Abstract
Objective
To provide a comprehensive analysis of the temporal structure of sucking in full term neonates.
Design
Descriptive study.
Setting
Newborn nursery in a city teaching institution.
Patients/Participants
Fifty-six full term infants with a mean birth weight of 3128 ± 370 grams completed sucking assessments on the first and second day of life.
Methods
A five-minute sucking assessment was completed on the first and second day of life. Instruments included an Infant Nutritive Sucking Apparatus and the Anderson Behavioral Assessment Scale.
Results
The number of sucks (p<.001), intersuck width (p=.008) and interburst width (p<0.05) were significantly different between the first and second day of life. On the second day of life the infants generated significantly more sucks, a decrease in interburst width and a decrease in intersuck width. There was a significant increase in the presence of an alert behavioral state from the first to second sucking assessment (p<.01). In addition, with a more alert infant state there was an increased time spent bursting (p<.001).
Conclusion
Our results show that sucking analysis is sensitive to infant status and suggest that the development of sucking methodology can be considered as an useful clinical tool to assess the normal developmental course of sucking patterns.
Keywords: Full term infants, Sucking patterns, Behavioral state
Clinical experience has shown that many full term newborn infants are less likely to suckle on the first day than on the second day of life. Issues associated with feeding initiation in the newborn can arouse maternal concerns about adequate care and nourishment and are relevant to the discharge planning process. Most importantly, feeding difficulties during the neonatal period could be the first indication that an infant has neurological problems (da Costa et al., 2010). The factors that underlie the development of feeding skills are not well-understood. Infants are often described as sleepy during the first day of life, which has been associated with decreased feeding activities in full term infants (Crook, 1979). However, this lack of robust alert state does not take into account maturation and/or experience associated with feeding, which may contribute to the more systematic feeding patterns generated by an infant by the second day of life.
Changes in sucking organization microstructure over the first days of life have not been formally documented. In an early study, Dubingnon et al. (1969) examined the effects of perinatal and biographic factors on sucking and feeding during the first four days of life. Feeding, as measured by intake of calories or ounces, was influenced by length of labor, maternal sedation during labor, birth weight, gestational age, and Apgar score. Across the four days of that study, there was a consistent increase in the total intake from the first to the second through fourth day of life. da Costa and colleagues (2010) assessed feeding behaviors from day 2 to 3 of life using the Neonatal Oral-Motor Assessment Scale (NOMAS) (Palmer, 1993) but did not describe sucking pattern transitions from the first day of feeding. Paul and colleagues (1996) reported that the parameters of sucking, breathing and swallowing significantly changed during the first 6 months of age. These findings suggesedt a maturation of sucking patterns over the first months of life (Lau, Alagugurusamy, Schanler, Smith, & Shulman, 2000; Medoff-Cooper, Bilker, & Kaplan, 2001). However, specific sucking parameters such as duration of sucking or time between bursts were not assessed.
One challenge for the interpretation of any changes in sucking pattern over the first two days of life concerns the importance of behavioral state. For the healthy newly born infants, behavioral state appears to follow a well defined sequence. Behavioral state has been reported to be a powerful influencing factor on a variety of infant behaviors (Thoman, 1990). For example, Medoff-Cooper et al. (2002) found that behavioral state during a neurobehavioral assessment was correlated with infant tone and responsiveness to the environment. Arousal or state must be considered as a central factor in sucking performance. For the full term infant, it appears that behavioral state is an indicator of how long an infant will suck at a given feeding. However, this relationship may not be as fixed during the first days of life when transitions between state are not well defined, and a robust alert state may not be as easily achieved as compared to an older infant.
The goals of this study were to: provide a more comprehensive analysis of the temporal structure of sucking in healthy full term infants during the first 48 hours of life, and determine whether changes in sucking patterns from the first to second day of life can be dissociated from behavioral state changes.
