Abstract
Dietary factors might affect the risk of atrial fibrillation (AF), but available studies have provided inconsistent results. In a review of published observational studies and randomized trials, we identified four dietary exposures that had been investigated regarding AF risk: alcohol, fish-derived n-3 polyunsaturated fatty acids, caffeine, and ascorbic acid. Though studies were highly heterogeneous in their design and results, they showed a consistently increased risk of AF in heavy alcohol drinkers, but no risk associated with moderate alcohol intake. High coffee intake was not clearly associated with an increased risk of AF, and a potential U-shaped association (lower AF risk in moderate drinkers) could exist. High intake of fish-derived n-3 polyunsaturated fatty acids from diet or supplements might prevent AF episodes following cardiovascular events, but no consistent evidence supports an effect in primary prevention. Additional large, well-conducted randomized experiments are necessary to address the role of diet in AF prevention.
Keywords: Atrial fibrillation; Diet; Alcohol drinking; Fatty acids, omega-3; Caffeine
INTRODUCTION
Atrial fibrillation (AF) is a common cardiac dysrhythmia affecting more than 2.6 million Americans, with a lifetime risk of 1 in 4.1 Individuals with AF have six times the risk of stroke and twice the risk of all cause mortality compared to those without AF.2
Though structural heart disease underlies most cases of AF,2 its pathogenesis is not well understood. It is hypothesized that initiation of AF may be due to the effect of specific triggers (e.g. ectopic activity, generally, but not always, in one or more of the pulmonary veins) on a susceptible substrate produced by structural and electrical remodeling of the cardiac tissue that allows the arrhythmia to be maintained.2 A number of physiopathological processes, including inflammation and fibrosis, might play a contributory role in the initiation of ectopic activity.3
Additionally, some evidence suggests that lifestyle, such as physical activity or diet, could affect the risk of developing AF.4 Among dietary factors, four group foods and nutrients have been studied with respect to incident AF: alcohol; caffeine; fish/fish-derived n-3 polyunsaturated fatty acids (n-3 PUFAs); and ascorbic acid. In this article, we review the epidemiologic evidence linking diet with the risk of AF and summarize the potential mechanisms underlying these associations, schematically outlined in Figure 1.
Figure 1.
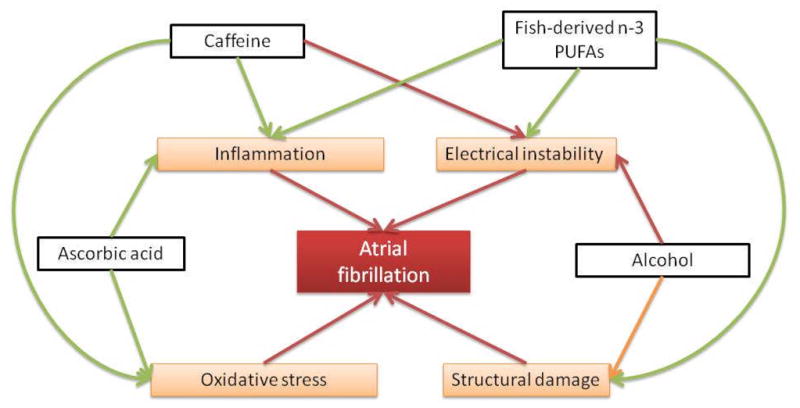
Interaction of dietary exposures, pathophysiology, and atrial fibrillation. Green arrows represent protective effects (inhibition), red arrows deleterious effects (stimulation), and orange arrows, mixed effects.
LITERATURE SEARCH METHOD
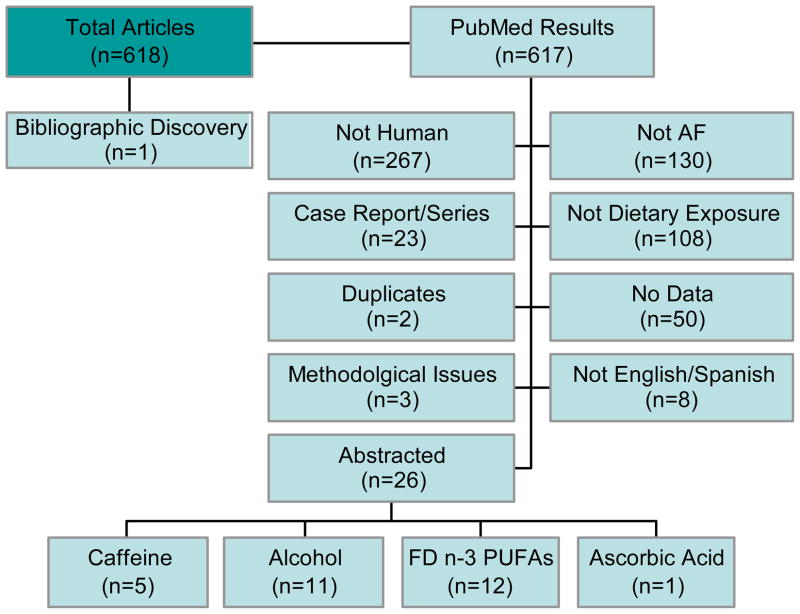
We searched PubMed from 1950 to June 2010 using the following search string: (Ethanol OR Caffeine OR Fatty Acids Omega-3 OR Fish Oils OR Seafood OR Food and Beverages) AND (Atrial Fibrillation) (all MeSH terms). Only articles published in English or Spanish were considered. Overall, our search resulted in 617 articles. One additional article not captured by our search was added based on bibliographic review.
Of these articles, we eliminated articles that were not done in humans (n=267), did not have AF as an outcome (n=130), did not consider dietary exposures (n=108), were case reports/series (n=23), did not contain data (n=50), were published in a language other than English or Spanish (n=8), or were duplicates (n=2). Additionally, three articles5–7 with results that were internally inconsistent (the odds ratios, 95% confidence intervals, and p-values contradicted) were excluded from consideration.
After exclusions, 26 articles were left for review. Those 26 covered 4 dietary exposures: alcohol (n=11), fish-derived n-3 PUFAs (n=12), caffeine (n=5), and ascorbic acid (n=1) (articles do not sum to 85 as some covered more than one exposure). Search details are presented in Figure 2.
Figure 2.
Flowchart of search results. AF, atrial fibrillation; FD n-3 PUFAs, fish-derived n-3 polyunsaturated fatty acids
ALCOHOL INTAKE AND AF
Alcohol is the dietary factor most frequently assessed in relation to AF. In fact, alcohol has been traditionally linked to AF through the “holiday heart” syndrome: AF episodes after periods of intense drinking on weekends or holidays.8 Although the structural damage caused by alcohol-related cardiomyopathy could account for some AF episodes, “holiday heart” can even occur in those without preexisting structural heart damage.8 In contrast, moderate alcohol drinking has been shown to be cardioprotective when compared to teetotalers and heavy drinkers.9, 10 Because of these potentially opposite effects, several epidemiologic studies have investigated the association of alcohol intake with AF risk. Their characteristics and main results are summarized in Table 1.
