 0 | 0 | 0 | 20(8) | 0 | 18(10) | 0 |
| Arches | 11(6) | 0 | 16(8) | 0 | 0 | 0 | 3(3) | 0 | 0 | 0 |
| Centra | 17(5) | 6(3) | 31(16) | 0 | 9(5) | 4(3) | 0 | 0 | 3(1) | 0 |
Compared to MD-treated controls, E-treated B6 fetuses (p < .01) and C3H fetuses (p < .03) had a greater percentage of digit malformations.
B6 fetuses had a greater percentage of Kidney malformations (p < .01).
ISS (p < .02) and A (p < .03) fetuses had a greater percentage of dilated brain ventricles; B6 (p < .065) just missed significance.
E-exposed fetuses had a higher percentage of rib malformations (P < .04).
Soft Tissue Malformations
After examination for gross morphological malformations, half the fetuses per litter were stored in Bouin’s fixative for subsequent soft tissue analyses. Soft tissue anomalies included dilated brain ventricles and hydronephrosis (Figures 1A and 1B). For dilated ventricles we found significant main effects of strain (F(4, 177) = 9.49, p < .001) and treatment (F(1, 177) = 14.23, p < .001) and a significant strain by treatment interaction (F(4, 177) = 4.50, p < .003). Compared to MD-controls, E-treated ISS (p < .02) and A (p < .024) fetuses had significantly more malformations. For kidney malformations (Figure 2) we also found significant main effects of strain (F(4, 177) = 6.44, p < .001) and treatment (F(1, 177) = 8.01, p < .01) and a significant strain by treatment interaction (F(4, 177) = 2.25, p < .05). Compared to MD-treated controls, E-treated B6 (p < .01) fetuses had significantly more kidney malformations (Table 2).
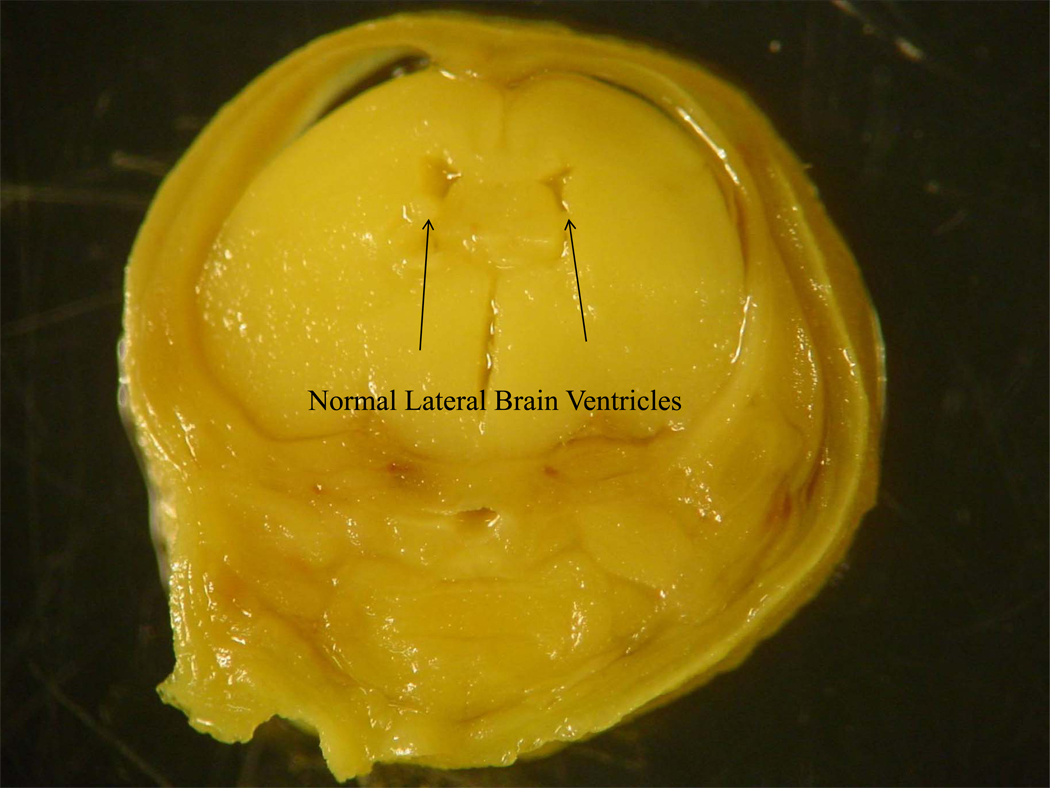
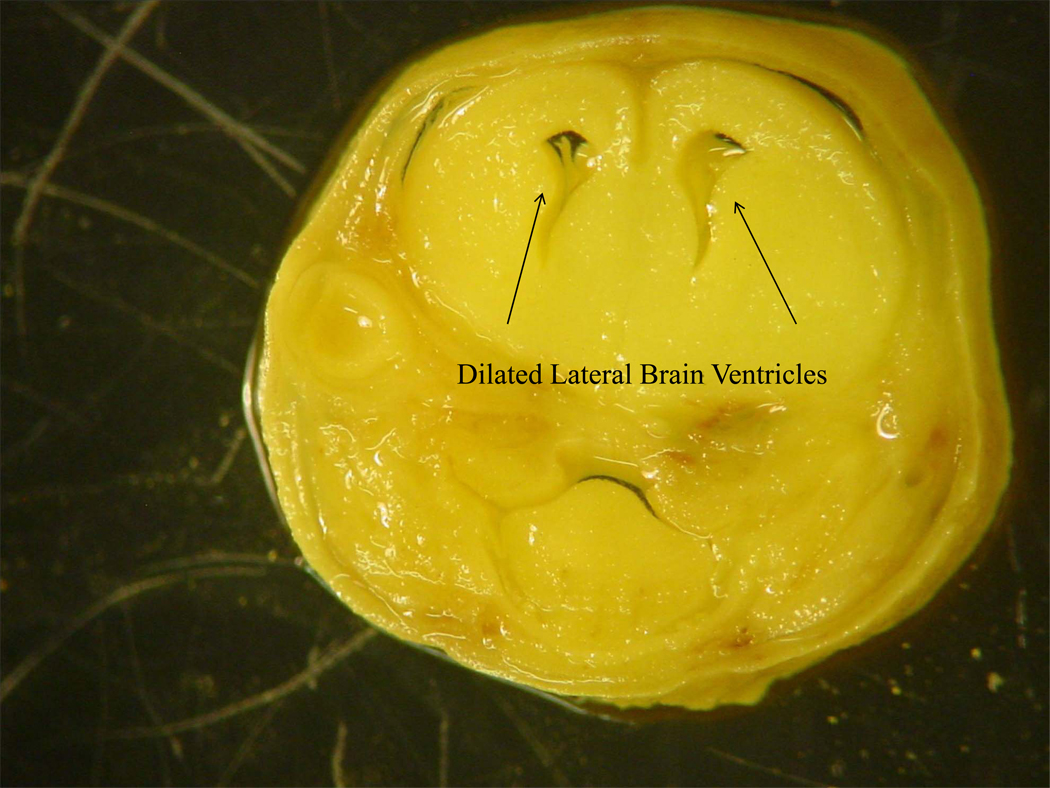
Figure 1.
Figure 1A. Normal lateral and third brain ventricles taken from a gestational day 18 mouse fetus exposed to maltose-dextrin prenatally.
Figure 1B. Dilated brain ventricles taken from a gestational day 18 mouse fetus exposed to alcohol prenatally.
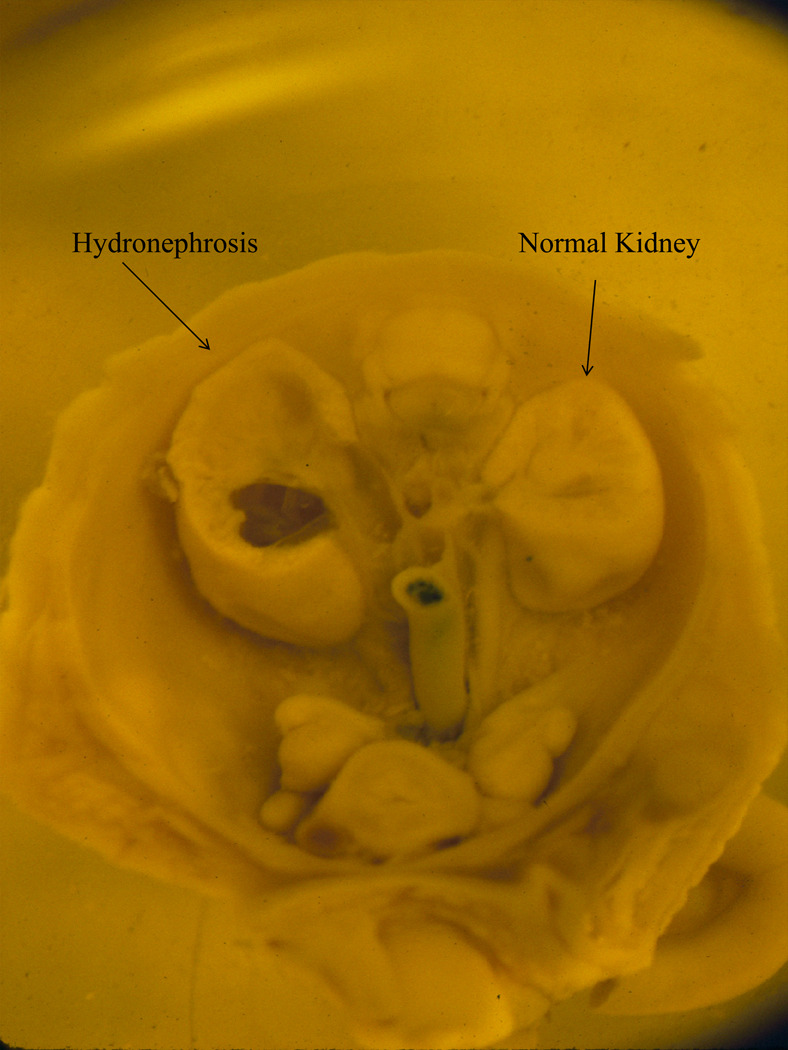
Figure 2.
Kidneys taken from a gestational day 18 mouse fetus exposed to alcohol prenatally. While the kidney on the right is normal, the kidney on the left exhibits hydronephrosis.
