Abstract
Background
Sleep loss can modify energy intake and expenditure.
Objective
To determine whether sleep restriction attenuates the effect of reduced-calorie diet on excess adiposity.
Design
Randomized two-period two-condition crossover study.
Setting
University clinical research center and sleep laboratory.
Patients
10 overweight nonsmoking adults (3F/7M); mean (SD) age 41 (5) y; body mass index 27.4 (2.0) kg/m2.
Intervention
14 days of moderate caloric restriction with 8.5 or 5.5-hour nighttime sleep opportunity.
Measurements
Primary: loss of fat and fat-free body mass. Secondary: changes in substrate utilization, energy expenditure, hunger, and 24-h metabolic hormone concentrations.
Results
Sleep curtailment decreased the fraction of weight lost as fat by 55% (1.4 vs. 0.6 kg with 8.5 vs. 5.5-h sleep opportunity, P=0.043) and increased the loss of fat-free body mass by 60% (1.5 vs. 2.4 kg, P=0.002). This was accompanied by markers of enhanced neuroendocrine adaptation to caloric restriction, increased hunger, and a shift in relative substrate utilization towards oxidation of less fat.
Limitations
The nature of the study limited its duration and sample size.
Conclusions
The amount of human sleep contributes to the maintenance of fat-free body mass at times of decreased energy intake. Lack of sufficient sleep may compromise the efficacy of typical dietary interventions for weight loss and related metabolic risk reduction.
Introduction
Mammalian sleep is closely integrated with the regulation of energy balance and metabolic survival of the organism (1). Compared to the robust catabolic effects of sleep deprivation in rodents (2, 3), the increase in energy expenditure in sleep-deprived humans is much smaller (4, 5). Nevertheless, emerging data suggest that lack of sufficient sleep may modify the human neuroendocrine response to reduced food intake and have an adverse impact on the metabolic effects of caloric restriction. Studies in volunteers who slept short vs. long hours show that sleep curtailment was accompanied by increased hunger, higher circulating concentrations of the orexigenic hormone, ghrelin, and reduced concentrations of the anorexigenic hormone, leptin, when their caloric intake during the testing period was restricted to ~20 kcal/kg/day (5g/kg/24h i.v. glucose infusion) (6), but not when they were in positive energy balance (5, 7).
Many people today are overweight or obese and diet-induced weight loss is a widely used strategy to reduce the health risks associated with excess adiposity. The neuroendocrine changes associated with sleep curtailment in the presence of caloric restriction (6), however, raise the possibility that lack of sufficient sleep may compromise the efficacy of commonly used dietary interventions in such individuals. For instance, higher ghrelin concentrations may facilitate the retention of fat (8–10) and increased hunger could compromise adherence to caloric restriction. This study tested the hypothesis that recurrent bedtime restriction can attenuate the effect of reduced-calorie diet on excess adiposity, enhance subjective hunger, and modify 24-h serum leptin and acylated ghrelin concentrations in overweight individuals. Since sleep loss may affect multiple neuroendocrine signals involved in the control of substrate utilization, we also examined the changes in circulating cortisol, epinephrine, norepinephrine, thyroid, and growth hormone concentrations.
Methods
Study participants
Sedentary non-smokers ages 35–49 with body mass index between 25–32 kg/m2 and self-reported sleep between 6.5–8.5 h/day were recruited through local newspaper advertisements. The study was conducted between July 2003 and July 2008 in parallel with other experiments in our laboratory (5) aimed at exploring the effects of sleep loss on human energy metabolism. Individuals were excluded from participation if they had: self-reported sleep problems (Pittsburgh Sleep Quality Index, PSQI, score >10), night work, variable sleep habits, or habitual daytime naps; physically demanding occupations or regular exercise; depressed mood (Center for Epidemiologic Studies of Depression, CES-D, score >15); excessive intake of alcohol (>14 drinks/week for men; >7 for women) or caffeine (>300 mg/day); smoking; use of prescription medications or over-the-counter drugs affecting sleep or metabolism; and abnormal findings on medical history, physical exam, and laboratory screening tests (including a 75g oral glucose challenge and one night of full polysomnography). Only non-pregnant women were studied and data collection was scheduled during the first half of their menstrual cycle. Twelve subjects (5 women and 7 men) were enrolled and 10 of them (3 women and 7 men; 3 Caucasians, 4 African Americans, and 3 Hispanics) completed the study. One female dropped out after completing the first half of the study to start a new job. The participation of a second female subject was stopped by the research team after she complained of palpitations during the period of combined caloric and sleep restriction and her electrocardiogram showed episodic premature atrial contractions in the absence of any serum electrolyte abnormalities. All volunteers gave written informed consent and were paid for their participation. The University of Chicago Institutional Review Board approved the study protocol.
