Abstract
Objectives
The aim of this study was to evaluate the effectiveness of a combination of dronedarone and ranolazine in suppression of atrial fibrillation (AF).
Background
Safe and effective pharmacological management of AF remains one of the greatest unmet medical needs.
Methods
The electrophysiological effects of dronedarone (10 μmol/l) and a relatively low concentration of ranolazine (5 μmol/l) separately and in combination were evaluated in canine isolated coronary-perfused right and left atrial and left ventricular preparations as well as in pulmonary vein preparations.
Results
Ranolazine caused moderate atrial-selective prolongation of action potential duration and atrial-selective depression of sodium channel–mediated parameters, including maximal rate of rise of the action potential upstroke, leading to the development of atrial-specific post-repolarization refractoriness. Dronedarone caused little or no change in electrophysiological parameters in both atrial and ventricular preparations. The combination of drone-darone and ranolazine caused little change in action potential duration in either chamber but induced potent use-dependent atrial-selective depression of the sodium channel–mediated parameters (maximal rate of rise of the action potential upstroke, diastolic threshold of excitation, and the shortest cycle length permitting a 1:1 response) and considerable post-repolarization refractoriness. Separately, dronedarone or a low concentration of ranolazine prevented the induction of AF in 17% and 29% of preparations, respectively. In combination, the 2 drugs suppressed AF and triggered activity and prevented the induction of AF in 9 of 10 preparations (90%).
Conclusions
Low concentrations of ranolazine and dronedarone produce relatively weak electrophysiological effects and weak suppression of AF when used separately but when combined exert potent synergistic effects, resulting in atrial-selective depression of sodium channel–dependent parameters and effective suppression of AF. (J Am Coll Cardiol 2010;56:1216–24)
Keywords: antiarrhythmic drugs, arrhythmias, electrophysiology, pharmacology, sodium-channel blockers
Atrial fibrillation (AF) is the most frequently encountered sustained clinical arrhythmia and is associated with significant morbidity and mortality. The efficacy and/or safety of currently available anti-AF drugs is less than optimal. Amiodarone remains the best available anti-AF agent for the long-term maintenance of sinus rhythm. Long-term use of amiodarone, however, is often associated with extracardiac toxicity. Dronedarone, a noniodinated derivative of amiodarone, has a better safety profile, although its anti-AF efficacy in the clinic is inferior to that of amiodarone (1). After numerous large clinical trials (2–5), the U.S. Food and Drug Administration approved dronedarone for the management of patients with AF without severe heart failure.
In experimental studies involving canine isolated atria, separate application of ranolazine and long-term amiodarone has been shown to exert atrial-selective depression of sodium channel current (INa)-dependent parameters and to effectively suppress AF (6–10). The combination of long-term amiodarone and acute ranolazine (5 μmol/l) caused marked atrial-selective depression of INa-dependent parameters and was very effective in suppressing AF (11). Because physicians are reticent to use amiodarone because of its adverse effects, we sought to determine whether a similar synergism exists between ranolazine and dronedarone. The present study was designed to test the hypothesis that a combination of acute dronedarone and a low concentration of ranolazine acts synergistically to cause potent atrial-selective electrophysiological actions that lead to the effective suppression and prevention of atrial arrhythmias.
Methods
Experiments were performed using isolated coronary-perfused canine right atrial (RA) and left ventricular (LV) preparations as well as superfused pulmonary vein (PV) sleeve preparations. Transmembrane action potential (AP) recordings were obtained using standard or floating glass microelectrodes. A pseudo-electrocardiogram was recorded using 2 electrodes consisting of silver/silver chloride half cells placed in the Tyrode's solution bathing the preparation, 1.0 to 1.2 cm from the 2 opposite sides of the atrial or ventricular coronary-perfused preparations. The diastolic threshold of excitation (DTE) was determined by increasing stimulus intensity in 0.01-mA steps. The effective refractory period (ERP) was measured by delivering premature stimuli after every 10th regular beat at a pacing cycle length (CL) of 500 ms (with 10-ms resolution; stimulation with a 2 × DTE amplitude, determined at each CL). Post-repolarization refractoriness (PRR) was recognized when ERP exceeded AP duration (APD) measured at 90% repolarization in the ventricles and APD measured at 70% repolarization in the atria. Note that ventricular ERP coincided with APD measured at 90% repolarization, whereas atrial ERP generally coincided with APD measured at 70% to 75% repolarization. The anti-AF efficacy of dronedarone and ranolazine separately as well as in combination was tested in an acetylcholine (ACh)–mediated model of persistent AF. Persistent ACh-induced AF was inducible in 100% of canine coronary-perfused RA preparations.
Detailed methods, experimental protocols, and statistical approaches are included in the online supplement.