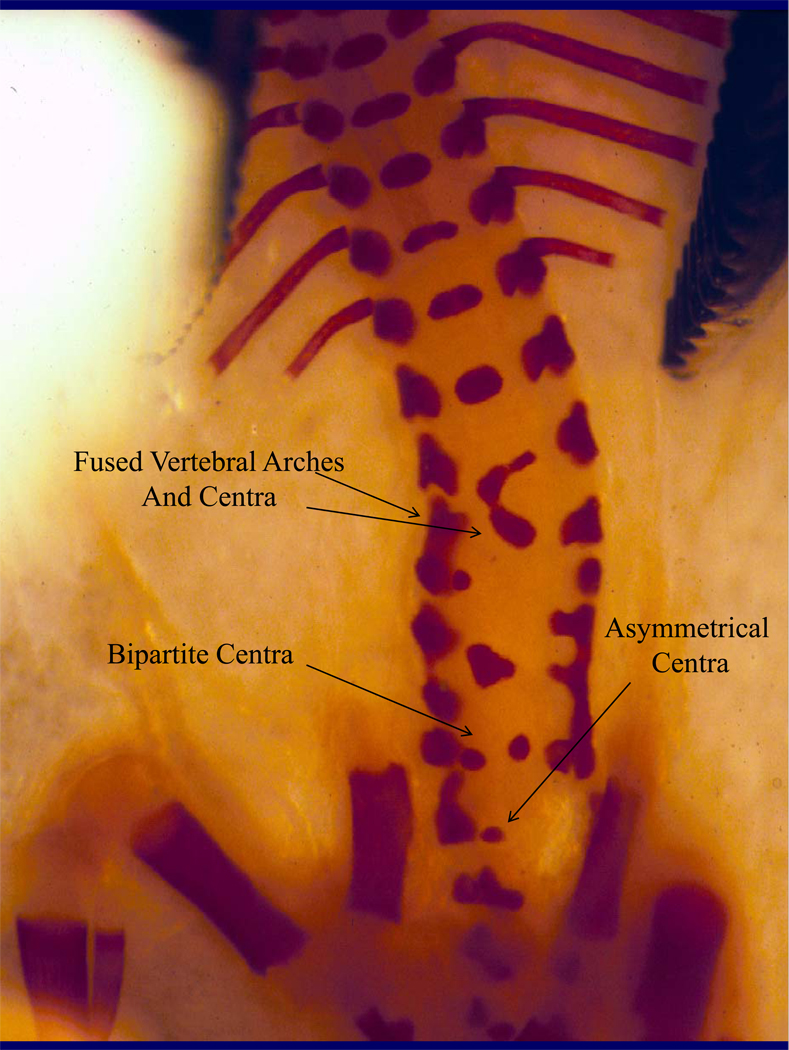
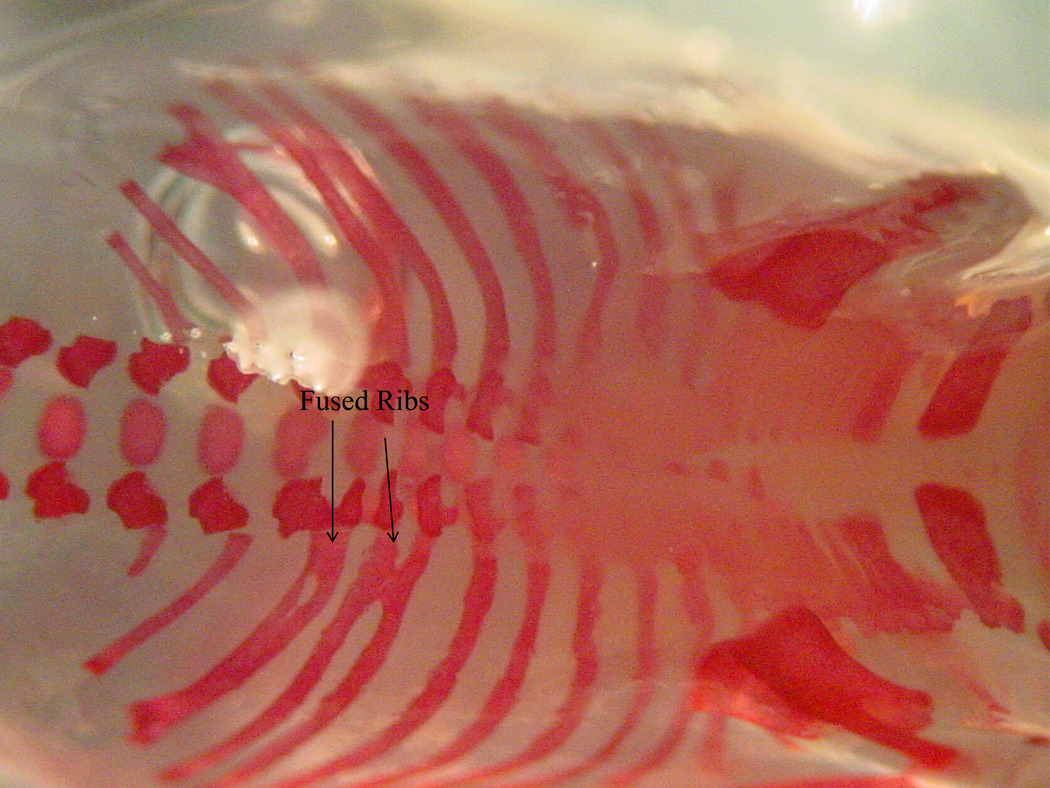
Skeletal Malformations
After gross examination, the remaining half of the fetuses in each litter were eviscerated and stored in ethanol for subsequent skeletal examinations. Rib malformations included missing or fused ribs, while vertebral malformations included missing, fused or asymmetrical arches and centra (Figures 3A–3C). For rib malformations we only found a significant main effect of treatment (F(1, 166) = 4.38, p < .04); E-exposed fetuses had a significantly higher percentage of rib malformations than MD-exposed fetuses (Table 2). For vertebral malformations, ANOVA showed no significant main effects or interactions.