Experimental protocol
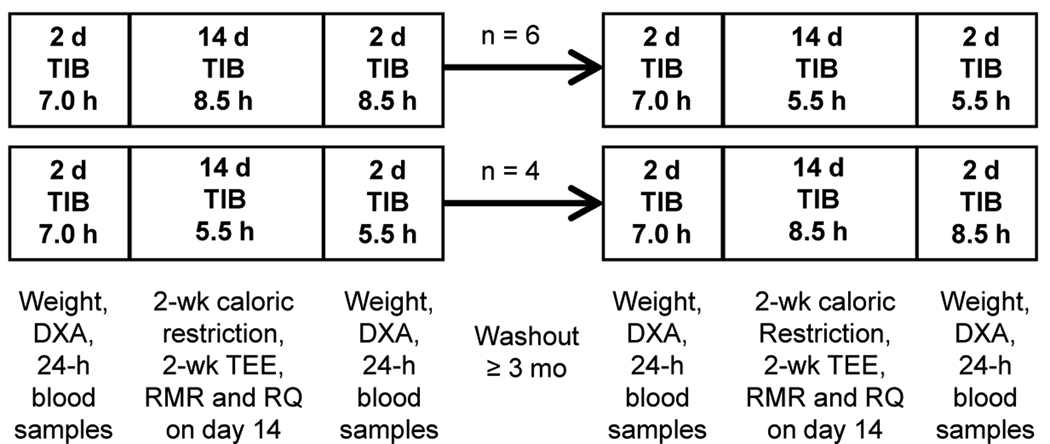
Figure 1 shows a schematic of the study protocol. Participants spent two 14-day periods in the laboratory with scheduled time-in-bed of 8.5 or 5.5 h/night in random order at least 3 months apart (mean ± SD time between treatments: 7 ± 3 months). To modify overnight sleep duration without changes in circadian phase, the usual going-to-bed and getting-out-of-bed times of the subjects were moved proportionally closer together or further apart. Order of treatment was determined using random number tables. Six subjects were studied in the 8.5-h time-in-bed condition first and 4 subjects started with the 5.5-h time-in-bed condition first (see APPENDIX Table I). Sleep was recorded every night (Neurofax-1100, Nihon-Kohden, Foothill Ranch, CA) and no daytime naps were allowed.
Figure 1. Experimental protocol.
Schematic diagram of the study protocol. TIB: assigned time-in-bed (hours per night); DXA: measurement of body composition using dual X-ray absorptiometry; TEE: measurement of total energy expenditure using doubly labeled water; RMR and RQ: measurement of resting metabolic rate and respiratory quotient by indirect calorimetry.
Food intake and energy expenditure
During each 14-day intervention period, participants consumed the same individualized diet with caloric content restricted to 90% of their resting metabolic rate at the time of screening. Daily calories were divided among breakfast (25%, 8:00–9:00), lunch (30%, 12:30–13:30), dinner (35%, 18:30–19:30) and an evening snack (10%, 21:00). This weight-reducing diet was supplemented with a daily multivitamin-plus-minerals (Theragran-M, Walgreen Co., Deerfield, IL) and 325 mg of ferrous sulphate. Food was weighed before and after each meal to determine actual consumption (Nutritionist-IV, Axxya Systems, Stafford, TX).