Table 1.
Selected characteristics of published studies evaluating the association of alcohol intake with atrial fibrillation
| Source | Study Population | Study Design | Alcohol Assessment | AF Ascertainment | Follow-up | Total N | AF Events | Main Results | Adjustment Variables |
|---|---|---|---|---|---|---|---|---|---|
| Rich 198511 | Both sexes; US subjects; patients hospitalized for acute idiopathic AF and 1:1 age, sex matched inpatient controls | Matched Case- control | Medical record reviews; heavy drinker was ≥70 ml alcohol/day, documented alcohol abuse, or obvious intoxication at admission; not heavy drinker was <70 mL alcohol/day or former alcohol abuser | Incident AF (acute idiopathic AF) as ascertained via discharge diagnoses - most patients had ECGs | N/A | 128 | 64 | 62% of AF cases reported heavy alcohol use compared to 33% of controls | Age, sex |
| Koskinen 198720 | Both sexes; Finnish subjects; average age = 48; hospitalized cases of AF and age/sex matched acute, hospitalized controls | Matched case- control | Interview regarding amount of alcohol in prior week (grams) and if different from previous weeks; categorized into abstainers, 1–30 g/day, and >30 g/day | Incident AF (idiopathic and known etiology) diagnosed by cardiologist (85% had ECGs) | N/A | 200 | 100 | Higher alcohol intake associated with higher risk of lone AF (p<0.05) but not AF associated with comorbidities | Age, sex |
| Koskinen 199019 | Both sexes; Finnish subjects; average age 51.5 years; patients hospitalized with recurrent AF, age/sex matched ER controls and age/sex frequency matched community controls | Matched Case- control | Interview regarding amount of alcohol in prior week (grams) and CAGE exam; weekly alcohol categorized into 0, 1–210 grams, and >210 g | Recurrent AF as ascertained by clinical evaluation | None | 246 | 98 | No association in women; in men, multivariate analyses showed that cases had higher odds of alcohol abuse (OR=3.25, p<0.05) compared with all controls | Age, sex, potassium levels, heart disease, stress/lack of sleep |
| Koskinen 199118 | Both sexes; Finnish subjects; average age 48 years; patients with new onset AF | Cohort | Interview regarding amount of alcohol in prior week, if different from previous weeks, and CAGE exam | Recurrent or chronic AF | 4 years | 98 | 39 subjects with recurrences and an additional 7 transitioned to chronic. | No association | None |
| Wilhelmsen 200121 | Men only; Swedish subjects; 47–55 at baseline; population based sample | Cohort | Alcohol abuse data were obtained via registries (yes/no) | Incident AF - Outpatient and Hospitalized AF via ICD-9 codes | 25.2 years | 7,495 | 754 | Alcohol abuse was associated with increased risk (RR=1.21, 95% CI 1.02–1.42) | Age |
| Djousse 200412 | Both sexes; U.S. subjects; average age 42–50 at baseline; Framingham cohort cases of AF and 1: ≥5 controls matched on age, sex, age at baseline, cohort, HTN, baseline CHF, and MI | Matched case- control of cohort data | Repeated questionnaires; frequency of alcohol intake; g/day averaged from baseline until visit prior to AF onset categorized into 0, 0.1–12, 12.1–24, 24.1–36, >36 | Incident AF obtained at study visit or by medical records - all confirmed with ECG | > 50 years | 10,333 | 1,055 | No effect with moderate intake. OR of AF in >36 g alcohol/day 1.33 (95% CI 1.01–1.78) | Multivariable |
| Frost 200413 | Both sexes; Danish subjects; average age 56 years; healthy population- based sample | Cohort | FFQ addressing amount (g/day), type (beer, wine, etc.), and frequency (times/week) of alcohol consumption. Broken out into sex-specific quintiles | Incident AF as identified by hospitalization discharge ICD-8 and ICD-10 codes, outpatient discharge codes after 1/1/95 | 5.7 years | 47,949 | 556 | No association in women. In men, 40% higher risk among those in quintiles 3–5 compared to quintile 1 (p for trend=0.04). | Multivariable |
| Mukamal 200514 | Both sexes; Danish subjects; median age 48–56; healthy, population-based sample | Cohort | Structured questionnaire given at three different time points | Incident AF from 1 of 3 exam visits (ECG) or nationwide hospitalization registries (ICD-9 codes) | >20 years | 1,645 | 1,071 | No association in women. In men higher risk among those taking ≥5 drinks/day (HR 1.45, 95% CI 1.02–2.04) | Multivariable |
| Mukamal 200715 | Both sexes; U.S. subjects; age > 65; population-based sample | Cohort | Yearly quantity/frequency questionnaire (until 1999) and then validated FFQ. Both had questions about frequency and amount of beer, wine, and spirits | Incident AF per annual visit or hospital discharge ICD-9 codes | 9.1 years | 5,609 | 1,232 | No association | Multivariable |
| Conen 200816 | Women only; U.S. subjects; >45 years at baseline; initially healthy | Cohort | FFQ measured at two time points; questions on alcohol intake amount and type; categorized into none, <1/day, 1-<2, and ≥2 | Incident AF via self-report and confirmed via ECG or medical report | 12.4 years | 34,715 | 653 | Increased risk if ≥2 drinks/day versus no alcohol (HR 1.49, 95% CI: 1.05–2.11) | Multivariable |
| Marcus 200817 | Both sexes; US subjects; AF/AFL cases; controls were patients with other arrhythmia and healthy controls | Matched case- control | Structured interview asking average amount and frequency of alcohol consumption (categorized into daily drinker vs. not daily drinker) | All AF/AFL patients reporting for ablation or cardioversion | None | 381 | 125 with AF, 70 with AFL | Increased risk of AFL in younger <60 daily drinkers vs. non-drinkers (OR 17, 95% CI 1.6–192.0) | Multivariable |
AF, atrial fibrillation; AFL, atrial flutter; CI: confidence interval; ECG, electrocardiogram; FFQ, food frequency questionnaire; N/A: not applicable; OR, odds ratio; RR: risk ratio
Epidemiological evidence
Overall, results from these studies are remarkably consistent across types of AF and different study designs. Specifically, it appears that moderate drinking—compared to no drinking—does not increase risk of AF but heavy drinking/alcohol abuse does.
Studies have defined exposure to alcohol in different ways: (1) typical intake11–17; (2) acute intake18–20; and (3) alcohol abuse.18, 19, 21 We describe each type separately.