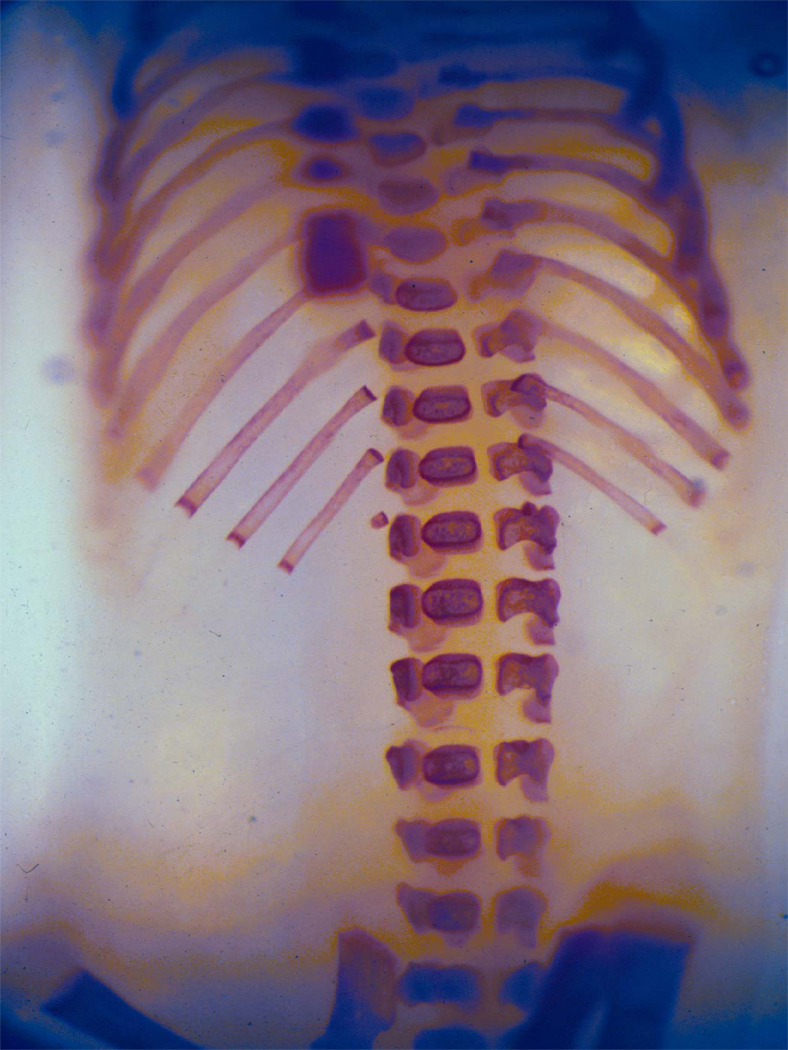
Figure 3.
Figure 3A. Skeleton from a gestational day 18 mouse fetus exposed to maltose-dextrin prenatally.
Figure 3B, 3C. Skeleton from a gestational day 18 mouse embryo exposed to alcohol prenatally. Note the fused and asymmetrical vertebral arches and centra (3B) and the fused ribs (3C).
Discussion
C57BL/6J is the most widely used inbred mouse strain in alcohol research and has been well characterized for several prenatal phenotypes. B6 are susceptible to fetal weight deficits, digit, kidney, brain ventricle and vertebral malformations following ethanol exposure during the period of organogenesis (Boehm et al. 1997; Downing and Gilliam 1999; Gilliam and Irtenkauf 1990; Gilliam et al. 1989b, 1997; Johnson et al. 2007; Persaud and Sam 1992; Randall et al. 1977, 1988; Webster et al. 1980, 1983; Zimmerman et al. 1990). Our results support these findings. Thus far, B6 seems to represent the extreme of ethanol teratogenesis, consistently showing effects on most measures.
Long-Sleep (LS) and Short-Sleep (SS) mice were selectively bred for sensitivity and resistance to the sedative properties of alcohol (McClearn and Kakihana 1981). Studies have shown that following acute or chronic ethanol exposure, LS mice are susceptible to fetal weight deficits and rib and skeletal malformations, but are resistant to PNM and digit, kidney and brain ventricle malformations (Gilliam and Irtenkauf 1990; Gilliam et al. 1989a, 1989b). In contrast, SS mice are resistant to fetal weight deficits, PNM, and digit, skeletal and soft-tissue malformations following prenatal ethanol exposure (Gilliam et al. 1989a, 1989b, 1997). This suggests that one or more genes mediating susceptibility and resistance to the soporific effects of alcohol may also mediate, at least partly, the teratogenic effects of ethanol. Because pregnant LS and SS dams show similar blood ethanol concentrations at various timepoints following ethanol exposure, the differences in teratogenesis are likely not due to differences in ethanol metabolism (Gilliam et al. 1989b). While ILS mice were unavailable in the present study, we found that ISS mice are resistant to fetal weight deficits, PNM and digit, kidney, and skeletal malformations following in utero ethanol exposure. In contrast with SS, ISS mice are quite susceptible to dilated brain ventricles.