Sample and Procedure
This study was approved by the Institutional Review Board of the University of Pennsylvania, Philadelphia. All infants in this study were part of a larger project (N=315) to assess the development of sucking patterns in preterm and full term infants. Informed consent was obtained during the first 12 hours of life. Fifty-six full term infants, greater than 10 hours of age on the first day of the measurement and less than 48 hours on the second day, completed a five-minute sucking test on the first and second day of life. Feeding measurement was not conducted after any invasive procedure. Infants were all appropriate for gestational age at birth with no congenital anomalies. Fifty-eight percent of the women had an epidural or pudendal anesthesia, .08% general anesthesia, .05% local, and 27% had no anesthesia. The mean maternal age was 24 ± 6 years. Postmenstrual age ranged from 37–42 weeks with a mean of 39 ± 1 week. Mean birth weight was 3128 ± 370 grams. There were an equal number of males and females. Ninety-eight percent of the infants were African American. All mothers enrolled in this study had intended to bottle feed their infants. These fifty-six infants represented the entire sample of full term infants with sucking records on the first and second day of life.
Infants were fed either by their mothers or by a nurse in the nursery at 6 am. After the early morning feeding, all infants were brought back to the central nursery for physical examinations as per nursery routine. The sucking measurements were obtained using the Infant Nutritive Sucking Apparatus (Medoff-Cooper, 2000) exactly one half hour before the second morning feeding. The infant was lightly wrapped in one blanket and held by the researcher throughout the protocol. The 5-minute sucking test was preceded and followed by a five-minute period of time during which the first and fifth minutes were used to assess behavioral state.
Measures
Infant Nutritive Sucking Apparatus (INSA)
The hardware of the INSA continuously measures negative pressure generated by the infant during sucking. The sucking apparatus incorporates a calibrated capillary for metered flow of a nutrient into an ordinary nipple by embedding it in silicone rubber. The volume per suck (consumption) is proportional to the pressure-time integral, or area under the pressure-time curve of the suck. Flow is calibrated such that a sustained 100mg/Hg pressure yields a constant flow of 30 ml/min. All materials are non-toxic and easy to sterilize.
The pressure signal was fed on-line to a computer which displayed the pattern of sucks throughout the session and created a sucking record for off-line analysis. Customized software generated a range of session summary parameters including: number of sucks, number of bursts, sucks per burst, interburst interval (time between bursts), suck width (length of time for an individual suck), mean maximum sucking pressure and intersuck interval (Figure 1). In addition, the burst/pause organization and changes in sucking patterns over time within the session were characterized. For this purpose, the session was divided into five parts (epochs) with a mean duration equal to 58 seconds. An array of sucking parameters was generated for each quintile that include number of sucks per epoch, number of bursts, mean duration of sucking within a burst, percentage of time bursting, and suck frequency.
Figure 1.
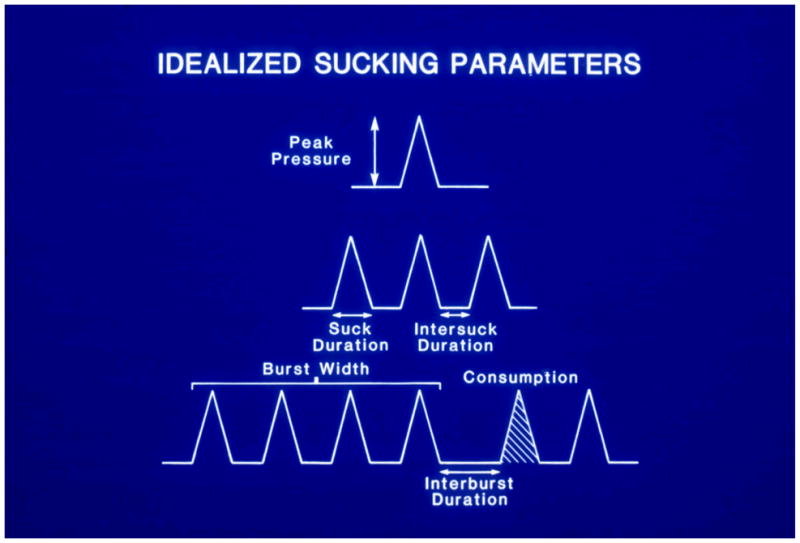
Sucking Parameters
Anderson Behavioral State Scale
The Anderson Behavioral State Scale (ABBS) (Gill, Behnke, Conlon, McNeely, & Anderson, 1988) was devised by Anderson as adapted from Parmelee and Stern (Parmalee & Stern, 1972) and from Burroughs et al., (Burroughs, Asonye, Anderson, & Vidyasagar, 1978). Inter-observer reliability was reported among five observers (r = 0.96) for the instrument standardization.