Participants spent their waking hours indoors engaged in home-office type work or leisure activities (5). Total energy expenditure plays an important role in the control of energy balance and has 3 principal components: 1) resting metabolic rate under basal conditions (RMR): the energy expenditure of an individual resting in bed awake and in the fasting state; 2) thermic effect of food: the energy expenditure associated with the digestion, absorption, metabolism and storage of food equal to approximately 10% of total energy expenditure; and 3) physical activity-related energy expenditure: the energy expended in all volitional and non-volitional daily activities. In parallel studies with ad libitum food intake (5), exposure to this same laboratory environment was accompanied by sedentary levels of physical activity corresponding to total energy expenditure equal to ~1.5 times the resting metabolic rate of the study participants (i.e. 25th centile physical activity level), irrespective of the presence or absence of sleep loss. In the present study, total energy expenditure was measured by doubly labelled water during each 14-day treatment (5) using individual respiratory quotients (RQ) and total body water changes derived from the individual food quotients and changes in body composition during the measurement period (11). On the last day of each dietary intervention, resting metabolic rate and RQ were measured by indirect calorimetry under fasting conditions and for 4h after breakfast, and the thermic effect of food was calculated as previously described (5). Technical problems caused loss of resting metabolic rate data from one participant during the 5.5-h time-in-bed condition. The RQ values of this subject were imputed using the means of the other participants. Hunger was measured daily before each meal and at 22:30 using 10-cm visual analog scales (12). Results were averaged to generate individual 24-h hunger scores. Treatment-related changes were determined by subtracting the 2-day mean hunger score before each intervention from the daily hunger scores during the study.
Body weight and composition and hormone measurements
Before and after each treatment, participants remained at rest for 48h with identical caloric intake including oral and intravenous doses of glucose at 9:00 (the results of these glucose tolerance tests will be reported in a separate report) and the same carbohydrate-rich meals at 14:00 and 19:00 (13). Time-in-bed during this 48-hour period was 7 h/night before, and 8.5 or 5.5 h/night after each 14-day intervention to match the assigned sleep condition (Figure 1). Fasting body weight and adiposity were assessed in the morning of the first day. Fat-free body mass was calculated as the difference between body weight measured by scale (Scale-Tronix Inc., Wheaton, IL) and body fat measured by dual X-ray absorptiometry (Lunar Prodigy, Madison, WI). During the last 24 hours of this 2-day period, blood was sampled every 30 min starting at 20:00. Serum leptin and acylated ghrelin concentrations were measured by radioimmunoassay (Linco Research, St. Charles, MO), and cortisol and growth hormone by chemiluminescent enzyme immunoassay (Immulite, Diagnostic Products, Los Angeles, CA). Plasma epinephrine and norepinephrine were measured by high-pressure liquid chromatography (13). Twenty-four-hour serum thyroid-stimulating hormone (TSH) and free thyroxine (T4) concentrations were analyzed only at the end of each intervention (Immulite, Diagnostic Products, Los Angeles, CA). Serum triiodothyronine (T3) and reverse T3 were measured in 7 of 10 participants who had sufficient residual serum pooled from 3–4 fasting morning samples.
Data analysis
Body weight and composition before and mean sleep parameters during each intervention were compared using paired t-tests. To control for differences in baseline body composition, the effect of 5.5-h vs. 8.5-h time-in-bed (a fixed factor) on the loss of fat and fat-free body mass as main outcome measures of this study was evaluated using mixed linear models with treatment period as repeated measure, initial fat and fat-free body mass as time-varying covariates, and participants as a random factor. Similar mixed model analyses controlling for order-of-treatment and differences in fat and fat-free body mass were used to explore the effects of sleep restriction on ancillary measures, such as resting metabolic rate, RQ, hunger, leptin, ghrelin, and other metabolic hormones, that can be influenced by changes in body composition. Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL). Results in the text are reported as mean (SD).
The funding sources played no role in the design of the study, analysis and interpretation of the data, writing of the manuscript, or the decision to submit it for publication.