Results
Electrophysiological effects of ranolazine and dronedarone
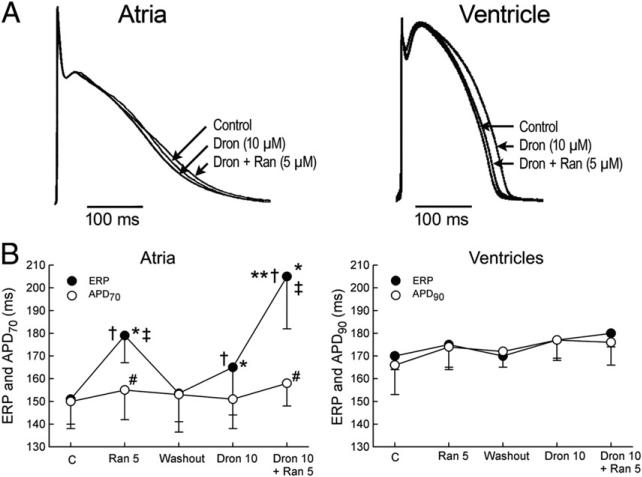
Ranolazine (5 μmol/l) and dronedarone (10 μmol/l) alone as well as combined caused little or no change in APD in either RA or LV coronary-perfused preparations (Figs. 1A and 1B). When applied separately, both ranolazine (5 μmol/l) and dronedarone (10 μmol/l) prolonged the ERP more than APD measured at 70% repolarization in the atria, thus leading to the development of PRR (Fig. 1B). The extent of the atrial PRR was greater after ranolazine than dronedarone. Ventricular ERP was not altered by either ranolazine or dronedarone at the concentrations tested. The combination of dronedarone and ranolazine caused a significant synergistic prolongation of ERP in the atria but did not change ERP in ventricles, thus resulting in a marked atrial-specific PRR (Fig. 1B).
Figure 1. Synergistic Effect of Ranolazine and Dronedarone to Atrial-Selectively Induce PRR.
Atrial-selective induction of post-repolarization refractoriness (PRR) by ranolazine (Ran) and dronedarone (Dron) alone and in combination (PRR was approximated by the difference between the effective refractory period [ERP] and the action potential duration measured at 70% repolarization [APD70] in the atria and between the ERP and the action potential duration measured at 90% repolarization [APD90] in the ventricles; ERP corresponds to APD70–75 in the atria and to APD90 in the ventricles). (A) Superimposed action potentials demonstrating relatively small changes with dronedarone and ranolazine and their combination. (B) Summary data of atrial-selective induction of PRR. Ventricular data were obtained from epicardium and atrial data from endocardial pectinate muscle (n = 7 to 8). *p < 0.05 versus respective control (C). †p < 0.05 versus washout. ‡p < 0.05 versus Dron 10 μmol/l. #p<0.05 versus respective ERP. **p < 0.05, change in ERP induced by combination of Ran and Dron (from washout) versus the sum of changes caused by Ran and Dron independently (both from washout). Cycle length = 500 ms.
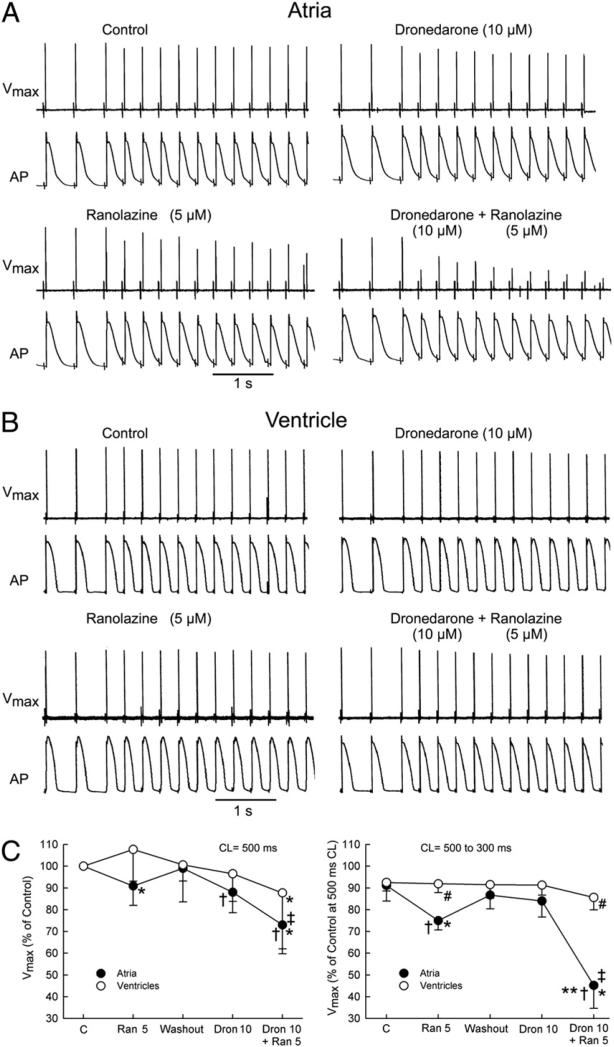
At a pacing CL of 500 ms, the maximal rate of rise of the action potential upstroke (Vmax) was reduced by ranolazine (5 μmol/l) and by dronedarone (10 μmol/l) in RA but not in LV preparations (Fig. 2). At this pacing rate, the combination of these agents led to a reduction of Vmax in both atria and ventricles but predominantly in the former (27 ± 11% vs. 12 ± 28% decrease, respectively). In the atria, acceleration of pacing rate from a CL of 500 to 300 ms caused a much greater depression of Vmax when dronedarone and ranolazine were combined than when each of these agents was applied alone (Fig. 2). In the ventricles, a similar acceleration of pacing rate led to only a modest reduction in Vmax under all conditions tested.