Current Study
Inter-rater reliability for the two nurse researchers in this study was established (r=0.95). The full 12-item scale was used to assess behavioral state before and after feeding assessment. The behavioral states were assessed as follows: 1= regular quiet sleep, 2= irregular quite sleep, 3= active sleep, 4= very active sleep, 5= drowsy, 6= alert inactivity, 7= quiet awake, 8= active, 9= very active; 10= fussing, 11= crying and 12= hard crying. The twelve behavioral states were collapsed into 3 groups for the regression analysis: sleeping, awake, and crying.
Data Analysis
Summary sucking parameters and behavioral state on the first and second days of life were compared using Wilcoxon signed-rank tests (a nonparametric alternative to the matched pairs t-test). Each time point was considered as independent of the other time points and was useful as a preliminary analysis.
There are numerous approaches for incorporating the longitudinal aspect of the data. A common approach is the repeated measures analysis of variance. However, this model makes specific assumptions about the correlation structure of the repeated measurements. Specifically, it is assumed that the correlation between the outcomes at any two points is constant. This assumption is difficult to evaluate and often violated as in this study. An alternative model, the generalized estimating equations regression (GEE) (Diggle, Liang, & Zeger, 1994), was used. This model permits multiple correlations structures between measurements at each epoch and over days to be considered. The correlation structure between the multiple measurements over time was unspecified and thus estimated within the regression.
Analysis using GEE was performed with the quintile data as the outcome variable and time (epoch number), gestational age, and infant behavioral state as the predictors. Interactions for day of life by epoch number and day of life by state were also considered for each model. The primary analysis used the model based on the categorical versions of these variables since they provided more flexibility and did not assume a linear effect on the outcomes for these variables. Two of the outcome variables required transformation to better approximate normality and to facilitate the use of the normal model. The square root transformation was used for percentage of time bursting (BDUR), and the log transformation was used for the mean burst duration (BDURX). Throughout the GEE analysis, behavioral state was used as a covariate, which allows for the examination of the sucking behaviors adjusted for behavioral state.
Sucking Patterns
In Table 1, the seven summary sucking parameters are compared for day 1 and day 2. Three of the 7 summary parameters changed significantly from the first to second day including: an increase in number of sucks, decrease in interburst width and a decrease in intersuck width.
Table 1.
Comparison of Sucking Parameters, Day 1 and 2 (N=56)
| Sucking Variable | Day 1 | Day 2 | P Value* |
|---|---|---|---|
| #Sucks | 163.41 ± 91.3 | 216.14 ± 87.1 | .001 |
| #Bursts | 19.26 ± 10.26 | 21.69 ± 12.04 | .20 |
| #Sucks/Burst | 10.27 ± 14.56 | 13.04 ± 13.72 | .27 |
| Interburst Width | 13.44 ± 29.7 | 6.19 ± 3.35 | .05 |
| Suck Width | .523 ± .33 | .487 ± .17 | .4 |
| Intersuck Width | .378 ± .12 | .332 ± .09 | .008 |
| Maximum Pressure | 107.81 ± 47.15 | 117.90 ± 41.57 | .1 |
Paired samples t-test
Quintile data for each parameter were analyzed by separate GEE regression models. Each model included day of life, epoch number, and state (three levels). Interactions for day of life by epoch number and day of life by state were also considered for each model, but were not significant in any models.
