Abstract
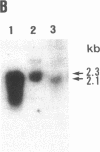
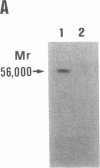
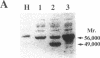
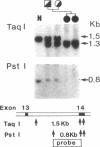
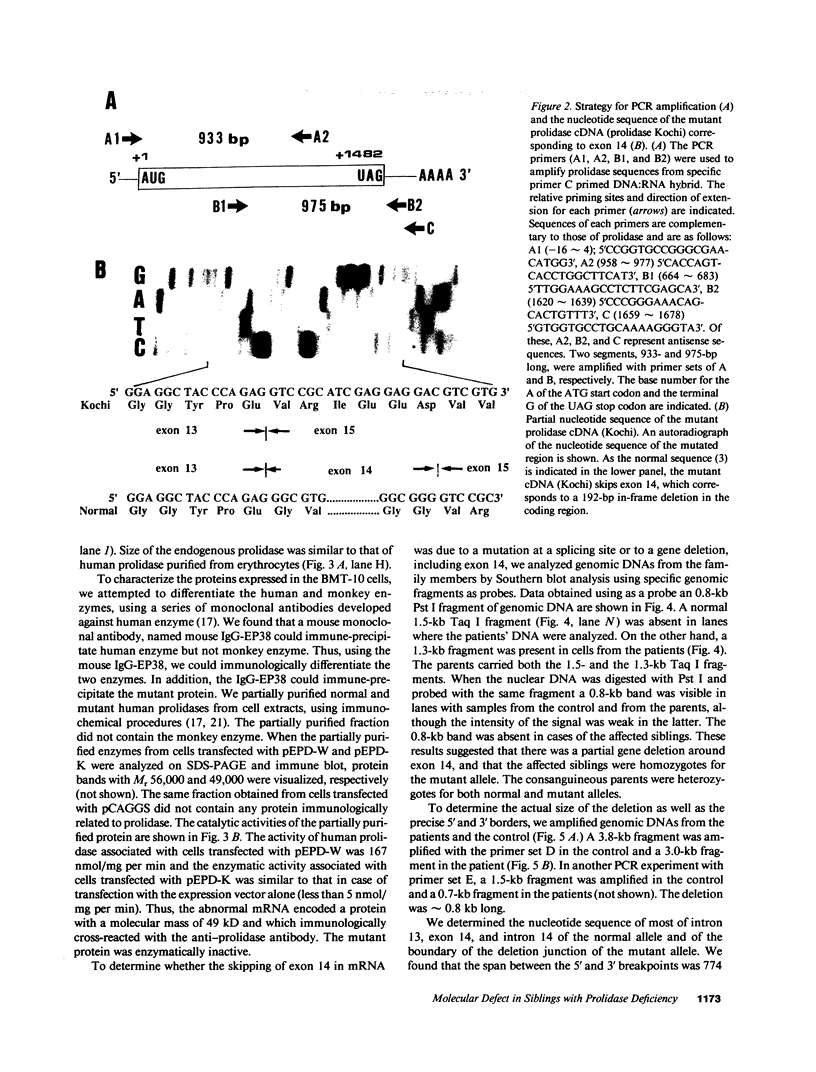
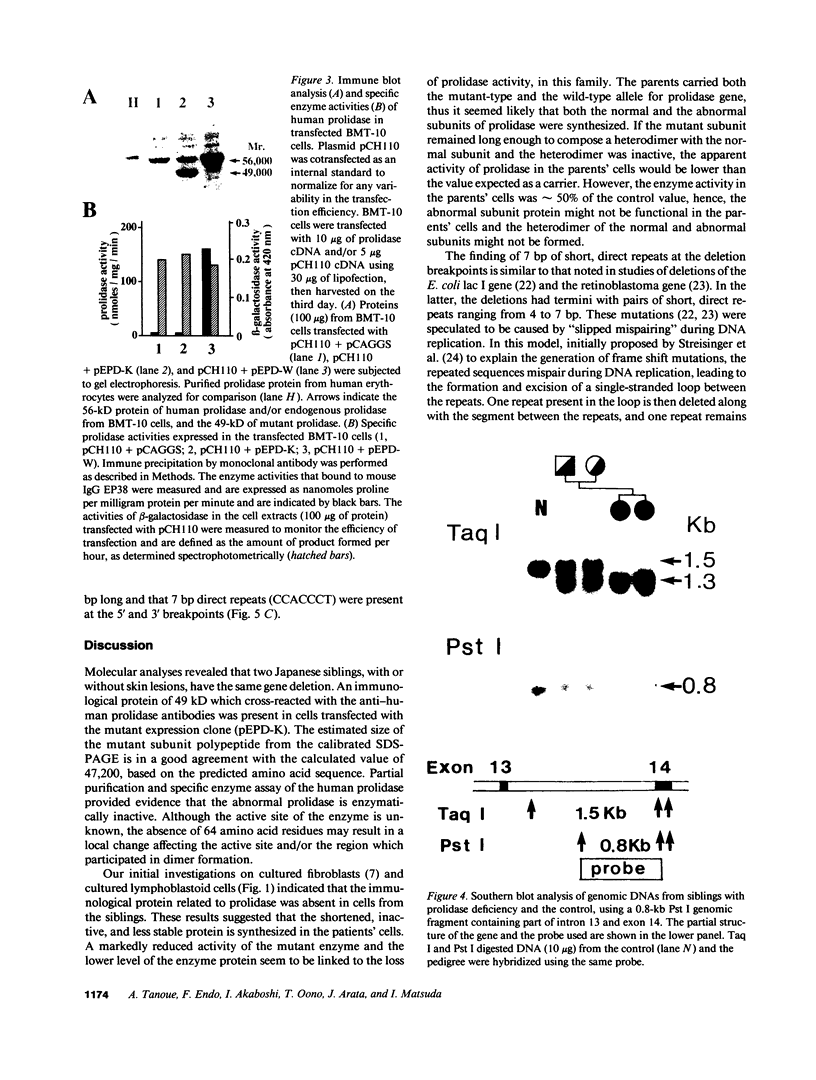
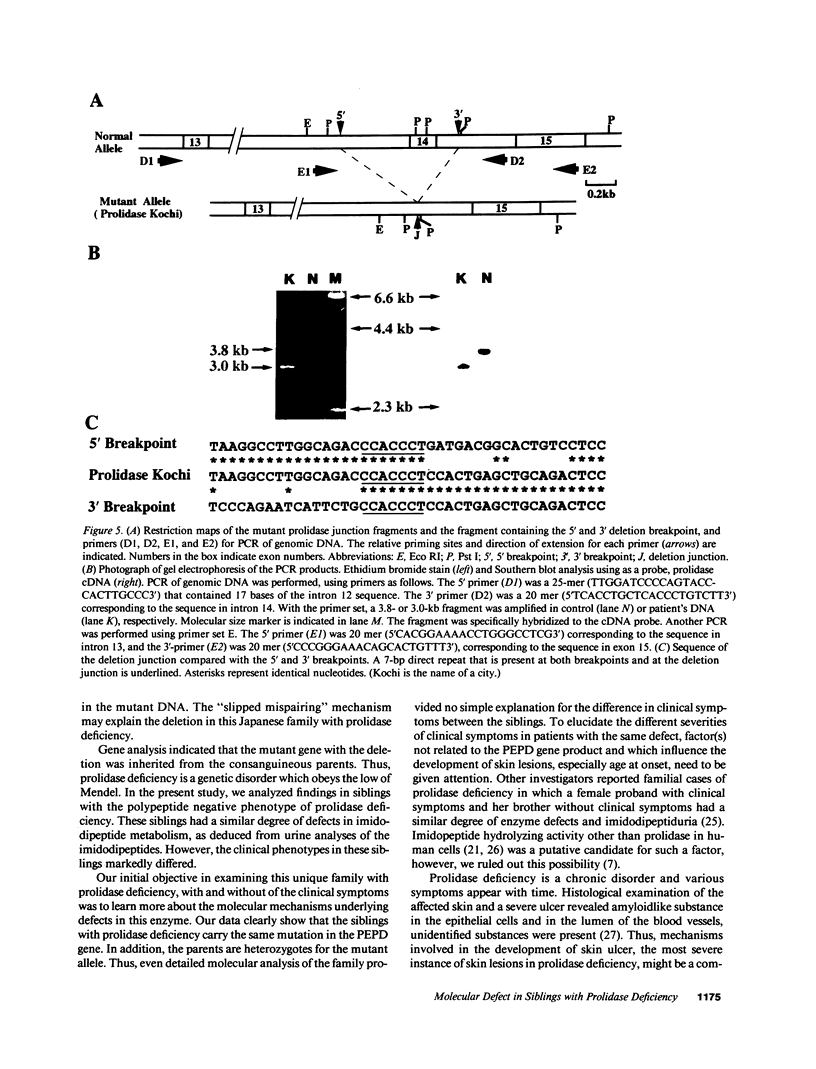
Prolidase deficiency is an autosomal recessive disorder with highly variable symptoms, including mental retardation, skin lesions, and abnormalities of collagenous tissues. In Japanese female siblings with polypeptide negative prolidase deficiency, and with different degrees of severity of skin lesions, we noted an abnormal mRNA with skipping of 192 bp sequence corresponding to exon 14 in lymphoblastoid cells taken from these patients. Transfection and expression analyses using the mutant prolidase cDNA revealed that a mutant protein translated from the abnormal mRNA had an Mr of 49,000 and was enzymatically inactive. A 774-bp deletion, including exon 14 was noted in the prolidase gene. The deletion had termini within short, direct repeats ranging in size of 7 bp (CCACCCT). The "slipped mispairing" mechanism may predominate in the generation of the deletion at this locus. This mutation caused a 192-bp in-frame deletion of prolidase mRNA and was inherited from the consanguineous parents. The same mutation caused a different degree of clinical phenotype of prolidase deficiency in this family, therefore factor(s) not related to the PEPD gene product also contribute to development of the clinical symptoms. Identification of mutations in the PEPD gene from subjects with prolidase deficiency provides further insight into the physiological role and structure-function relationship of this biologically important enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Arata J., Umemura S., Yamamoto Y., Hagiyama M., Nohara N. Prolidase deficiency: its dermatological manifestations and some additional biochemical studies. Arch Dermatol. 1979 Jan;115(1):62–67. doi: 10.1001/archderm.115.1.62. [DOI] [PubMed] [Google Scholar]