We found that following an acute dose of ethanol on GD9, C3H/Ibg mice are resistant to fetal weight deficits, PNM and kidney, brain ventricle and vertebral malformations; they are susceptible to digit and rib malformations. Using a paradigm similar to ours, Lochry et al. (1982) exposed C3H/HeJ mice to a single dose of ethanol (0, 2.5 or 5.0 g/kg) during gestation (GD 7, 8, 9, 10, 11, 12, 14, 16 or 18) and reported no effects on resorptions, dead fetuses or soft-tissue malformations. Chernoff (1977, 1980) reported that C3H/lgM1 mice are susceptible to fetal weight deficits, dilated brain ventricles and skeletal malformations following prenatal ethanol exposure. However, comparisons to the Chernoff studies are difficult due to differences in substrains used, dosing regimens, teratological assessment techniques and data analyses.
Boehm et al. (1997) reported that pregnant A/J dams put on less weight than controls following ethanol exposure and had increased PNM. Fetuses from the A strain were susceptible to weight deficits and rib and vertebral malformations, while they were resistant to digit and kidney malformations; they were not examined for dilated brain ventricles. We found pregnant A/Ibg females to be resistant to weight gain deficits and PNM. Offspring from the A strain were resistant to fetal weight deficits and digit, kidney and skeletal malformations following prenatal ethanol exposure; they were quite susceptible to dilated brain ventricles. The biggest difference in A mice between our study and the Boehm et al. (1997) study was for skeletal malformations. While Boehm et al. reported litter percentages for ethanol-induced rib, arch and centra malformations from 40–58%, our study found percentages 0–18%. Boehm et al. used A mice obtained from the Jackson Laboratory while our (IBG) mice, originally obtained from JAX, have been maintained separately for over 30 generations. It is possible that genetic drift has occurred in our colony and that our mice are slightly different (genetically) from the JAX stock. One or more of these mutations may explain the differences in skeletal malformations.
To the best of our knowledge, none of the many 129 substrains has been examined for ethanol teratogenesis. We found that 129 mice, when administered 5.8 g/kg on GD 9, are resistant to all measures of teratogenesis. This finding is important because 129 substrains of mice are often used to create targeted gene mutations (knockouts or KOs). Most mouse KO studies report the use of embryonic stem cells derived from 129 mice to create the KO construct; constructs are then injected into B6 blastocysts to create KOs. Rendering a gene nonfunctional is one way to test whether the gene has an effect on a phenotype of interest. Therefore, because most mouse KOs have at least some 129 genome in their background, it is important to characterize this strain for all phenotypes of interest, including prenatal ethanol traits.
Despite the uniformity in time of exposure, it is possible that the inbred strains used in the present study are at slightly different developmental stages. One of the only ways to definitively determine where mice are, developmentally, is to stage somites, something we did not do. However, between strains, between litters of the same strain and within litters of the same strain, there can be a great deal of variation in somitogenesis (Gossler and Tam, 2002; Tam 1981; Thiel et al. 1993). It has been shown that even in the same litter, embryos can be at different stages of development and (at any given time) can vary by 3–8 somites or ~ 6–17 hours (Gossler and Tam 2002). It is therefore impossible to ensure that all embryos are at the same developmental timepoint when we intubate on GD 9 and have all those fetuses for teratological examinations on GD 18. This may account for some of the effects, or lack thereof, in this study. Previous research from our laboratory has shown that following 5.8 g/kg ethanol on GD 9, GD 9 and GD 10, or GD 7 through GD 15, B6 mice are quite susceptible to ethanol teratogenesis while SS mice are relatively resistant (Gilliam et al. 1989a, 1989b, 1997). In addition, A mice are susceptible to skeletal malformations following ethanol exposure on GD 9 (Boehm et al. 1997). Thus, in the present study we are fairly certain that we are dosing ISS, B6 and A mice at a sensitive developmental timepoint. No such data exist for the 129 and C3H strains. Future studies need to examine a number of different inbred strains and timepoints to further characterize sensitive developmental timepoints for various organ systems.
Previous studies from our laboratory have shown that following a 5.8 g/kg dose of ethanol, pregnant SS, B6 and A dams show no differences in ethanol metabolism, averaging 350–450 mg% one hour after exposure (Boehm et al. 1997; Gilliam and Irtenkauf 1990; Gilliam et al. 1989b, 1997). Unpublished data from our laboratory has also shown that the inbred ISS and ILS do not differ in ethanol metabolism from the selectively bred SS and LS. Therefore, we are fairly confident that the differential teratogenesis seen in the ISS, B6 and A strains are not due to differences in ethanol metabolism. However, pregnant C3H and 129 dams have not been examined for BECs following a similar dosing regimen so it is possible that the teratogenesis we observed in C3H and the lack of effect seen in 129 are due to differential ethanol metabolism.