The GEE regression model parameters and corresponding p-values are presented in Table 2. After adjusting for state, differences between day 1 and 2 of life were seen in mean frequency of sucks within a burst, number of sucks per epoch and sucks per minute. No significant differences were found between days 1 and 2 for the time spent bursting, the mean time in bursting activity or the number of bursts.
Table 2.
Results of GEE analysis of sucking variables between first and second day of life
| Sucking Variable | Significance level |
|---|---|
| Frequency of sucks within a burst | .005 |
| Number of sucks per epoch | .002 |
| Number of sucks per minute | .002 |
For many individual infants there were dramatic differences in patterns of sucking over the course of a session from the 1st to 2nd day of life. However, these individual patterns were not clearly reflected in the aggregate analysis. Indeed, the effect of day on the sucking parameters did not change over epochs for most of the sucking parameters (i.e., no statistical interaction between day and epoch for most of the sucking parameters). However, as described below, the sucking parameters did differ between day 1 and 2 as a function of behavioral state.
The number of sucks per epoch showed a significant decrease at epoch 2, 3, 4, and 5 compared to epoch 1 (Table 3). There was a significant increase in the number of sucks per epoch for state 3 compared to 1 but not for state 2 compared to 1. A trend is present with the sucks per epoch, which increased with awake or crying (increasing state levels). As expected, the results from sucks per minute are quite similar to those for sucks per epoch, in that there is an increase in sucks per minute on the second day of life as compared to the first day of life.
Table 3.
Sucking Variables with significant differences in patterns over the 5 epochs
| Variable - Comparison of epoch 1 to other epochs | Epoch 2 | Epoch 3 | Epoch 4 | Epoch 5 |
|---|---|---|---|---|
| Number of sucks per epoch* | p=0.001 | p<0.002 | P<0.001 | p<0.001 |
| Burst Duration* | p<.031 | p=.001 | p=.008 | p=.026 |
| Number of bursts* | p=0.111 | p=.099 | p=.178 | p<.001 |
Decrease in all sucking parameters in each epoch compared to the first epoch or first minute of sucking except for number of bursts in epoch 5.
The burst duration decreased over epochs 2–4 compared to epoch 1. The mean time in bursting activity did not change significantly over epochs. There were, however, differing effects of state on the mean time in burst duration for state 1 compared to 2 and for state 1 compared to 3. Thus, infants in state 2 (awake) and 3 (crying) demonstrated an increase in burst duration relative to those infants in state 1 (asleep or drowsy).
The number of bursts showed a significant increase at epoch 5 compared to epoch 1 but no differences for epochs 2, 3, or 4 compared to epoch 1. State did not significantly impact the number of bursts. The mean frequency of sucks within a burst showed no significant increase from epoch 1 compared to epoch 2, 3, 4, and 5. There were also no significant differences in mean frequency of sucks within a burst.
State
Based on the original 12-state scale, infants were more awake on the day 2 versus day 1 assessment as seen in differences in the 1 minute state (6.55 ± 3.06 vs. 8.17 ± 3.48, p<.01). As described above, the twelve behavioral states were collapsed into 3 groups for the regression analysis: state 1= sleeping, state 2= awake, and state 3= crying. The day 2 - day 1 difference was also highly significant when based on the 3-state scale used for these regression models.
Discussion
Although adequate feeding skills are necessary for survival, many infants are unable to demonstrate effective feeding before discharge, usually within the first 24 to 48 hours of life. With few objective criteria for assessing their progress in the hospital and little or no organized home follow-up care, it is possible that poor feeding skills go undetected far too long. Several recent studies have suggested that poor feeding skills also may reflect the integrity of the central nervous system and objectively measured sucking parameters may offer useful clinical indices of maturation in newborn infants (Crook, 1979; S. da Costa, van den Engle-Hock, & Bos, 2008; da Costa et al., 2010; Wolff, 1966).