Typical Intake of Alcohol
All studies included beer, wine, and hard liquor when assessing alcohol intake. Some studies assessed typical alcohol consumption at one time point,5, 11–13, 17 while other studies utilized repeated measures.14–16 Only one study considered former alcohol use as a category of alcohol intake.15
Most studies found a positive association between high levels of alcohol consumption and AF11–13, 16 Of these, three studies investigated sex-specific associations12–14 (two with sex-specific alcohol cutpoints13, 14) and one did not.11
An analysis of the US Framingham Heart Study found 33% increased odds of incident AF for those with highest consumption (>36 g/day) versus those with no consumption (95% CI: 1.01–1.78), without large differences between men and women.12 Two prospective studies conducted in Denmark found an increased risk of AF with higher alcohol intake in men but no association in women – the first, the Danish Diet, Cancer and Health Study, found a dose-response effect (p for trend = 0.04) with a 46% increase in risk (95% CI: 1.05–2.04) in the highest quintile of alcohol intake compared to the lowest13 and the second, the Copenhagen City Heart Study, showed a dose-response relationship (p for trend = 0.05) with a 45% increase in risk (95% CI 1.02–2.04) in men who consumed >36 drinks/week (>5 drinks/day) compared with <1 drink/week, but no increased risk with lower consumption.14
An additional case-control study conducted in the US found that rates of heavy drinking in cases of incident AF were significantly higher than in controls (62% vs. 33%).11 This study defined heavy drinking as either ≥70 mL alcohol/day, documented alcohol abuse, or obvious intoxication at admission; not heavy drinkers drank <70 mL alcohol/day or were former alcohol abusers.
An analysis of the prospective Cardiovascular Health Study did not find an association between alcohol intake and AF risk, but in this older population (>65 years old at baseline) few individuals had a high alcohol intake.15 A case-control study in the US showed an increased risk of atrial flutter (but not AF) in daily drinkers younger than 60 compared to non-drinkers (OR=17, 95% CI 1.6–192.0), and no association in older individuals.17 A potential explanation for the lack of association in this study is the limited sample size (125 AF and 70 atrial flutter cases.
Finally, an analysis of the Women’s Health Study, which included initially healthy women, found that ≥2 drinks/day (versus no alcohol) resulted in 49% increased risk for AF (HR=1.49, 95% CI: 1.05–2.11) after adjustment for multiple confounders.16 This is the only study that found an increased risk of AF among heavy drinkers among women. Potential differences with the Danish studies that might explain the association are the larger number of women (and AF cases) who consumed ≥2 drinks/day, diversity in the patterns of alcohol consumption, and use of varied tools for assessment of alcohol intake.
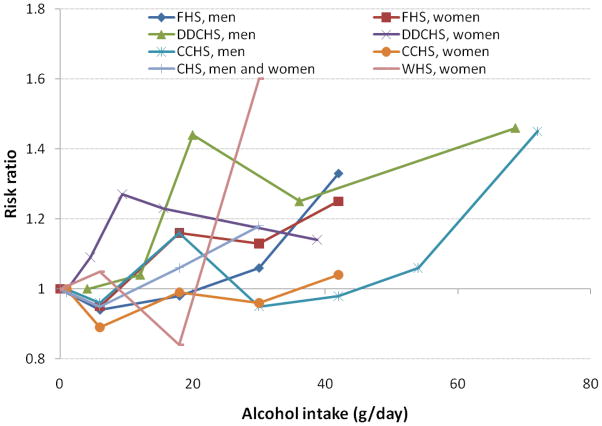
Overall the studies are quite consistent, showing an elevated risk of AF among individuals with high intake of alcohol. This association appears to be stronger in men, who have higher average alcohol intake. No evidence exists that the alcohol/AF association differs by alcohol type13–15 or that there is an age-by-alcohol interaction.16, 17 Results were similar across differing study designs, indicating that recall bias was probably not an issue. Figure 3 shows the association of alcohol intake with AF risk in prospective studies
Figure 3.
Risk ratios of AF by alcohol intake (g/day) in prospective studies. FHS: Framingham Heart Study, Djousse, 200412; DDCS: Danish Diet, Cancer and Health Study, Frost 200413; CCHS: Copenhagen City Heart Study, Mukamal 200514; CHS: Cardiovascular Health Study, Mukamal 200715; WHI: Women’s Health Study, Conen 200816.
Acute Intake
Three studies considered acute alcohol intake in the week prior to hospitalization for AF.18–20 A prospective Finnish study in 98 cases of AF found no association between alcohol intake and recurrence of AF. 18 In a case-control study, also in Finland, researchers found a dose-response (categorized recent alcohol intake as 0, 1–30, and >30 g/day) relationship in lone AF cases but not in cases of incident AF with known etiology.20 Finally, a case-control study compared patients with recurrent AF to both hospital controls and population controls. There was no association in women, no association comparing men with population controls, but there was higher alcohol use in male cases when compared with hospital controls.19
Potential reasons for these discrepancies include differential associations by sex and recall bias. Two of the studies did not report sex-specific associations and both showed null associations when considering all cases of AF (vice idiopathic only).18, 20 Failure to stratify by sex might obscure a true association if the effect of acute alcohol intake differs in women and men. Also, case-control studies in subjects with incident AF may be subject to recall bias. AF patients believe alcohol is a precipitating factor of their AF22 and differential exposure misclassification may bias associations upwards.
Alcohol Abuse
Three studies considered alcohol abuse as an exposure, defined as either alcoholism in men21 or responses to the CAGE questionnaire23 in both sexes.18, 19 A prospective study in Swedish men showed increased odds of incident AF among alcoholics (OR=1.21, 95% CI: 1.02–1.42) in age-adjusted analysis.21 This result is not likely due to recall bias as alcoholism was established via official registries. In a case-control study19 men (but not women) with recurrent AF had higher odds of alcohol abuse (OR=3.25, p<0.05) compared with controls. Finally a prospective study18 of both men and women showed null associations between alcohol abuse and recurrent AF. It should be noted, however, that the CAGE questionnaire has been shown to be less sensitive in women,24 which might explain some of the inconsistencies.
Biological mechanisms
Several potential biological mechanisms may explain how alcohol consumption can affect risk of AF, although the actual mechanism is unknown (Figure 1). As previously stated, moderate alcohol consumption is associated with decreased risk of coronary disease.9, 10 Because previous cardiac events can result in structural damage conducive to AF, moderate alcohol intake may be protective. However, heavy drinkers are prone to cardiomyopathy,25 which might lead to heart failure and a consequently increased risk of AF. Additionally, a study showed that those with a previous history of alcohol-induced AF have impaired vagal heart rate and increased beta-adrenoceptor density when exposed to alcohol26 implying that alcohol may be proarrhythmic in these individuals. Alcohol’s proarrhythmic properties are further supported by evidence that alcohol increases QT interval prolongation,27 though it is unclear how this would relate to an increased risk of AF.