Results from this study further the knowledge of genetic variation in ethanol teratogenesis. Such knowledge will facilitate identification of susceptibility and resistance genes in mice, which can then be examined in human populations. We and others have outlined a strategy, using mus musculus, which allows one to go from identifying a genetic influence on a phenotype of interest to identifying the causal gene(s) and DNA sequence polymorphisms (Downing et al. 2005). The first step is demonstrating genetic variation for the phenotype of interest, usually by identifying inbred strain differences or through selective breeding. Then susceptible and resistant strains are crossed to create populations for quantitative trait locus (QTL mapping). After initial QTL mapping, various recombinant strategies are used to narrow QTL regions, typically to one megabase or smaller, in order to identify candidate genes mediating phenotypic differences. Finally, several strategies can be used to functionally evaluate candidate genes. These strategies have been used to identify and evaluate candidate genes for many alcohol-related phenotypes, including consumption and preference, locomotor activation, loss-of-righting reflex and several measures of tolerance (Bennett et al. 2006; Crabbe et al. 1999; Downing et al. 2005; Treadwell 2006). Somewhat surprisingly, for all prenatal ethanol phenotypes, researchers are still at step one: identifying genetic variation. Towards this end, we have recently reported preliminary results from QTL mapping for ethanol teratogenesis in recombinant inbred strains derived from a cross between the susceptible B6 strain and the resistant DBA/2J (D2) strain (BXD RIs; Downing et al. 2008)
Acknowledgements
This study was supported by grants AA016676 and AA01466. The authors would like to thank Ms. Alexi Kimball for her excellent technical assistance.
References
- Abel EL. An update on the incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol. 1995;17:437–443. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Abel EL, Hannigan JH. Maternal risk factors in Fetal Alcohol Syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995;17:445–462. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Assadi FK. Renal tubular dysfunction in fetal alcohol syndrome. Pediatr Nephrol. 1990;4:48–51. doi: 10.1007/BF00858439. [DOI] [PubMed] [Google Scholar]
- Bennett B, Downing C, Parker C, Johnson TE. Mouse genetic models in alcohol research. Trends Genet. 2006;22:367–374. doi: 10.1016/j.tig.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Lundahl KR, Caldwell J, Gilliam DM. Ethanol teratogenesis in the C57BL/6J, DBA/2J and A/J inbred mouse strains. Alcohol. 1997;14:389–395. doi: 10.1016/s0741-8329(97)87950-5. [DOI] [PubMed] [Google Scholar]
- Cassells B, Wainwright P, Blom K. Heredity and alcohol-induced brain anomalies: effects of alcohol on anomalous prenatal development of the corpus callosum and anterior commissure in BALB/c and C57BL/6 mice. Exper Neurol. 1987;95:587–604. doi: 10.1016/0014-4886(87)90301-3. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ. Fetal Alcohol Syndrome in twin pregnancy. Acta Genet Med Gemellol (Roma) 1985;34:229–232. doi: 10.1017/s0001566000004797. [DOI] [PubMed] [Google Scholar]
- Chernoff GF. The Fetal Alcohol Syndrome in mice: an animal model. Teratology. 1977;15:223–230. doi: 10.1002/tera.1420150303. [DOI] [PubMed] [Google Scholar]
- Chernoff GF. The Fetal Alcohol Syndrome in mice: maternal variables. Teratology. 1980;22:71–75. doi: 10.1002/tera.1420220110. [DOI] [PubMed] [Google Scholar]
- Christoffel KK, Salafsky I. Fetal alcohol syndrome in dizygotic twins. J Pediatr. 1975;87:963–967. doi: 10.1016/s0022-3476(75)80919-x. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC, Jr, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92:64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- Coles CD. Fetal alcohol exposure and attention: Moving beyond ADHD. Alcohol Res Health. 2001;25:199–203. [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- Das UG, Cronk CE, Martier SS, Simpson PM, McCarver DG. Alcohol dehydrogenase 2*3 affects alterations in offspring facial dysmorphology associated with maternal ethanol intake in pregnancy. Alcohol Clin Exp Res. 2004;28:1598–1606. doi: 10.1097/01.alc.0000141816.14776.97. [DOI] [PubMed] [Google Scholar]
- DeBeukelaer MM, Randall CL, Stroud DR. Renal anomalies in the fetal alcohol syndrome. J Pediatr. 1977;91:759–760. doi: 10.1016/s0022-3476(77)81033-0. [DOI] [PubMed] [Google Scholar]
- Downing C, Gilliam D. Cytoplasmic factors do not contribute to a maternal effect on ethanol teratogenesis. Behav Genet. 1999;29:31–39. doi: 10.1023/a:1021485821842. [DOI] [PubMed] [Google Scholar]
- Downing C, Bennett B, Johnson TE. Murine models of alcoholism: From QTL to gene. In: Peltz G, editor. Computational Genetics and Genomics: Tools for Understanding Disease. Totawa, NJ: Humana Press; 2005. pp. 199–252. [Google Scholar]