Figure 2. Synergistic Effect of Ranolazine and Dronedarone to Atrial-Selectively Depress Vmax.
Atrial-selective synergetic depression of the maximal rate of rise of the action potential (AP) upstroke (Vmax) by the combination of dronedarone (Dron) and ranolazine (Ran) at rapid activation rates. Shown are AP tracings and corresponding Vmax values recorded during acceleration of pacing rate from a cycle length (CL) of 500 to 300 ms in atrial (A) and ventricular (B) perfused preparations. Composite data of Vmax of atrial and ventricular preparations paced at a CL of 500 ms (C, left) expressed as percentage of control (C). Composite data of Vmax of atrial and ventricular APs after acceleration from a CL of 500 to 300 ms expressed as percentage of Vmax value recorded at a CL of 500 ms in controls (C, right). “Atria” represent combined pectinate muscle and crista terminalis data. “Ventricles” represent combined epicardial and M-cell data from ventricular wedge preparations (n = 7 to 8). *p < 0.05 versus respective control. †p < 0.05 versus washout. ‡p < 0.05 versus Dron 10 μmol/l. #p < 0.05 versus respective atrial values. **p < 0.01, change in Vmax induced by the combination of Ran and Dron (from washout) versus the sum of changes caused by Ran and Dron independently (both from washout).
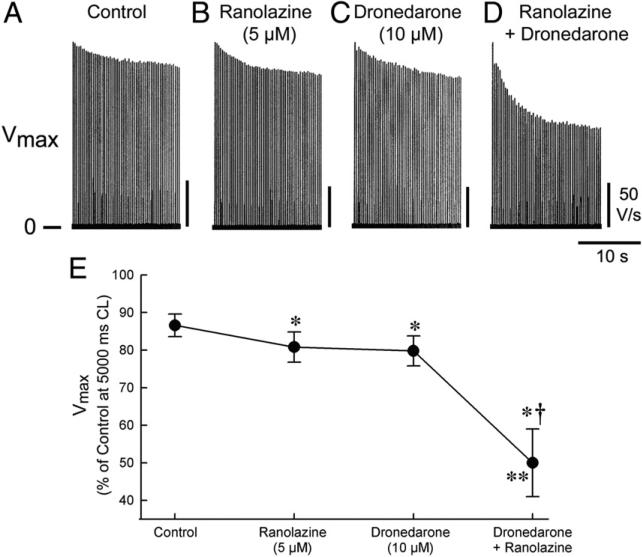
In PV sleeves, a change of rate from a CL of 5,000 ms to a CL of 300 ms caused a 13 ± 3% reduction in Vmax under control conditions and 19 ± 4%, 20 ± 3%, and 50 ± 9% reductions after ranolazine (5 μmol/l), dronedarone (10 μmol/l), and dronedarone plus ranolazine, respectively (Fig. 3).
Figure 3. Synergistic Effect of Ranolazine and Dronedarone to Depress Vmax in PV.
Synergistic effect of the combination of ranolazine and dronedarone on maximal rate of rise of the action potential upstroke (Vmax) after an abrupt change in rate in pulmonary vein (PV) sleeve preparations. (A) Vmax traces recorded after a change in cycle length (CL) from 5,000 to 300 ms. (B) Graph displaying composite data of Vmax changes after acceleration of pacing rate from a CL of 5,000 to 300 ms (n = 4 to 8). *p < 0.05 versus control. †p < 0.05 versus ranolazine or dronedarone alone. **p < 0.05, change in Vmax induced by combination ranolazine plus dronedarone (from washout) versus the sum of changes caused by ranolazine and dronedarone independently (both from washout).