- Boright A. P., Scriver C. R., Lancaster G. A., Choy F. Prolidase deficiency: biochemical classification of alleles. Am J Hum Genet. 1989 May;44(5):731–740. [PMC free article] [PubMed] [Google Scholar]
- Butterworth J., Priestman D. A. Presence in human cells and tissues of two prolidases and their alteration in prolidase deficiency. J Inherit Metab Dis. 1985;8(4):193–197. doi: 10.1007/BF01805434. [DOI] [PubMed] [Google Scholar]
- Canning S., Dryja T. P. Short, direct repeats at the breakpoints of deletions of the retinoblastoma gene. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5044–5048. doi: 10.1073/pnas.86.13.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo F., Matsuda I. Screening method for prolidase deficiency. Hum Genet. 1981;56(3):349–351. doi: 10.1007/BF00274691. [DOI] [PubMed] [Google Scholar]
- Endo F., Motohara K., Indo Y., Matsuda I. Immunochemical studies of human prolidase with monoclonal and polyclonal antibodies: absence of the subunit of prolidase in erythrocytes from a patient with prolidase deficiency. Pediatr Res. 1987 Dec;22(6):627–633. doi: 10.1203/00006450-198712000-00002. [DOI] [PubMed] [Google Scholar]
- Endo F., Tanoue A., Kitano A., Arata J., Danks D. M., Lapière C. M., Sei Y., Wadman S. K., Matsuda I. Biochemical basis of prolidase deficiency. Polypeptide and RNA phenotypes and the relation to clinical phenotypes. J Clin Invest. 1990 Jan;85(1):162–169. doi: 10.1172/JCI114407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo F., Tanoue A., Nakai H., Hata A., Indo Y., Titani K., Matsuda I. Primary structure and gene localization of human prolidase. J Biol Chem. 1989 Mar 15;264(8):4476–4481. [PubMed] [Google Scholar]
- Endo F., Tanoue A., Ogata T., Motohara K., Matsuda I. Immunoaffinity purification of human erythrocyte prolidase. Clin Chim Acta. 1988 Aug 31;176(2):143–149. doi: 10.1016/0009-8981(88)90201-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard R. D., Gluzman Y. New host cell system for regulated simian virus 40 DNA replication. Mol Cell Biol. 1985 Nov;5(11):3231–3240. doi: 10.1128/mcb.5.11.