FISH-DERIVED N-3 FATTY ACIDS AND AF
Ample evidence suggests that long-chain fish-derived n-3 polyunsaturated fatty acids (PUFAs) could reduce the risk of cardiovascular disease, particularly cardiac sudden death.28 This protection could be due to an anti-arrhythmic effect of fish-derived n-3 PUFAs via stabilization of the myocyte cell membrane. Therefore, it has been hypothesized that n-3 PUFAs could also reduce the risk of AF through their anti-arrhythmic effect. Several epidemiologic studies have explored this association. Their characteristics and results are summarized in Table 2.
Table 2.
Selected characteristics of published studies evaluating the association of fish derived n-3 fatty acids intake with atrial fibrillation
| Source | Study Population | Study Design | Exposure Assessment | AF Ascertainment | Follow-up | Total N | AF Events | Main Results | Adjustment Variables |
|---|---|---|---|---|---|---|---|---|---|
| Mozaffarian 200429 | Both sexes; U.S. subjects; age=65+ at baseline; population based cohort | Cohort | Fish via FFQ - 2 measures (tuna, other fish, and fried fish sandwiches) | Incident AF via hospital discharge records and annual ECGs | 12 years | 4,815 | 980 | No association of fried fish/fish sandwich consumption with AF. Higher consumption of tuna or other boiled/baked fish associated with decreased risk of AF (5+ servings/wk vs. <1/month RR=0.7, 95% CI: 0.53–0.93) | Multivariable |
| Calo 200537 | Both sexes; patients undergoing CABG | Trial | Fish-derived n- 3 PUFA 2 g/day capsule | Post Operative AF - ascertained via continuous rhythm monitoring | 8 days | 160 | 39 | Fish-derived n-3 PUFAs supplementation associated with decreased risk of post-CABG AF (33.5% vs. 15.2%, p=0.01) | None |
| Frost 200532 | Both sexes; Danish subjects; 50–64 at baseline | Cohort | Fish-derived n- 3 PUFAs intake measured via FFQ | Hospitalized incident AF - identified by ICD-8 and ICD-10 discharge codes | 5.7 years | 47,949 | 556 | Higher risk of AF in 5th quintile vs. 1st quintile of n-3 PUFA intake (RR=1.34, 95% CI: 1.02 – 1.76) | Multivariable |
| Brouwer 200631 | Both sexes; Dutch subjects; 55+ at baseline; population based cohort | Cohort | Fish (g/day) via FFQ; EPA and DHA via FFQ | Incident AF - defined as AF on ECG at study visits, PCPs provided medical records, hospital discharge ICD-9 codes | 6.4 years | 5,184 | 312 | No association between total DHA+EPA or fish intake and AF | Multivariable |
| Macchia 200841 | Both sexes; Italian subjects; avg age=65.1; patients who had survived an MI and were free of AF during MI | Population study (all residents in the Italian civil registry) | Prescription for fish-derived n-3 PUFAs (per prescription databases - no dosage given) | Post MI AF (per hospital outcomes) | 360 days | 3,242 | 471 | RR 0.15, 95% CI 0.05–0.46 comparing those with ≥1 fish-derived n-3 PUFA prescriptions vs. no prescription | Propensity score matching |
| Patel 200939 | Both sexes; U.S. subjects; avg age=59; patients undergoing PVAI | Matched nested case- control | Prescription for fish-derived n-3 PUFAs with at least 655 mg fish oil any time during the follow-up | Post Operative AF - early (0–8 weeks post ablation) and late (8+ weeks after ablation) | 5 years | 258 | 92 with early POAF and 70 with late POAF | Those treated with n-3 PUFAs had less early and late POAF than those not treated | Multivariable |
| Virtanen 200933 | Finnish men only; age 42–60 at baseline; population based cohort | Cohort | n-3 PUFAs measured in serum | Incident AF (hospitalization ICD-8 and ICD-10 discharge codes) | 17.7 years | 2,174 | 240 | Total fish-derived PUFAs (Q4 vs. Q1) associated with 40% lower risk of AF (95% CI: 0.31 – 0.8). Association restricted to DHA (not to EPA or DPA) | Multivariable |
| Berry 201030 | Women only; U.S. subjects; 50–79 years at baseline; postmenopausal | Cohort | Fish (tuna, dark, and non-fried fish) via FFQ; Intake of fish- derived n-3 PUFAs via FFQ | Incident AF - defined by AF or AFL on ECG at study visits | 6 years | 44,720 | 378 | No association fish intake and AF, and fish-derived n-3 PUFAs and AF | Multivariable |
| Heidarsdottir 201035 | Both sexes; Icelandic subjects; avg age = 67; patients admitted for CABG or valvular repair surgery | Trial | 2.2 g of EPA+DHA pre- and post- surgery; fish- derived n-3 fatty acids as measured in plasma | POAF - diagnosed via continuous ECG monitoring where an AF episode lasted >5 minutes | Until hospital discharge. | 168 | 91 | No association (52.4% vs. 52.1%) | None |
| Mariscalco 201038 | Both sexes; Italian subjects; avg age = 66.4; patients scheduled for initial or redo cardiac surgeries | Cohort | Pre-operative fish-derived n-3 PUFA supplements (1 g/day) | Early POAF (continuous ECG monitoring during hospitalization) and late POAF (ECGs given at least every 7 days) | Until conclusion of rehabilitation | 530 | early AF = 237, late AF = 78 | Preop PUFA supplement associated with lower risk of early POAF (31.0% vs. 47.3%, p=0.008) but no association with late POAF 11.9% vs. 15.2%, p=0.43) | Propensity score matching |
| Saravanan 201036 | Both sexes; U.K. subjects; median age 64–68; patients undergoing CABG | Trial | 2 g/day fish- derived n-3 PUFAs pre- and post-surgery | Post Operative AF (>30 seconds) measured via continuous ECG monitoring for 5 days and then daily ECGs. | Until conclusion of hospital stay | 103 | 51 | No association | None |
| Viviani Anselmi 201034 | Both sexes; Italian subjects; avg age 57–63; cases of AF/AFL and healthy controls | Case-control | Fish-derived n- 3 PUFAs as measured in erythrocytes cell membranes | Idiopathic AF (not stated if incident or not) based on referral for ablation | None | 93 | 40 | AF and AFL cases had higher erythrocyte n-3 FA compared to controls (5.3 vs. 2.8, p<0.001) | None |
AF, atrial fibrillation; AFL, atrial flutter; CABG, coronary artery bypass graft surgery; CI, confidence interval; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; ECG, electrocardiogram; EPA, eicosapentaenoic acid; FFQ, food frequency questionnaire; POAF, post-operative AF; PUFA, polyunsaturated fatty acids; PVAI, pulmonary vein antrum isolation; RR, risk ratio.