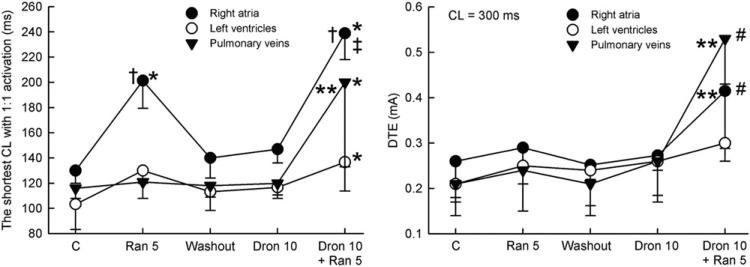
The shortest S1 to S1 interval permitting 1:1 activation was increased by ranolazine in RA preparations preferentially, but not by dronedarone in either RA or LV preparations (Fig. 4A). When dronedarone and ranolazine were combined, the shortest S1 to S1 interval was significantly increased in all preparations, but the change was many times greater in atrial (RA and PV sleeves) than in ventricular preparations. The DTE was not significantly affected by either ranolazine (5 μmol/l) or dronedarone (10 μmol/l) in either atrial or ventricular preparations (Fig. 4B). However, when these drugs were combined, the DTE was increased significantly in RA and PV sleeves, but not in LV preparations.
Figure 4. Synergistic Effect of Ranolazine and Dronedarone to Atrial-Selectively Depress Excitability.
Effect of ranolazine (Ran) (5 μmol/l), dronedarone (Dron) (10 μmol/l), and their combination to prolong the shortest cycle length (CL) permitting 1:1 activation and to increase the diastolic threshold of excitation (DTE). The combination of Dron and Ran caused greater changes in these parameters in atrial than in ventricular preparations (n = 4to8). *p < 0.05 versus respective control (C). †p < 0.05 versus washout and Dron. ‡p < 0.05 versus Ran. #p < 0.001 versus respective atrial values. **p < 0.05, change in Vmax caused by combination of ranolazine and dronedarone (relative to washout) versus the sum of changes induced by ranolazine and dronedarone independently.
Antiarrhythmic effects in coronary-perfused atria and superfused PV sleeves
Persistent AF was induced in 100% of coronary-perfused atrial preparations in the presence of 1 μmol/l ACh (6). Dronedarone (10 μmol/l) and a low concentration of ranolazine (5 μmol/l) were poorly effective in preventing the induction of persistent ACh-mediated AF and in terminating persistent AF when administered separately (Fig. 5, Tables 1 and 2). However, when dronedarone (10 μmol/l) and ranolazine (5 μmol/l) were combined, the success rate for preventing the induction of persistent AF was dramatically augmented to 90% (9 of 10 atrial preparations) (Table 1). The drug combination terminated persistent AF in 6 of 10 atria (Table 2) and prevented the reinduction of AF in 100% of preparations (6 of 6). Persistent atrial flutter or tachycardia (with a CL ≥160 ms) could be induced by rapid pacing and/or programmed electrical stimulation in 2 of the 6 preparations.
Figure 5. Effect of Dronedarone and Ranolazine to Suppress AF.
The combination of dronedarone (10 μmol/l) and ranolazine (5 μmol/l) is effective in terminating persistent atrial fibrillation (AF) and/or preventing its induction in coronary-perfused right atria. (A) Persistent acetylcholine (ACh) (0.5 μmol/l)–mediated AF is terminated by the drug combination. AF is initially converted to flutter and then to sinus rhythm. (B) The combination of dronedarone and ranolazine prevents rapid-pacing induction of AF after pretreatment with ACh (1 μmol/l), likely because of depression of the sodium channel (see reduction of maximal rate of rise of the action potential [AP] upstroke). Acceleration of pacing rate from a cycle length (CL) of 500 to 130 ms leads to failure of a 1:1 response. ECG = electrocardiogram.
Table 1.
Effects of Ranolazine (5 μmol/l), Dronedarone (10 μM), and Their Combination on Atrial Excitability and Induction of ACh-Mediated Persistent AF in the Isolated Canine Coronary-Perfused Right Atria
| Protocol | APD90 (ms) | ERP (ms) | Shortest S1 to S1 Interval Permitting 1:1 Activation (ms) | Induction of Persistent AF |
|---|---|---|---|---|
| Control | 198 ± 7 | 153 ± 8 | 130 ± 10 | 0% |
| Ranolazine (5 μmol/l) | 210 ± 10* | 181 ± 11* | 201 ± 22* | 0% |
| Dronedarone (10 μmol/l) | 202 ± 9 | 171 ± 11* | 147 ± 11* | 0% |
| Ranolazine + dronedarone | 212 ± 11* | 211 ± 16* | 239 ± 21 | 0% |
| ACh (1 μmol/l) | 41 ± 6 | 52 ± 9 | 56 ± 7 | 100% (10/10) |
| Ranolazine (5 μmol/l) + ACh | 52 ± 6† | 72 ± 13† | 94 ± 11† | 71% (5/7) |
| Dronedarone (10 μmol/l) + ACh | 45 ± 5 | 59 ± 7 | 88 ± 13† | 83% (5/6) |
| Ranolazine + dronedarone + ACh | 67 ± 14†‡ | 99 ± 18†‡ | 120 ± 24†‡ | 10% (1/10) |
APD90 and ERP data presented were obtained from the pectinate muscle region of coronary-perfused atria at a CL of 500 ms (n = 5 to 15).