Results
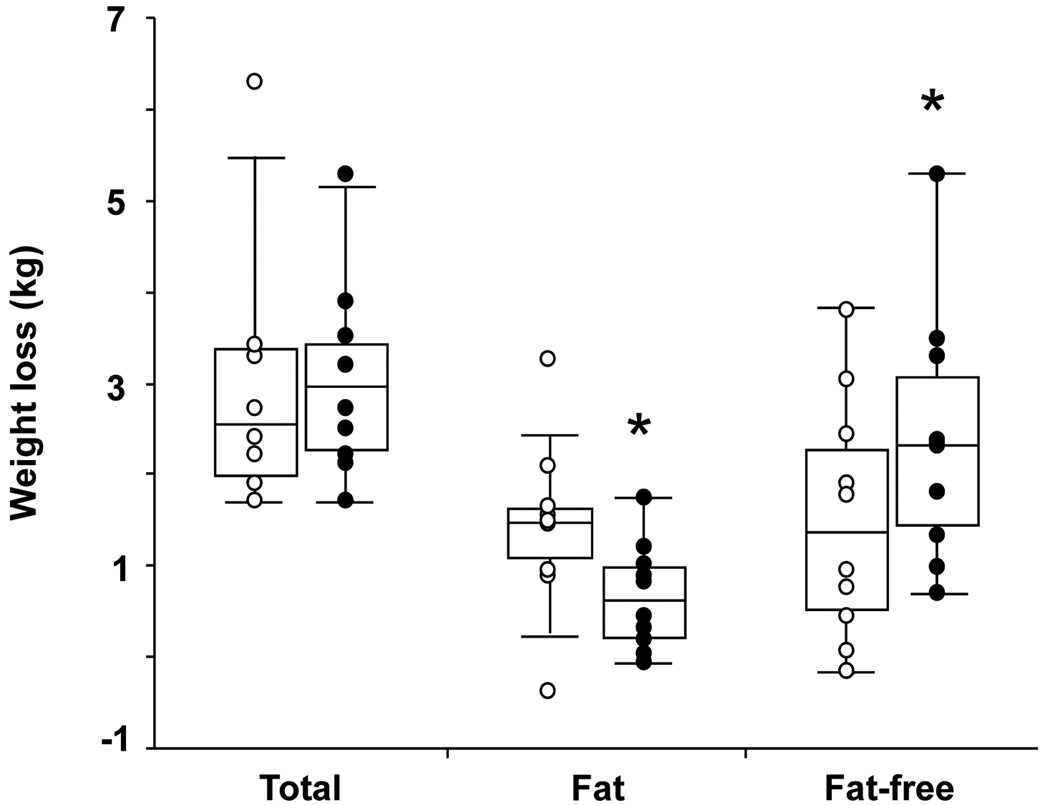
Study participants had mean age 41 (5) y, self-reported habitual sleep 7.7 (0.7) h/day, CES-D score 4 (5), PSQI score 3 (2), sleep respiratory disturbance index 3 (3) events/hour, and resting metabolic rate 1624 (210) kcal/day. Mean sleep duration was reduced by 131 (30) min/day from 7h 25min (32 min) during the 8.5-h time-in-bed condition to 5h 14min (6 min) during the 5.5-h time-in-bed condition (P<0.001, Table 1). When time-in-bed was restricted to 5.5 hours, subjects went to bed later, 0:43 (37 min) vs. 23:23 (43 min), and got out of bed earlier, 6:14 (36 min) vs. 7:52 (45 min). The body weight and composition of the participants before each treatment are summarized in Table 1. Participants consumed similar energy levels (1447 (227) vs. 1450 (236) kcal/day) during the 8.5-h and 5.5-h time-in-bed condition, which as intended were considerably lower than their corresponding doubly labeled water-based measures of total energy expenditure (2136 (342) vs. 2139 (393) kcal/day). Carbohydrate, fat, and protein contributed 48 (1), 34 (1), and 18 (1) % of energy during each study period. Both treatments were accompanied by comparable weight loss (approximately 3 kg, Table 1), however, more than half of the weight loss during the 8.5-h time-in-bed condition and only a quarter of the weight loss during the 5.5-h time-in-bed condition was fat (55% reduction in fat loss, 1.4 (0.9) vs. 0.6 (0.6) kg, 8.5 vs. 5.5-h sleep opportunity, P=0.043, Table 1, Figure 2). Instead, sleep restriction resulted in considerably increased loss of fat-free body mass when compared to the 8.5-h time-in-bed condition (60% increase in fat-free weight loss, 1.5 (1.3) vs. 2.4 (1.4) kg, 8.5 vs. 5.5-h sleep opportunity, P=0.002, Table 1, Figure 2).
Table 1.