- Downing C, Carosone-Link P, Gaudreau C, Kimball A, Broncucia H, Biers J, Johnson TE, Gilliam DM. Quantitative trait locus mapping for ethanol teratogenesis in BXD recombinant inbred mice. Alcohol Clin Exp Res. 2008;32(6) Supplement:11A. doi: 10.1111/j.1530-0277.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: Comparability of effects in humans and animal models. Neurotoxicol Teratol. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Froster UG, Baird PA. Congenital defects of the limbs and alcohol exposure in pregnancy: Data from a population based study. Am J Med Genet. 1992;44:782–785. doi: 10.1002/ajmg.1320440612. [DOI] [PubMed] [Google Scholar]
- Giknis MLA, Damjanov I, Rubin E. The differential transplacental effects of ethanol in four mouse strains. Neurobehav Toxicol. 1980;2:235–237. [Google Scholar]
- Gilliam DM, Kotch LE, Dudek BC, Riley EP. Ethanol teratogenesis in mice selected for differences in alcohol sensitivity. Alcohol. 1989a;5:513–519. doi: 10.1016/0741-8329(88)90091-2. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Kotch LE, Dudek BC, Riley EP. Ethanol teratogenesis in selectively bred Long-Sleep and Short-Sleep mice: A comparison to inbred C57BL/6J mice. Alcohol Clin Exp Res. 1989b;13:667–672. doi: 10.1111/j.1530-0277.1989.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Irtenkauf KT. Maternal genetic effects on ethanol teratogenesis and dominance of relative embryonic resistance to malformations. Alcohol Clin Exp Res. 1990;14:539–545. doi: 10.1111/j.1530-0277.1990.tb01196.x. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Mantle MA, Barkhausen DA, Tweden DR. Effects of acute prenatal ethanol administration in a reciprocal cross of C57BL/6J and Short-Sleep mice: Maternal effects and nonmaternal factors. Alcohol Clin Exp Res. 1997;21:28–34. [PubMed] [Google Scholar]
- Gilliam DM, Kotch LE. Alcohol-related birth defects in Long- and Short-Sleep mice: Postnatal litter mortality. Alcohol. 1990;7:483–487. doi: 10.1016/0741-8329(90)90036-c. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Kotch LE. Dose-related growth deficits in LS but not SS mice prenatally exposed to alcohol. Alcohol. 1996;13:47–51. doi: 10.1016/0741-8329(95)02010-1. [DOI] [PubMed] [Google Scholar]
- Gossler A, Tam PPL. Somitogenesis: Segmentation of the paraxial mesoderm and the delineation of tissue compartments. In: Rossant J, Tam PPL, editors. Mouse Development: Patterning, Morphogenesis and Organogenesis. San Diego, CA: Academic Press; 2002. pp. 127–149. [Google Scholar]
- Johnson CS, Zucker RM, Hunter ES, III, Sulik KK. Perturbation of retinoic acid (RA)-mediated limb development suggests a role for diminished RA signaling in the teratogenesis of ethanol. Birth Defect Res (Part A) 2007;79:631–641. doi: 10.1002/bdra.20385. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. The fetal alcohol syndrome. Teratology. 1975;12:1–10. doi: 10.1002/tera.1420120102. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth AP. Pattern of malformations in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Pierce DR, West JR. Microencephaly and hyperactivity in adult rats can be induced by neonatal exposure to high blood alcohol concentrations. Exp Neurol. 1987;1987:580–593. doi: 10.1016/0014-4886(87)90220-2. [DOI] [PubMed] [Google Scholar]
- Konovalov HV, Kovetsky NS, Bobryshev YV, Ashwell KWS. Disorders of brain development in the progeny of mothers who used alcohol during pregnancy. Early Hum Develop. 1997;48:153–166. doi: 10.1016/s0378-3782(96)01848-8. [DOI] [PubMed] [Google Scholar]
- Koren G, Nulman I, Chudley AE, Loocke C. Fetal alcohol spectrum disorder. CMAJ. 2003;169:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Kvigne VL, Leonardson GR, Neff-Smith M, Brock E, Borzelleca J, Welty TK. Characteristics of children who have full or incomplete fetal alcohol syndrome. J Pediatr. 2004;145:635–640. doi: 10.1016/j.jpeds.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Leonardson GR, Loudenberg R. Risk factors for alcohol use during pregnancy in a multistate area. Neurotoxicol Teratol. 2003;25:651–658. doi: 10.1016/j.ntt.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Lochry EA, Randall CL, Goldsmith AA, Sutker PB. Effects of acute alcohol exposure during selected days of development in C3H mice. Neurobehav Toxicol Teratol. 1982;4:15–19. [PubMed] [Google Scholar]
- Lowry RB. The Klippel-Feil anomalad as part of the Fetal Alcohol Syndrome. Teratology. 1977;16:53–56. doi: 10.1002/tera.1420160109. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of Fetal Alcohol Syndrome. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- McCarver DG, Thomasson HR, Martier SS, Sokol RJ, Li T-K. Alcohol dehydrogenase- 2*3 allele protects against alcohol-related birth defects among African Americans. J Pharmacol Exp Ther. 1997;283:1095–1101. [PubMed] [Google Scholar]
- McClearn GE, Kakihana R. Selective breeding for ethanol sensitivity: Short-Sleep and Long-Sleep mice. In: McClearn GE, Deitrich RA, Erwin VG, editors. Development of Animal Models as Pharmacogenetic Tools. Washington, DC: US Department of Health and Human Services; 1981. pp. 147–159. NIAAA Research Monograph No. 6. [Google Scholar]