p < 0.05 versus control.
p < 0.05 versus ACh (10 μmol/l) alone.
p < 0.05 versus ranolazine plus ACh and dronedarone plus ACh.
ACh = acetylcholine; AF = atrial fibrillation; APD90 = action potential duration measured at 90% repolarization; CL = cycle length; ERP = effective refractory period.
Table 2.
Effects of Ranolazine (5 μmol/l), Dronedarone (10 μmol/l), and Their Combination to Terminate Persistent ACh-Mediated AF and Prevent Its Reinduction in the Isolated Canine Coronary-Perfused Right Atria
| Protocol | Termination of Persistent AF | Prevention of AF Reinduction |
|---|---|---|
| ACh (1 μmol/l) | 0% (0/10) | — |
| ACh + ranolazine (5 μmol/l) | 20% (1/5) | 100% (1/1) |
| ACh + dronedarone (10 μmol/l) | 17% (1/6) | 0% (0/1) |
| ACh + ranolazine + dronedarone | 60% (6/10) | 100% (6/6) |
ACh = acetylcholine; AF = atrial fibrillation.
Delayed afterdepolarization (DAD)–induced triggered activity induced in PV sleeves was reduced by ranolazine 5 μmol/l and dronedarone 10 μmol/l and abolished when the drugs were combined (6 of 6) (Fig. 6).
Figure 6. Synergistic Effect of Ranolazine and Dronedarone to Suppress DAD.
The combination of ranolazine (5 μmol/l) and dronedarone (10 μmol/l) abolishes delayed afterdepolarization (DAD)–induced triggered activity in pulmonary vein sleeve preparations. (A) Isoproterenol (1 μmol/l) and high calcium (5.4 mmol/l) induce DAD and triggered response. Ranolazine (5 μmol/l) eliminates the triggered response but not the DAD. Washout of ranolazine restores the triggered response, followed by a DAD. Dronedarone (10 μmol/l) abolishes the triggered response, but the DAD persists. The combination of ranolazine and dronedarone eliminates all DAD activity and induces 2:1 activation failure; an increase of stimulus intensity restores 1:1 activation but not the DAD. (B) In another preparation, the same protocol yielded multiple triggered beats. Ranolazine is seen to abolish all of ectopic activity, but not the DAD, whereas dronedarone (5 umol/l) reduces the number of triggered responses. A single triggered beat followed by a DAD persists. The combination of ranolazine and dronedarone eliminates all DAD activity and induces 2:1 activation failure; an increase of stimulus intensity restores 1:1 activation, but not the DAD. Basic cycle length (BCL) = 120 ms (A) and 150 ms (B).
Discussion
Our data demonstrate a potent atrial-selective effect of the combination of dronedarone (10 μmol/l) and ranolazine (5 μmol/l) to depress sodium channel– dependent parameters and to suppress AF and triggered activity in experimental models of AF. When dronedarone (10 μmol/l) or ranolazine (5 μmol/l) was used alone, the observed electro-physiological changes in both atria and ventricles were either small or absent, and the anti-AF efficacy of the drug was poor (<30%). Considering the relatively good safety profiles of both agents, our results suggest that the striking synergistic atrial-selective action of ranolazine and dronedarone may offer a unique combination therapy for AF that is both safe and effective.
Electrophysiology and antiarrhythmic efficacy of dronedarone
Acute dronedarone has been reported to produce variable but a generally small or no effect to alter APD (12–16). In rabbit superfused cardiac preparations, acute dronedarone abbreviated APD in both atria and ventricles (14,15). APD was not altered in superfused ventricular preparations isolated from canine and guinea pig hearts at concentrations of dronedarone up to 10 μmol/l (12,13). Thus, our data on the acute effect of dronedarone on APD are generally consistent with those reported previously.