We found significant differences in sucking patterns from day to day as well as across the feeding session. Parameters that varied systematically from the first to second day of life were the number of sucks generated over the feeding session, interburst width and intersuck width. The changes were independent of the number of hours after birth and the amount of feeding experience. These findings formally confirm the clinical impression that infant feeding does change over the first days of life. Equally interesting, and to date undocumented, were the changes in the sucking patterns over the feeding session. Infants tended to decrease the number of sucks over time, but generated more bursts, albeit shorter ones, towards the end of the feeding session, as compared to the first minutes.
A variety of factors are thought to influence sucking rate other than hours of life or maturation, such as satiety and state at the time of feeding (Watt & Strongman, 1985). As satiety concerns were controlled for in this study (with the infants tested one half hour prior to a routine feeding), it was possible to examine the interrelationship between infant behavioral state and sucking patterns. There were indeed significant differences in sucking patterns related to the various behavioral states, as demonstrated by the increase in sucks generated by infants who began the feeding session crying versus those infants who were in a sleep or quiet awake state. In addition, the amount of time spent bursting was also affected by behavioral state. In this case, there were significant differences across the feeding session for infants that began the session in a sleep state as compared to those infants who were either awake or crying. Clearly, infant state is a determinant of the sucking pattern and should be taken into account when teaching parents about feeding skills in the newly born infant.
There were, however, changes in the sucking pattern that were independent of behavioral state. With the use of an appropriate longitudinal analysis method, GEE regression analysis, it was possible to begin to dissociate state and maturational influences on sucking. With state held constant, significant changes from the first to the second day were found for number of sucks, pauses between sucking bursts, and average suck pressure. Thus, sucking differences between the first and second day of life were confirmed in this population of healthy infants.
Study Limitations
Given that only bottle-fed infants were included in this study, we are not able to generalize findings to breast-fed infant feeding transitions from the first to second day of life. In addition, a five minute feeding once a day may not be sufficient to capture the influence of experience from the first morning feeding throughout the day. Although all of the infants on day one were less than 24 hours old, hours of life varied depending on the time of birth.
Clinical Implications/Conclusions
These findings suggest that feeding or sucking patterns may serve as an index of maturation during the first days of life and should be taken into account during hospitalization.
With few objective measures to evaluate the well-being of an infant after only 24 hours of life, feeding patterns may help fill the gap. Although most full term infants develop sucking patterns that allow for adequate nutritional intake by the second or third day of life, infants who persist in exhibiting disorganized sucking patterns should be carefully evaluated prior to discharge. Ongoing development of the sucking instrumentation for clinical and research use are in progress. In addition, further testing is necessary to establish normative data on a cross section of full term infants.
Call Outs.
Clinical experience has shown that many full term newborn infants are less likely to suckle on the first day than on the second day of life.
Changes in sucking organization microstructure over the first days of life have not been formally documented.
Feeding or sucking patterns may serve as an index of maturation during the first days of life and should be taken into account during hospitalization.
Acknowledgments
Funded by National Institute of Health, NCNR-R01NR02093.
Contributor Information
Barbara Medoff Cooper, Pediatric Nursing, The Children’s Hospital of Philadelphia, professor, and director of Biobehavioral Research Center, University of Pennsylvania, School of Nursing, Philadelphia, PA.
Warren Bilker, Department of Biostatistics and Epidemiology and in the Department of Psychiatry, University of Pennsylvania, School of Medicine, Philadelphia, PA.
Joel M. Kaplan, Division of Psychology, Department of Clinical Neuroscience Karolinska Institute, Stockholm, Sweden.