Sleep and reduction in body weight and adiposity
| Baseline characteristics | TIB-8.5h | TIB-5.5h | Differencea | P |
| Body weight (kg) | 82.0 (11.2) | 80.5 (10.3) | −1.5 (2.1) | 0.057 |
| Body mass index (BMI, kg/m2) | 27.5 (2.2) | 27.1 (2.0) | −0.5 (0.7) | 0.069 |
| Body fat (kg) | 26.4 (6.4) | 25.0 (6.3) | −1.4 (1.6) | 0.022 |
| Fat-free body mass (kg) | 55.6 (11.5) | 55.5 (11.2) | −0.1 (0.7) | 0.84 |
| 14-day mean sleep parameters | TIB-8.5h | TIB-5.5h | Differencea | P |
| Total sleep time (h:min) | 7:25 (0:32) | 5:14 (0:06) | −2:11 (0:30) | <.001 |
| Sleep efficiency (%) | 87 (6) | 95 (2) | 8 (5) | 0.001 |
| Sleep onset latency (min) | 21 (9) | 7 (4) | −14 (6) | <.001 |
| Wake (min) | 66 (31) | 16 (5) | −50 (29) | <.001 |
| Stage 1 sleep (min) | 29 (10) | 12 (3) | −17 (9) | <.001 |
| Stage 2 sleep (min) | 264 (37) | 180 (42) | −84 (36) | <.001 |
| Slow wave sleep (stages 3+4; min) | 43 (28) | 46 (34) | 3 (24) | 0.66 |
| REM sleep (min) | 108 (22) | 76 (13) | −32 (16) | <.001 |
| End of treatment | TIB-8.5h | TIB-5.5h | B (95% CI) | P |
| Weight loss (kg) | 2.9 (1.4) | 3.0 (1.0) | 0.2 (−0.2, 0.7)b | 0.24 |
| Loss of fat-free mass (kg) | 1.5 (1.3) | 2.4 (1.4) | 1.0 (0.4, 1.5)b | 0.002 |
| Loss of fat (kg) | 1.4 (0.9) | 0.6 (0.6) | −0.7 (−1.4, −0.03)b | 0.043 |
| Fraction of weight loss as fat (%) | 56 (35) | 25 (24) | −31 (−49, −12)b | 0.004 |
| Fasting RQ | 0.80 (0.04) | 0.83 (0.04) | 0.03 (0.01, 0.06)c | 0.042 |
| Postprandial RQ (4-h mean) | 0.80 (0.04) | 0.83 (0.05) | 0.03 (.002, 0.06)c | 0.038 |
| RMR (kcal/day, n=9) | 1505 (262) | 1391 (180) | −147 (−253, −41)c | 0.010 |
Data are mean (SD) values (n=10 except for RMR where n=9). TIB-5.5h and TIB-8.5h: 5.5-h and 8.5-h time-in-bed conditions;
paired t-test comparisons;
beta coefficient (B) for the effect of sleep restriction (TIB-5.5h vs. TIB-8.5h) and its 95% confidence interval (CI) based on a mixed linear model controlling for crossover study design (treatment period as repeated measure) and baseline fat and fat-free body mass as time-varying covariates;
effect of sleep restriction in a mixed linear model controlling for treatment period and body composition at the end of each intervention.
REM: rapid eye movement; RQ: respiratory quotient; RMR: resting metabolic rate
Figure 2. Changes in body weight and composition.
Box plots of the weight loss and its composition during the 8.5-h (open circles) and 5.5-h (solid circles) time-in-bed condition (n=10). Asterisk: significant difference in loss of fat (P=0.043) and fat-free body mass (P=0.002) between the two sleep conditions controlling for study period (initial vs. crossover) and pre-treatment body composition.