Ventricular and atrial ERP have been reported to be prolonged to a similar extent after acute dronedarone in dogs in vivo (17). In our present in vitro investigation, dronedarone prolonged both atrial and ventricular ERP, with preferential lengthening in the atria (9% and 4%, respectively). ERP prolongation by dronedarone and ranolazine in the ventricles was associated with a comparable prolongation of APD measured at 90% repolarization (Fig. 1). In contrast, the increase of ERP in the atria greatly exceeded the increase in APD measured at 70% repolarization, because of the development of marked PRR.
Acute dronedarone (10 μmol/l) has been reported to produce no changes or a relatively small reduction in AP Vmax in both atrial and ventricular rabbit superfused preparations as well as in canine and guinea pig ventricular and Purkinje fiber superfused preparations (12,13,15). Even at rapid pacing rates (a CL of 125 ms), dronedarone 10 μmol/l reduced the AP Vmax by only 16% in superfused rabbit atrial preparations (15). Thus, our data showing a modest effect of acute dronedarone on AP Vmax are consistent with those previously reported.
Interestingly, although several clinical investigations have reported on the anti-AF efficacy of dronedarone for the long-term maintenance of sinus rhythm (2,3), we could not find a full-length report evaluating the efficacy of dronedarone against AF in any experimental model (acutely or long term). We are also not aware of any clinical study testing the anti-AF effect of acute dronedarone. Thus, we are unable to compare our results of the relatively weak actions of acute dronedarone to suppress AF with any previous preclinical or clinical study.
Available clinical data indicate that the long-term efficacy of dronedarone for the maintenance of sinus rhythm in AF patients is inferior to that of amiodarone (1). In the DIONYSOS (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation) trial, a direct comparison between amiodarone and dronedarone showed that the rate of recurrence of AF was 63% with dronedarone and 42% with amiodarone (at 6-month follow-up). In the combined EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australasian Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm), AF recurrence occurred in 64% of patients treated with dronedarone compared with 75% patients taking placebo at 1-year follow-up (3). The rate of conversion of persistent AF with dronedarone ranged from 5.8% to 14.8% (800 to 1,600 mg/day) versus 3.1% in the placebo arm, as determined on the fifth to seventh days after the start of drug treatment (2). The relatively weak actions of acute dronedarone to suppress AF in the present study are consistent with the modest effects of the drug on AF in the clinic.
Electrophysiology and antiarrhythmic efficacy of ranolazine
We have previously reported that ranolazine 5 μmol/l causes moderate electrophysiological effects in canine atrial preparations, with little to no effect in ventricular preparations (6,18). This concentration of ranolazine is well within the therapeutic range of the drug (2 to 10 μmol/l). Ranolazine has also been shown to cause predominant atrial prolongation of ERP in anesthetized closed-chest pigs (10).
The anti-AF efficacy of ranolazine 5 μmol/l was not tested in the ACh-mediated AF model in our previous studies. Higher concentrations of ranolazine (10 μmol/l), at the upper end of the therapeutic range, were shown to exert potent anti-AF effects in experimental models of vagally mediated AF in canine atria in vitro (10 μmol/l) (6) and porcine atria in vivo (about 9 μmol/l plasma concentration) (10) models. In an ischemia-reperfusion-isoproterenol model of AF, ranolazine 5 μmol/l was observed to prevent the induction of AF in 60% of atrial preparations (6). In superfused PV preparations ranolazine 10 μmol/l effectively suppressed intracellular calcium-dependent DAD and late phase 3 early afterdepolarization–induced triggered activity (8). Consistent with results of our in vitro experimental models of AF, ranolazine has also been shown to reduce the onset of new AF and to terminate AF in the clinic (19–21).
Ranolazine's antiarrhythmic action in the ventricle is thought to be due largely to its potent inhibition of late INa, which serves to normalize abnormal repolarization encountered under pathophysiologic conditions (18). In contrast, the anti-AF efficacy of ranolazine is ascribed to its potent atrial-selective inhibition of early INa (6). Although not the predominant mechanism, blockade of late INa likely contributes to ranolazine's antiarrhythmic action in the atria.
Drug combination for antiarrhythmic therapy: efficacy and safety
The concept of combining dronedarone and ranolazine in the management of AF derives from our recent demonstration of a synergetic effect of a combination of chronic amiodarone and acute ranolazine (11). This combination was found to depress INa-dependent parameters, thus acting to terminate and prevent the reinduction of AF in coronary-perfused canine atrial preparations as well as to suppress DAD-induced and early afterdepolarization–induced triggered activity in PVs (11). Ectopic activity arising from the PV has been shown to play a prominent role in the development of AF (22). Early afterdepolarization–induced as well as DAD-induced triggered activity originating from PV sleeves has been proposed as a potential trigger in the initiation of AF (9,23,24). When dronedarone (5 to 10 μmol/l) or ranolazine (5 μmol/l) is used separately, DAD-induced triggered activity is reduced but is totally abolished when the drugs are used in combination.