Epidemiologic evidence
Epidemiologic studies have focused on four exposure measures and three outcomes. Exposure measures are: (1) fish consumption measured via food-frequency questionnaires (FFQ) as a proxy for fish-derived n-3 PUFA intake; (2) fish-derived n-3 PUFA consumption estimated via FFQ; (3) fish-derived n-3 PUFAs as measured in blood plasma or cell membranes; and (4) fish-derived n-3 PUFA supplements. The three outcomes are incident/idiopathic AF, postoperative AF, and post myocardial infarction (MI) AF.
Incident/Idiopathic AF
An analysis of the US-based Cardiovascular Health Study including individuals older than 65 found that higher consumption of tuna or other non-fried fish was associated with a lower risk of AF (5+ servings/week vs. <1 serving/month, RR=0.7, 95% CI 0.53–0.93).29 No association was found for fried fish or fish sandwiches. However, three prospective studies could not replicate this association. In an analysis of post-menopausal American women enrolled in the Women’s Health Initiative, the HR (95% CI) of AF in women who consumed 2 or more servings/week of non-fried fish compared to those with less than 0.5 servings/week was 1.02 (0.73 – 1.42). In this same analysis, intake of fish-derived n-3 PUFAs was not associated with AF risk (RR=1.02, 95% CI: 0.73 – 1.44, comparing ≥ 0.16 g/day vs. < 0.05 g/day).30 Similar results were observed in Dutch individuals participating in the Rotterdam Study (RR=1.17, 95% CI: 0.87–1.57, comparing ≥20 grams fish/day vs. no fish intake, and RR=1.18, 95% CI 0.88–1.57, comparing ≥144 mg/d of n-3 PUFAs vs. ≤43 mg/d).31 Finally, an analysis of the Danish Diet, Cancer and Health Study reported an increased risk of AF among those with the highest intake of fish-derived n-3 PUFAs and AF risk (RR=1.34, 95% CI 1.02–1.76, comparing extreme quintiles).32
Two studies have explored the associations between various fish-derived n-3 PUFAs (docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and docosapentaenoic acid (DPA)) measured in erythrocyte membranes or blood serum, as biomarkers of fish intake, and AF risk, with mixed results. A Finnish prospective study found an inverse association between blood serum levels of total fish-derived n-3 PUFAs (RR=0.5, 95% CI 0.3–0.8, comparing extreme quartiles) and DHA (RR=0.51, 95% CI: 0.32 - 0.82, comparing extreme quartiles) with incident AF but no association was seen for other n-3 PUFAs (EPA, DPA) in this population.33 In contrast, a recent small case-control study in Italy found that levels of n-3 PUFA in erythrocyte membranes were higher in cases of idiopathic AF than in controls (31.4% vs. 23.5%, p<0.001).34 A major limitation of this later study is the lack of adjustment for potential confounders.
Several potential reasons might explain these discrepancies across studies. Assessment of fish intake using food frequency questionnaires may not be an accurate measure of fish-derived n-3 PUFA intake, diluting measures of association. Additionally, heterogeneity in case definition (AF hospitalization,32 AF referred for ablation,34 or AF detected through routine electrocardiogram (ECG) monitoring29–31) might lead to inconsistent results across studies if different types of AF cases have different risk profiles.
Post-operative AF
Various studies, mostly randomized trials, have explored the effect of fish-derived n-3 PUFA supplements on the risk of post-operative AF – defined as the first ECG-diagnosed episode of AF after a cardiac surgery.
A randomized trial in Icelandic subjects undergoing coronary artery bypass graft (CABG) surgery and/or valvular repair surgery administered 2.2 g of EPA+DHA or placebo pre- and post- surgery and determined the association with post-operative AF35 - incident rates were 52.4% in the supplement group versus 52.1% in the placebo group. A similar study in the UK including patients undergoing CABG surgery administered 2 g/day fish-derived n-3 PUFAs pre-and post-surgery or placebo. No differences in post-operative AF were found between groups.36 In contrast, a third randomized trial in Italy found that 2 g/day of fish-derived n-3 PUFAs pre-and post-surgery was associated with a reduction in the incidence of post-operative AF (15.2% in the treatment vs. 33.3% in the placebo group, p=0.01).37
Two observational studies have explored whether supplementation with n-3 PUFAs reduced the risk of post-operative AF. In an Italian study, the use of at least 1 g/day of fish-derived n-3 PUFA was associated with lower risk of early (e.g., before 8 weeks, 31.0% vs. 47.3%, p=0.008) post-operative AF but not late (e.g., after 8 weeks, 11.9% vs. 15.2%, p=0.43) post-operative AF.38 A second study in the US found less post-operative AF in subjects receiving fish-derived n-3 PUFA supplementation at some point during their follow-up.39 However, a major limitation of this last study is its potential for ‘immortal time bias’, since an individual who did not develop post-operative AF was more likely to be considered exposed to n-3 PUFAs.40
Notably, supplementation was not standardized across interventions and supplements differed with respect to amount and ratios of the fish-derived n-3 PUFAs. Given the differential associations by fatty acid type shown in observational studies,33 this distinction may be important and could explain the mixed results for fish-derived n-3 PUFAs and post-operative AF. Additionally, follow-up time differed across trials, which might be relevant since some studies have found different associations for early and late post-operative AF;38, 39 non-standard follow-up times may lead to mixed results.
Post-MI AF
One Italian study assessed the association of prescriptions for low dose n-3 fatty acids with AF following myocardial infarction in 3,242 patients.41 The study found an 85% lower risk of AF in those who had at least one prescription for n-3 fatty acids either before or after their MI compared to those patients who had never received a prescription. A major limitation, however, is that this study results might be affected by immortal time bias.40 The fish-derived n-3 PUFA supplement/post-MI AF relationship has not been studied in other populations.
Biological mechanisms
Fish-derived n-3 PUFAs may prevent AF through several mechanisms: (1) preventing structural heart damage; (2) inhibiting inflammation; and (3) inhibiting electrical currents via myocyte cell membrane stabilization (Figure 1). Previous research has shown that fish-derived n-3 PUFAs protect against coronary heart disease (CHD),42, 43 particularly cardiac sudden death.44 As such n-3 PUFAs may prevent the structural heart damage that is often a precursor to AF. Additionally, fish-derived n-3 PUFAs may inhibit the inflammatory triggers that occasionally initiate the ectopic activity in AF.3 Even once that electrical activity has been stimulated, fish-derived n-3 PUFAs may inhibit the fast, voltage-dependent sodium current and the L-type calcium currents45, 46 that would allow the arrhythmia to be sustained.47
CAFFEINE INTAKE AND AF
Caffeine is a known stimulant and can increase heart rate. Anecdotal evidence points to caffeine consumption as a trigger of AF22, 48, 49 but observational studies have had conflicting results. Results of observational studies exploring this association are summarized in Table 3.