- Neidengard L, Carter T, Smith DW. Klippel-Feil malformation complex in Fetal Alcohol Syndrome. Am J Dis Child. 1978;132:929–930. doi: 10.1001/archpedi.1978.02120340105024. [DOI] [PubMed] [Google Scholar]
- Palmer RH, Ouellette EM, Warner L, Leichtman SR. Congenital malformations offspring of a chronic alcoholic mother. Pediatrics. 1974;53:490–494. [PubMed] [Google Scholar]
- Pauli RM, Feldman PF. Major limb malformations following intrauterine exposure to ethanol: Two additional cases and literature review. Teratology. 1986;33:273–280. doi: 10.1002/tera.1420330304. [DOI] [PubMed] [Google Scholar]
- Persaud TV, Sam GO. Prenatal influence of alcohol following a single exposure in two inbred strains of mice. Ann Anat. 1992;174:301–303. doi: 10.1016/s0940-9602(11)80286-4. [DOI] [PubMed] [Google Scholar]
- Qazi Q, Masakawa A, Milman D, McGann B, Chua A, Haller J. Renal anomalies in fetal alcohol syndrome. Pediatr. 1979;63:886–889. [PubMed] [Google Scholar]
- Randall CL, Taylor WJ, Walker DW. Ethanol-induced malformations in mice. Alcohol Clin Exp Res. 1977;1:219–223. doi: 10.1111/j.1530-0277.1977.tb05876.x. [DOI] [PubMed] [Google Scholar]
- Randall CL, Anton RF, Becker HC. Role of alcohol-induced hypothermia in mediating the teratogenic effects of alcohol in C57BL/6J mice. Alcohol Clin Exp Res. 1988;12:412–416. doi: 10.1111/j.1530-0277.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Riikonen RS. Difference in susceptibility to teratogenic effects of alcohol in discordant twins exposed to alcohol during the second half of gestation. Pediatr Neurol. 1994;11:332–336. doi: 10.1016/0887-8994(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Schilgen M, Loeser H. Klippel-Feil anomaly combined with fetal alcohol syndrome. Eur Spine J. 1994;3:289–290. doi: 10.1007/BF02226582. [DOI] [PubMed] [Google Scholar]
- Smith DF, Sandor GG, MacLeod PM, Tredwell S, Wood B, Newman DE. Intrinsic defects in the Fetal Alcohol Syndrome: Studies on 76 cases from British Columbia and the Yukon Territory. Neurobehav Toxicol Teratol. 1981;3:145–152. [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Stoler JM, Ryan LM, Holmes LB. Alcohol dehydrogenase 2 genotypes, maternal alcohol use, and infant outcome. J Pediatr. 2002;141:780–785. doi: 10.1067/mpd.2002.128112. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Dehaene P. Fetal alcohol syndrome in twins of alcoholic mothers: concordance of diagnosis and IQ. Am J Med Genet. 1993;47:857–861. doi: 10.1002/ajmg.1320470612. [DOI] [PubMed] [Google Scholar]
- Tam PPL. The control of somitogenesis in mouse embryos. J Embryol Exp Morph. 1981 Supp:103–128. [PubMed] [Google Scholar]
- Taylor CL, Jones KL, Jones MC, Kaplan GW. Incidence of renal anomalies in children prenatally exposed to ethanol. Pediatr. 1994;94:209–212. [PubMed] [Google Scholar]
- Thiel R, Chahoud I, Jurgens M, Neubert D. Time-dependent differences in the development of somites of four different mouse strains. Teratogen, Carcinogen, Mutagen. 1993;13:247–257. doi: 10.1002/tcm.1770130602. [DOI] [PubMed] [Google Scholar]
- Treadwell JA. Integrative strategies to identify candidate genes in rodent models of human alcoholism. Genome. 2006;49:1–7. doi: 10.1139/g05-083. [DOI] [PubMed] [Google Scholar]
- Tredwell SJ, Smith DF, MacLeod PJ, Wood BJ. Cervical spine anomalies in Fetal Alcohol Syndrome. Spine. 1982;7:331–334. doi: 10.1097/00007632-198207000-00002. [DOI] [PubMed] [Google Scholar]
- Tsukahara M, Kajii T. Severe skeletal dysplasias following intrauterine exposure to ethanol. Teratology. 1988;37:79–81. doi: 10.1002/tera.1420370112. [DOI] [PubMed] [Google Scholar]
- van Rensburg JL. Major skeletal defects in the fetal alcohol syndrome. S Afr Med J. 1981;59:687–688. [PubMed] [Google Scholar]
- Viljoen DL, Carr LG, Foroud TM, Brooke L, Ramsay M, Li T-K. Alcohol dehydrogenase-2*2 allele is associated with decreased prevalence of Fetal Alcohol Syndrome in the mixed-ancestry population of the Western Cape Province, South Africa. Alcohol Clin Exp Res. 2001;25:1719–1722. [PubMed] [Google Scholar]
- Wainwright P, Gagnon M. Moderate prenatal ethanol exposure interacts with strain affecting brain development in BALB/c and C57BL/6 mice. Exper Neurol. 1985;88:84–94. doi: 10.1016/0014-4886(85)90115-3. [DOI] [PubMed] [Google Scholar]
- Webster WS, Walsh DA, Lipson AH, McEwen SE. Teratogenesis after acute alcohol exposure in inbred and outbred mice. Neurobehav Toxicol. 1980;2:227–234. [Google Scholar]
- Webster WS, Walsh DA, McEwen SE, Lipson AH. Some teratogenic properties of ethanol and acetaldehyde in C57BL/6J mice: Implications for the study of Fetal Alcohol Syndrome. Teratology. 1983;27:231–243. doi: 10.1002/tera.1420270211. [DOI] [PubMed] [Google Scholar]
- Wilson JG. Methods for administering agents and detecting malformations in experimental animals. In: Wilson JG, Warkany J, editors. Teratology, Principles and Techniques. Chicago: Chicago Press; 1965. p. 262. [Google Scholar]
- Zimmerman EF, Scott WJ, Jr, Collins MD. Ethanol-induced limb defects in mice: Effect of strain and Ro15-4513. Teratology. 1990;41:453–462. doi: 10.1002/tera.1420410410. [DOI] [PubMed] [Google Scholar]