References
- Burroughs A, Asonye U, Anderson G, Vidyasagar D. The effects of nutritive sucking on transcutaneous oxygen tension in noncrying, preterm neonates. Research in Nursing and Health. 1978;1:69–75. doi: 10.1002/nur.4770010204. [DOI] [PubMed] [Google Scholar]
- Crook C. The Organization and Control of Infant Sucking. Advances in Child Development and Behavior. 1979;14:209–253. doi: 10.1016/s0065-2407(08)60115-9. [DOI] [PubMed] [Google Scholar]
- da Costa S, van den Engle-Hock L, Bos A. Sucking and swallowing in infants and diagnostic tools. Journal of Perinatology. 2008;28:247–257. doi: 10.1038/sj.jp.7211924. [DOI] [PubMed] [Google Scholar]
- da Costa SP, van der Schans CP, Boelema SR, van der Meij E, Boerman MA, Bos AF. Sucking patterns in fullterm infants between birth and 10 weeks of age. Infant Behavior and Development. 2010;33(1):61–67. doi: 10.1016/j.infbeh.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Diggle P, Liang K, Zeger Sd. Anaylsis of Longitudinal Data. London: Oxford University Press; 1994. [Google Scholar]
- Dubignon JM, Campbell D, Partington MW. The development of non-nutritive sucking in premature infants. Biologia Neonatorum. 1969;14(5):270–278. doi: 10.1159/000240194. [DOI] [PubMed] [Google Scholar]
- Gill NE, Behnke M, Conlon M, McNeely JB, Anderson GC. Effect of nonnutritive sucking on behavioral state in preterm infants before feeding. Nursing Research. 1988;37(6):347–350. [PubMed] [Google Scholar]
- Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr. 2000;89(7):846–852. [PubMed] [Google Scholar]
- Medoff-Cooper B. Multi-system approach to the assessment of successful feeding. Acta Paediatr. 2000 April/May; doi: 10.1080/080352500750028050. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Bilker W, Kaplan J. Suckling behavior as a function of gestational age: A cross-sectional study. Infant Behavior and Development. 2001;24:83–94. [Google Scholar]
- Medoff-Cooper B, McGrath J, Shults J. Feeding patterns of full term and preterm infants at forty weeks post-conceptional age. Developmental and Behavioral Pediatrics. 2002;23(1):231–236. doi: 10.1097/00004703-200208000-00007. [DOI] [PubMed] [Google Scholar]
- Palmer MM. Identification and management of the transitional suck pattern in premature infants. Journal of Perinatal & Neonatal Nursing. 1993;7(1):66–75. doi: 10.1097/00005237-199306000-00009. [DOI] [PubMed] [Google Scholar]
- Parmalee AH, Stern E. Development of states in infants. In: Clemente CD, Purpura DP, Mayer FE, editors. Sleep and the Maturing Nervous System. New York: Academic Press; 1972. pp. 199–228. [Google Scholar]
- Paul K, Dittrichová J, Papouscaronek H. Infant feeding behavior: Development in patterns and motivation. Developmental Psychobiology. 1996;29(7):563–576. doi: 10.1002/(SICI)1098-2302(199611)29:7<563::AID-DEV2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Thoman EB. Sleeping and waking states in infants: a functional perspective. Neuroscience & Biobehavioral Reviews. 1990;14(1):93–107. doi: 10.1016/s0149-7634(05)80165-4. [DOI] [PubMed] [Google Scholar]
- Watt J, Strongman K. The organization and stability of sleep states in fullterm, preterm, and small-for-gestational-age infants: A comparative study. Developmental Psychobiology. 1985;18(2):151–162. doi: 10.1002/dev.420180207. [DOI] [PubMed] [Google Scholar]
- Wolff P. Causes, controls, and organization of behaviors in the neonate. Psychological Issues. 1966;5(mograph 17):1–105. [PubMed] [Google Scholar]