Table 3.
Selected characteristics of published studies evaluating the association of caffeine intake with atrial fibrillation
| Source | Study Population | Study Design | Caffeine Assessment | AF Ascertainment | Follow- up | Total N | AF Events | Main Results | Adjustment Variables |
|---|---|---|---|---|---|---|---|---|---|
| Koskiren 198720 | Both sexes; Finnish subjects; average age = 48; hospitalized cases of AF and age/sex matched acute, hospitalized controls | Matched case- control | Coffee only; interview regarding coffee drinking habits categorized into daily and non- daily drinkers | Incident AF (idiopathic and known etiology) diagnosed by cardiologist (85% had ECGs) | N/A | 200 | 100 | No association | Age, sex |
| Wilhelmsen 200121 | Men only; Swedish subjects; 47–55 at baseline; population based sample | Cohort | Coffee only; questionnaire regarding coffee consumption; categorized into 0, 1–5, and 5+ cups/day | Incident AF - Outpatient and Hospitalized AF via ICD-9 codes | 25.2 years | 7,495 | 754 | No association | Not specified |
| Frost 200551 | Both sexes; Danish subjects; age 50–54 at baseline; population based sample | Cohort | Total caffeine; FFQ including information on consumption of coffee, tea, cola, cocoa, and chocolate categorized into quintiles of g/day | Incident AF as identified by hospitalization discharge ICD- 8 and ICD-10 codes, outpatient discharge codes after 1/1/95 | 5.7 years | 47,949 | 555 | No association | Multivariable |
| Mukamal 200954 | Both sexes; Swedish subjects; aged 45–70; patients hospitalized with first MI | Cohort | Coffee only; questionnaire about daily consumption of coffee categorized into 0-<1, 1-<3, 3- <5, 5-<7, 7+ cups/day | Post MI AF - Hospital discharge ICD- 9 and ICD-10 codes for AF. | 6.9–9.9 years | 1,369 | 163 | No association | Multivariable |
| Conen 201052 | Women only; U.S. subjects; age >45 years at baseline | Cohort | Total caffeine; FFQ including chocolate and decaf/caffeinated versions of the following: coffee, tea, cola, low-cal cola; categorized into quintiles of caffeine intake | Incident AF via self-report and confirmed via ECG or medical report | Median 14.4 years | 33,638 | 945 | Suggest a U- shaped association between total caffeine intake and AF with lowest risk in the 2nd and 3rd quintiles (p<0.01 for quadratic trend) | Multivariable |
AF, atrial fibrillation; ECG, electrocardiogram; FFQ, food frequency questionnaire; MI, myocardial infarction; N/A, not applicable.
Epidemiological evidence
Studies have investigated the association between caffeine intake and two types of AF: (1) overall incident AF; and (2) post-MI AF. In these studies, incident AF was defined as the first diagnosed AF occurrence. Usually, but not always, these diagnoses were done in hospitalized patients. Post-MI AF was defined as the first hospital-diagnosed episode of AF after hospitalization for MI. Each of these outcomes will be addressed separately.
These studies used hospitalization or outpatient records for AF ascertainment, which lead to underascertainment of AF since an important proportion of AF patients will never experience any symptoms.50 Thus, results from these studies might not be generalized to all AF cases.
Incident AF
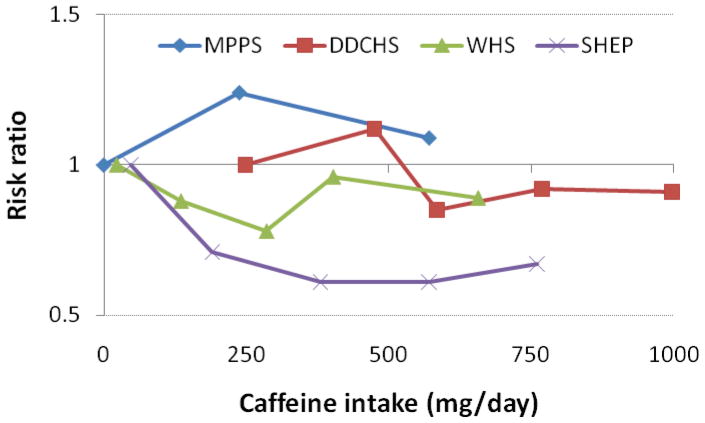
Most studies conducted to date suggest that caffeine intake is not associated with incident AF. An investigation in the Danish Diet, Cancer and Health Study, a large cohort in Denmark, showed independence between caffeine intake and AF incidence (HR 0.9, 95% CI 0.7–1.2, comparing extreme intake quintiles).51 Similar results were found in a cohort study of Swedish men.21 Finally, an analysis of the Women’s Health Study also supported lack of association, though its results supported a potential U-shaped association (lowest AF risk in the 2nd and 3rd quintiles of caffeine intake, p<0.01 for quadratic trend).52 No differences were found for different dietary sources of caffeine. Figure 4 summarizes results from prospective studies exploring the association of caffeine intake and AF incidence.
Figure 4.
Risk ratios for AF given caffeine intake (mg/day). MPPS: Multifactor Primary Prevention Study, Wilhelmsen 200121 (coffee only); DDCHS: Danish Diet, Cancer and Health Study, Frost 200551; WHS: Women’s Health Study, Conen 201052; SHEP: Stockholm Heart Epidemiology Program, Mukamal 200954 (coffee only).
One explanation why these studies have failed to find an association is the potential for exposure misclassification, particularly in studies that used habitual coffee intake as the main exposure. 21 Using coffee as a surrogate for total caffeine intake can lead to exposure misclassification bias,53 which would be expected to bias results towards the null (no association).
Post-MI AF
One Swedish study assessed the association of caffeine intake with AF following myocardial infarction in 1,369 patients.54 The study found a non-significant 33% lower risk of AF with an intake of at least 7 cups/day compared to <1 cup/day (95% CI: 0.33–1.34). This cohort study used coffee as an exposure (vice caffeine) but did use standardized portion size (1 cup = 1dL) and distinguished between boiled, unfiltered, and filtered coffee.
Biological mechanisms
There are two potential mechanisms that may explain caffeine’s effect on atrial fibrillation: (1) antioxidants in tea and coffee; and (2) proarrhythmic effects of caffeine (figure 1). Tea and coffee are predominant sources of caffeine,52 and are high in antioxidants55 which may reduce the risk of AF.56 In contrast, caffeine has been shown to have many proarrhythmic effects including changes in the activation of cardiac channels.57, 58 Thus, while the mechanism driving the relationship between caffeine intake and AF is unclear, it may be multifactorial, and could have competing effects.