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Isemura M., Hanyu T., Gejyo F., Nakazawa R., Igarashi R., Matsuo S., Ikeda K., Sato Y. Prolidase deficiency with imidodipeptiduria. A familial case with and without clinical symptoms. Clin Chim Acta. 1979 May 2;93(3):401–407. doi: 10.1016/0009-8981(79)90291-2. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Harris H. Peptidase D (prolidase) variants in man. Ann Hum Genet. 1969 May;32(4):317–322. doi: 10.1111/j.1469-1809.1969.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Takaki S., Araki K., Tashiro F., Tominaga A., Takatsu K., Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989 Jul 15;79(2):269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- O'Brien T., Ball S., Sarfarazi M., Harper P. S., Robson E. B. Genetic linkage between the loci for myotonic dystrophy and peptidase D. Ann Hum Genet. 1983 May;47(Pt 2):117–121. doi: 10.1111/j.1469-1809.1983.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Ogata A., Tanaka S., Tomoda T., Murayama E., Endo F., Kikuchi I. Autosomal recessive prolidase deficiency. Three patients with recalcitrant ulcers. Arch Dermatol. 1981 Nov;117(11):689–697. [PubMed] [Google Scholar]
- Ohhashi T., Ohno T., Arata J., Kodama H. Biochemical studies on prolidase in sera from control, patients with prolidase deficiency and their mother. J Inherit Metab Dis. 1988;11(2):166–173. doi: 10.1007/BF01799867. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Tanoue A., Endo F., Kitano A., Matsuda I. A single nucleotide change in the prolidase gene in fibroblasts from two patients with polypeptide positive prolidase deficiency. Expression of the mutant enzyme in NIH 3T3 cells. J Clin Invest. 1990 Jul;86(1):351–355. doi: 10.1172/JCI114708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue A., Endo F., Matsuda I. Structural organization of the gene for human prolidase (peptidase D) and demonstration of a partial gene deletion in a patient with prolidase deficiency. J Biol Chem. 1990 Jul 5;265(19):11306–11311. [PubMed] [Google Scholar]