The rationale for the amiodarone-ranolazine combination stemmed from the observation that both agents are atrial-selective sodium-channel blockers (6,7) and that amiodarone is a predominantly inactivated-state blocker of the sodium channel, and ranolazine is a predominantly open-state blocker (25). Ranolazine, amiodarone, and dronedarone all display relatively rapid dissociation from the sodium channel, which contributes prominently to their atrial-selective sodium channel blockade (26,27). We hypothesized that a combination of open-state and inactivated-state sodium-channel blockade with rapid kinetics could produce a synergistic atrial-selective sodium-channel inhibition and thus be effective against AF without producing significant electrophysiological effects in the ventricles. After validation of this hypothesis (11), we considered the combination of dronedarone and ranolazine, in that dronedarone is a congener of amiodarone with far fewer adverse effects and a similar electrophysiological profile. The results of the present study provide validation of this concept as well, presenting further evidence in support of the hypothesis that a combination of predominantly open-state and inactivated-state blockers of the sodium channel can lead to synergistic effects to selectively inhibit INa-dependent parameters and thus exert potent atrial-selective actions to terminate and prevent the induction and reinduction of AF (Table 1). The combination of the 2 drugs is expected to act synergistically because the sodium channel is inhibited via 2 independent mechanisms. Note that although there are INa blockers that can inhibit both open and inactivated sodium channels to a similar extent (e.g., quinidine or disopyramide), these agents cause an unfavorable shift in the balance of inward and outward currents, leading to QT prolongation, dispersion of repolarization, and ventricular proarrhythmia. The slow dissociation of these drugs from the sodium channel can also lead to a pronounced slowing of conduction, contributing to the development of ventricular arrhythmias. In contrast, ranolazine as well as amiodarone and dronedarone have rapid unbinding kinetics from the sodium channel and produce a shift in the balance of currents that does not, or very rarely, cause ventricular proarrhythmia (26,27).
A major concern in the pharmacological management of AF agents is the risk for the induction of ventricular arrhythmias and/or organ toxicity. Although amiodarone only rarely produces ventricular proarrhythmia and is generally safe in patients with structurally compromised ventricles, this agent causes extracardiac complications. Dronedarone, a noniodinated derivative of amiodarone, is generally considered to be safer compared with amiodarone in patients with AF (1). However, in patients with preexisting severe congestive heart failure (New York Heart Association functional class III and IV), dronedarone has been reported to worsen heart failure symptoms, leading to increased mortality (4).
Clinical use of ranolazine, both acute and long term, has not been associated with serious adverse effects, not even in patients with structural heart disease and no reported organ toxicity (28). The rationale for combining dronedarone and ranolazine in the present study stems from the superiority of dronedarone over amiodarone with respect to safety (1,3). Like the combination of amiodarone and ranolazine (11), the combination of dronedarone and ranolazine produces potent atrial-selective anti-AF effects but is likely to be associated with fewer adverse effects and no organ toxicity.
Study limitations
As with all data derived from experimental animal models, extrapolation to the clinic must be done with great caution. It is noteworthy that our data were obtained acutely in “healthy” atrial and ventricular preparations. Most patients with AF have structural and electrical cardiac abnormalities capable of modulating the pharmacological response to chronic treatment. Although the vagally mediated AF model used in the present study does not recapitulate all forms of clinical AF, the anti-AF efficacy of antiarrhythmic drugs previously studied using this model, including amiodarone, propafenone, lidocaine, and sotalol, is consistent with the efficacy of these agents in the clinic (6,7). Finally, although dronedarone and ranolazine are relatively safe and well tolerated when used individually (with the exception of dronedarone use in cases of advanced heart failure [4]), the safety and tolerability of the combination of ranolazine and dronedarone is unknown.
Supplementary Material
Acknowledgments
The authors thank the Judy Hefferon and Robert J. Good-row for their expert technical assistance.
This study was supported by grants from Gilead Sciences, the National Institutes of Health (grant HL-47678 to Dr. Antzelevitch), and the New York State and Florida Grand Lodges of Free and Accepted Masons. Dr. Antzelevitch received research support from Gilead Sciences, AstraZeneca, Merck, and Cardiome; and consulting support from Gilead Sciences and AstraZeneca. Dr. Belardinelli is an employee of Gilead Sciences. All other authors have reported that they have no relationships to disclose.
Abbreviations and Acronyms
- ACh
acetylcholine
- AF
atrial fibrillation
- AP
action potential
- APD
action potential duration
- CL
cycle length
- DAD
delayed afterdepolarization
- DTE
diastolic threshold of excitation
- ERP
effective refractory period
- INa
sodium channel current
- LV
left ventricular
- PRR
post-repolarization refractoriness
- PV
pulmonary vein
- RA
right atrial
- Vmax
maximal rate of rise of the action potential upstroke
Footnotes
APPENDIX
For supplemental methods, experimental protocols, and statistical approaches, please see the online version.