OTHER DIETARY FACTORS AND AF
Our search revealed one other dietary exposure that is associated with AF post CABG: ascorbic acid.59 It was hypothesized that ascorbic acid may reduce the inflammation and oxidative stress that potentially precede AF. In a trial including 100 post-CABG patients, those who received oral doses ascorbic acid (2 g before surgery, and 1g/day for 5 days after surgery) in addition to beta blockers pre- and post-surgery had 4% incidence of AF whereas those who received beta blockers only had 25% incidence (OR 0.1, 95% CI 0.02–0.4).59 This specific hypothesis, however, has not been tested in other populations.
PUBLIC HEALTH IMPLICATIONS AND FUTURE DIRECTIONS
Existing evidence does not demonstrate a higher risk of AF in moderate alcohol drinkers. Given other potential cardiovascular benefits of moderate alcohol drinking, our review suggests that moderate alcohol should not necessarily be restricted in those at risk of AF. Heavy drinking, however, remains a consistent risk factor for AF (and other diseases) and should be considered in strategies for the prevention of AF in the general population.4
The relationship between fish-derived n-3 PUFAs and AF is unclear. While a few studies suggest that these fatty acids might have a protective effect for post-operative and post-MI AF, the associations between habitual intake and incident AF were generally null. More evidence is needed before recommendations can be made regarding intake of fish-derived n-3 PUFAs and primary or secondary prevention of AF. Specifically, large, placebo-controlled, blinded randomized trials are necessary before making evidence-based recommendations.
Taken together, studies of the association of caffeine with AF suggested a lack of caffeine effect. Thus, not evidence exists to support a reduction in caffeine intake in the prevention of AF.
Finally, no previous studies have explored whether dietary patterns—associated with different cardiovascular and metabolic outcomes in numerous observational studies—might be associated with the risk of AF. Future studies need to address this gap.
CONCLUSION
Our review shows that higher alcohol intake is consistently related with an increased AF risk, while moderate intake of alcohol and caffeine seem to have no effect. The association between fish-derived n-3 PUFAs and AF was inconsistent, though some evidence exists that these fatty acids might have a protective effect. Further research to clarify the role of diet in the prevention of AF is warranted.
Acknowledgments
Supported by grants T32 HL07779 and RC1HL099452 from the National Heart, Lung and Blood Institute and grant 09SDG2280087 from the American Heart Association
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Markides V, Schilling RJ. Atrial fibrillation: classification, pathophysiology, mechanisms and drug treatment. Heart. 2003;89:939–943. doi: 10.1136/heart.89.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattioli AV, Bonatti S, Zennaro M, Mattioli G. The relationship between personality, socio-economic factors, acute life stress and the development, spontaneous conversion and recurrences of acute lone atrial fibrillation. Europace. 2005;7:211–220. doi: 10.1016/j.eupc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Mattioli AV, Bonatti S, Zennaro M, Melotti R, Mattioli G. Effect of coffee consumption, lifestyle and acute life stress in the development of acute lone atrial fibrillation. J Cardiovasc Med (Hagerstown) 2008;9:794–798. doi: 10.2459/JCM.0b013e3282f64554. [DOI] [PubMed] [Google Scholar]
- 7.Mattioli AV, Farinetti A, Miloro C, Pedrazzi P, Mattioli G. Influence of coffee and caffeine consumption on atrial fibrillation in hypertensive patients. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Nissen MB, Lemberg L. The “holiday heart” syndrome. Heart Lung. 1984;13:89–92. [PubMed] [Google Scholar]
- 9.Ellison RC. Balancing the risks and benefits of moderate drinking. Ann N Y Acad Sci. 2002;957:1–6. doi: 10.1111/j.1749-6632.2002.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 10.Klatsky AL. Moderate drinking and reduced risk of heart disease. Alcohol Res Health. 1999;23:15–23. [PMC free article] [PubMed] [Google Scholar]
- 11.Rich EC, Siebold C, Campion B. Alcohol-related acute atrial fibrillation. A case-control study and review of 40 patients. Arch Intern Med. 1985;145:830–833. doi: 10.1001/archinte.145.5.830. [DOI] [PubMed] [Google Scholar]
- 12.Djousse L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, et al. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93:710–713. doi: 10.1016/j.amjcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Frost L, Vestergaard P. Alcohol and risk of atrial fibrillation or flutter: a cohort study. Arch Intern Med. 2004;164:1993–1998. doi: 10.1001/archinte.164.18.1993. [DOI] [PubMed] [Google Scholar]
- 14.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736–1742. doi: 10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 15.Mukamal KJ, Psaty BM, Rautaharju PM, Furberg CD, Kuller LH, Mittleman MA, et al. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J. 2007;153:260–266. doi: 10.1016/j.ahj.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300:2489–2496. doi: 10.1001/jama.2008.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus GM, Smith LM, Whiteman D, Tseng ZH, Badhwar N, Lee BK, et al. Alcohol intake is significantly associated with atrial flutter in patients under 60 years of age and a shorter right atrial effective refractory period. Pacing Clin Electrophysiol. 2008;31:266–272. doi: 10.1111/j.1540-8159.2008.00985.x. [DOI] [PubMed] [Google Scholar]
- 18.Koskinen P. A 4-year prospective follow-up study of the role of alcohol in recurrences of atrial fibrillation. J Intern Med. 1991;230:423–426. doi: 10.1111/j.1365-2796.1991.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 19.Koskinen P, Kupari M, Leinonen H. Role of alcohol in recurrences of atrial fibrillation in persons less than 65 years of age. Am J Cardiol. 1990;66:954–958. doi: 10.1016/0002-9149(90)90932-q. [DOI] [PubMed] [Google Scholar]
- 20.Koskinen P, Kupari M, Leinonen H, Luomanmaki K. Alcohol and new onset atrial fibrillation: a case-control study of a current series. Br Heart J. 1987;57:468–473. doi: 10.1136/hrt.57.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250:382–389. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 22.Hansson A, Madsen-Hardig B, Olsson SB. Arrhythmia-provoking factors and symptoms at the onset of paroxysmal atrial fibrillation: a study based on interviews with 100 patients seeking hospital assistance. BMC Cardiovasc Disord. 2004;4:13. doi: 10.1186/1471-2261-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 24.Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women: a critical review. JAMA. 1998;280:166–171. doi: 10.1001/jama.280.2.166. [DOI] [PubMed] [Google Scholar]