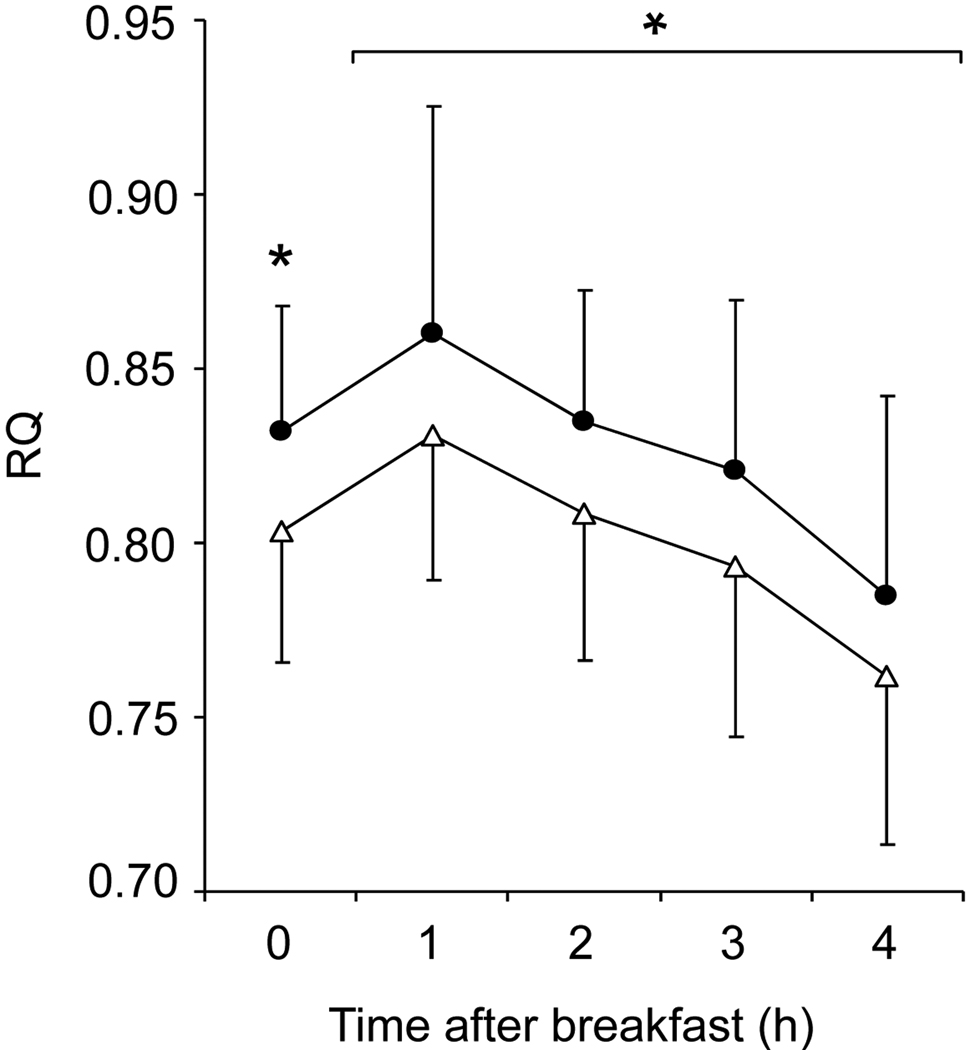
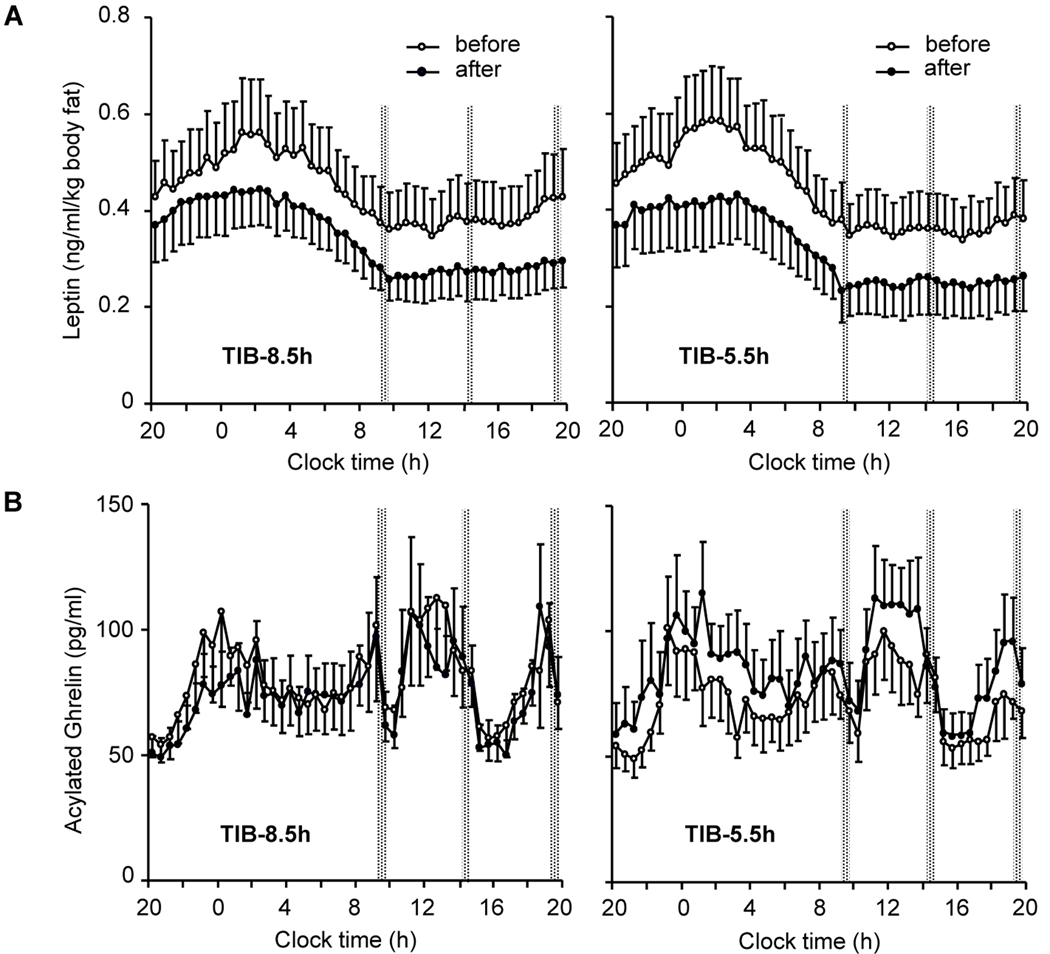
The differential loss of fat and fat-free mass between the two sleep conditions was accompanied by changes in several secondary endpoints, including increased hunger during the period of sleep restriction (−0.1 (1.2) vs. 0.7 (1.2) cm, mixed linear model P=0.043, 8.5 vs. 5.5-h sleep opportunity) and higher fasting and postprandial RQ (Table 1, Figure 3) and 24-h acylated ghrelin concentrations (APPENDIX Figure I and Table II) at the end of the 5.5-h time-in-bed condition (P=0.04 for each measure). In contrast, resting metabolic rate (P=0.01; Table 1) and 24-h plasma epinephrine concentrations (P=0.005; APPENDIX Table II) were lower at the end of the 5.5-h compared to the 8.5-h time-in-bed condition. Leptin concentrations declined in parallel with the loss of weight and adiposity (P=0.001; APPENDIX Figure I) without a significant independent effect of sleep loss (APPENDIX Table II). There were no differences in the fractional thermic effect of food (10.2 (7.5) vs. 12.8 (6.7) %), 24-h norepinephrine, cortisol, growth hormone, and thyroid hormone concentrations at the end of the 8.5-h vs. 5.5-h time-in-bed condition (APPENDIX Table II).
Figure 3. Substrate utilization.
Mean (±SD) respiratory quotient (RQ) under fasting conditions (time 0) and during four consecutive 1-h intervals after breakfast at the end of the 8.5-h (open triangles) and 5.5-h (solid circles) time-in-bed condition (n=10). Asterisk: significant difference in fasting (P=0.042) and 4-h postprandial RQ (P=0.038) controlling for study period and body composition at the end of each intervention.
APPENDIX Figure I. Selected metabolic hormone profiles.