REFERENCES
- 1.Piccini JP, Hasselblad V, Peterson ED, et al. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol. 2009;54:1089–95. doi: 10.1016/j.jacc.2009.04.085. [DOI] [PubMed] [Google Scholar]
- 2.Touboul P, Brugada J, Capucci A, et al. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J. 2003;24:1481–7. doi: 10.1016/s0195-668x(03)00321-x. [DOI] [PubMed] [Google Scholar]
- 3.Singh BN, Connolly SJ, Crijns HJ, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–99. doi: 10.1056/NEJMoa054686. [DOI] [PubMed] [Google Scholar]
- 4.Kober L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–87. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 5.Hohnloser SH, Crijns HJ, van Eickles M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–78. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 6.Burashnikov A, Di Diego JM, Zygmunt AC, et al. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–57. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burashnikov A, Di Diego JM, Sicouri S, et al. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;5:1735–42. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicouri S, Glass A, Belardinelli L, et al. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–26. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sicouri S, Belardinelli L, Carlsson L, et al. Potent antiarrhythmic effects of chronic amiodarone in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2009;20:803–10. doi: 10.1111/j.1540-8167.2009.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar K, Nearing BD, Carvas M, et al. Ranolazine exerts potent effects on atrial electrical properties and abbreviates atrial fibrillation duration in the intact porcine heart. J Cardiovasc Electrophysiol. 2009;20:796–802. doi: 10.1111/j.1540-8167.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 11.Sicouri S, Burashnikov A, Belardinelli L, et al. Synergistic electro-physiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circ Arrhythm Electrophysiol. 2010;3:88–95. doi: 10.1161/CIRCEP.109.886275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varro A, Takacs J, Nemeth M, et al. Electrophysiological effects of dronedarone (SR 33589), a noniodinated amiodarone derivative in the canine heart: comparison with amiodarone. Br J Pharmacol. 2001;133:625–34. doi: 10.1038/sj.bjp.0704106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautier P, Guillemare E, Marion A, et al. Electrophysiologic characterization of dronedarone in guinea pig ventricular cells. J Cardiovasc Pharmacol. 2003;41:191–202. doi: 10.1097/00005344-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Sun W, Sarma JS, Singh BN. Electrophysiological effects of drone-darone (SR33589), a noniodinated benzofuran derivative, in the rabbit heart: comparison with amiodarone. Circulation. 1999;100:2276–81. doi: 10.1161/01.cir.100.22.2276. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Sarma JS, Singh BN. Chronic and acute effects of dronedarone on the action potential of rabbit atrial muscle preparations: comparison with amiodarone. J Cardiovasc Pharmacol. 2002;39:677–84. doi: 10.1097/00005344-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Moro S, Ferreiro M, Celestino D, et al. In vitro effects of acute amiodarone and dronedarone on epicardial, endocardial, and M cells of the canine ventricle. J Cardiovasc Pharmacol Ther. 2007;12:314–21. doi: 10.1177/1074248407306906. [DOI] [PubMed] [Google Scholar]
- 17.Manning A, Thisse V, Hodeige D, et al. SR 33589, a new amiodarone-like antiarrhythmic agent: electrophysiological effects in anesthetized dogs. J Cardiovasc Pharmacol. 1995;25:252–61. doi: 10.1097/00005344-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Antzelevitch C, Belardinelli L, Zygmunt AC, et al. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110:904–10. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scirica BM, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–52. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 20.Murdock DK, Overton N, Kersten M, et al. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J. 2008;8:175–81. [PMC free article] [PubMed] [Google Scholar]
- 21.Murdock DK, Kersten M, Kaliebe J, et al. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a review of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9:260–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 23.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:290–5. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson E, Po SS, Scherlag BJ, et al. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Zygmunt AC, Nesterenko VV, Rajamani S, et al. Mechanism of the preferential block of the atrial sodium current by ranolazine. Biophys J. 2009;96:250a. [Google Scholar]
- 26.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel block for the treatment of atrial fibrillation. Expert Opin Emerg Drugs. 2009;14:233–49. doi: 10.1517/14728210902997939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burashnikov A, Antzelevitch C. New development in atrial antiarrhythmic drug therapy. Nat Rev Cardiol. 2010;7:139–48. doi: 10.1038/nrcardio.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koren MJ, Crager MR, Sweeney M. Long-term safety of a novel antianginal agent in patients with severe chronic stable angina: the Ranolazine Open Label Experience (ROLE). J Am Coll Cardiol. 2007;49:1027–34. doi: 10.1016/j.jacc.2006.10.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.