- 25.Klatsky A. Alcohol and cardiovascular diseases. Ann N Y Acad Sci. 2002;957:7–15. doi: 10.1111/j.1749-6632.2002.tb02901.x. [DOI] [PubMed] [Google Scholar]
- 26.Maki T, Toivonen L, Koskinen P, Naveri H, Harkonen M, Leinonen H. Effect of ethanol drinking, hangover, and exercise on adrenergic activity and heart rate variability in patients with a history of alcohol-induced atrial fibrillation. Am J Cardiol. 1998;82:317–322. doi: 10.1016/s0002-9149(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 27.Kupari M, Koskinen P. Alcohol, cardiac arrhythmias and sudden death. Novartis Found Symp. 1998;216:68–79. doi: 10.1002/9780470515549.ch6. discussion 79–85. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am J Clin Nutr. 2008;87:1991S–1996S. doi: 10.1093/ajcn/87.6.1991S. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry JD, Prineas RJ, van Horn L, Passman R, Larson J, Goldberger J, et al. Dietary fish intake and incident atrial fibrillation (from the Women’s Health Initiative) Am J Cardiol. 2010;105:844–848. doi: 10.1016/j.amjcard.2009.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JC. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J. 2006;151:857–862. doi: 10.1016/j.ahj.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Frost L, Vestergaard P. n-3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:50–54. doi: 10.1093/ajcn/81.1.50. [DOI] [PubMed] [Google Scholar]
- 33.Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009;120:2315–2321. doi: 10.1161/CIRCULATIONAHA.109.852657. [DOI] [PubMed] [Google Scholar]
- 34.Viviani Anselmi C, Ferreri C, Novelli V, Roncarati R, Bronzini R, Marchese G, et al. Fatty acid percentage in erythrocyte membranes of atrial flutter/fibrillation patients and controls. J Interv Card Electrophysiol. 2010;27:95–99. doi: 10.1007/s10840-009-9466-8. [DOI] [PubMed] [Google Scholar]
- 35.Heidarsdottir R, Arnar DO, Skuladottir GV, Torfason B, Edvardsson V, Gottskalksson G, et al. Does treatment with n-3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace. 2010;12:356–363. doi: 10.1093/europace/eup429. [DOI] [PubMed] [Google Scholar]
- 36.Saravanan P, Bridgewater B, West AL, O’Neill SC, Calder PC, Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electrophysiol. 2010;3:46–53. doi: 10.1161/CIRCEP.109.899633. [DOI] [PubMed] [Google Scholar]
- 37.Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 38.Mariscalco G, Braga SS, Banach M, Borsani P, Bruno VD, Napoleone M, et al. Preoperative n-3 Polyunsatured Fatty Acids Are Associated With a Decrease in the Incidence of Early Atrial Fibrillation Following Cardiac Surgery. Angiology. 2010 doi: 10.1177/0003319710370962. [DOI] [PubMed] [Google Scholar]
- 39.Patel D, Shaheen M, Venkatraman P, Armaganijan L, Sanchez JE, Horton RP, et al. Omega-3 polyunsaturated Fatty Acid supplementation reduced atrial fibrillation recurrence after pulmonary vein antrum isolation. Indian Pacing Electrophysiol J. 2009;9:292–298. [PMC free article] [PubMed] [Google Scholar]
- 40.Alonso A. Immortal time bias in the association of n-3 fatty acid supplementation and atrial fibrillation. Eur J Clin Pharmacol. 2009;65:431. doi: 10.1007/s00228-009-0615-x. [DOI] [PubMed] [Google Scholar]
- 41.Macchia A, Monte S, Pellegrini F, Romero M, Ferrante D, Doval H, et al. Omega-3 fatty acid supplementation reduces one-year risk of atrial fibrillation in patients hospitalized with myocardial infarction. Eur J Clin Pharmacol. 2008;64:627–634. doi: 10.1007/s00228-008-0464-z. [DOI] [PubMed] [Google Scholar]
- 42.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 43.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 44.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 45.Kang JX, Leaf A. Protective effects of free polyunsaturated fatty acids on arrhythmias induced by lysophosphatidylcholine or palmitoylcarnitine in neonatal rat cardiac myocytes. Eur J Pharmacol. 1996;297:97–106. doi: 10.1016/0014-2999(95)00701-6. [DOI] [PubMed] [Google Scholar]
- 46.Xiao Y, Gomez A, Morgan J, Lederer W, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1997;94:4182. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 48.Artin B, Singh M, Richeh C, Jawad E, Arora R, Khosla S. Caffeine-Related Atrial Fibrillation. Am J Ther. 2010 doi: 10.1097/MJT.0b013e3181df8cf8. [DOI] [PubMed] [Google Scholar]
- 49.Josephson GW, Stine RJ. Caffeine intoxication: a case of paroxysmal atrial tachycardia. JACEP. 1976;5 :776–778. doi: 10.1016/s0361-1124(76)80308-5. [DOI] [PubMed] [Google Scholar]
- 50.Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369–382. doi: 10.1023/a:1009823001707. [DOI] [PubMed] [Google Scholar]
- 51.Frost L, Vestergaard P. Caffeine and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:578–582. doi: 10.1093/ajcn/81.3.578. [DOI] [PubMed] [Google Scholar]
- 52.Conen D, Chiuve SE, Everett BM, Zhang SM, Buring JE, Albert CM. Caffeine consumption and incident atrial fibrillation in women. Am J Clin Nutr. 2010 doi: 10.3945/ajcn.2010.29627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown J, Kreiger N, Darlington G, Sloan M. Misclassification of exposure: coffee as a surrogate for caffeine intake. Am J Epidemiol. 2001;153:815. doi: 10.1093/aje/153.8.815. [DOI] [PubMed] [Google Scholar]
- 54.Mukamal KJ, Hallqvist J, Hammar N, Ljung R, Gemes K, Ahlbom A, et al. Coffee consumption and mortality after acute myocardial infarction: the Stockholm Heart Epidemiology Program. Am Heart J. 2009;157:495–501. doi: 10.1016/j.ahj.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Ho CT. Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem. 2009;57:8109–8114. doi: 10.1021/jf804025c. [DOI] [PubMed] [Google Scholar]
- 56.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 57.Zhang YA, Tuft RA, Lifshitz LM, Fogarty KE, Singer JJ, Zou H. Caffeine-activated large-conductance plasma membrane cation channels in cardiac myocytes: characteristics and significance. Am J Physiol Heart Circ Physiol. 2007;293:H2448–2461. doi: 10.1152/ajpheart.00032.2007. [DOI] [PubMed] [Google Scholar]
- 58.Cai B, Shan L, Gong D, Pan Z, Ai J, Xu C, et al. Homocysteine modulates sodium channel currents in human atrial myocytes. Toxicology. 2009;256:201–206. doi: 10.1016/j.tox.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 59.Eslami M, Badkoubeh RS, Mousavi M, Radmehr H, Salehi M, Tavakoli N, et al. Oral ascorbic acid in combination with beta-blockers is more effective than beta-blockers alone in the prevention of atrial fibrillation after coronary artery bypass grafting. Tex Heart Inst J. 2007;34:268–274. [PMC free article] [PubMed] [Google Scholar]