A: Mean ± SE (n=10) 24-hour profiles of serum leptin at the beginning (open circles) and the end (solid circles) of the 8.8-h time-in-bed condition (TIB-8.5h, left panels) and 5.5-h time-in-bed condition (TIB-5.5h, right panels) condition; B: Mean ± SE (n=10) 24-hour profiles of serum acylated ghrelin at the beginning (open circles) and the end (solid circles) of the TIB-8.5h (left panels) and TIB-5.5h (right panels) condition. Times of controlled identical calorie intake are marked with vertical dotted bars.
Discussion
This study examined whether experimental sleep restriction, designed to approximate the short sleep times of a growing number of people in modern society, may compromise the effect of reduced-calorie diets on excess adiposity. The fraction of body weight lost as fat during the 8.5-h time-in-bed condition (56%) is consistent with other published short-term observations (14); however, the combination of energy and sleep restriction in overweight adults resulted in a modified state of negative energy balance characterized by decreased loss of fat and considerably increased loss of fat-free body mass (Table 1, Figure 2). Although rodent studies have established that sleep deprivation can have considerable catabolic effects that resemble protein malnutrition (2), so far, the possibility that sleep loss may have a similar negative impact in humans has not received much attention: a single report found that 1–2 nights of total sleep deprivation was accompanied by elevated 24-h urinary nitrogen excretion (15), whereas the effects of reduced sleep duration have not been tested. Our experimental data now indicate that sleep plays an important role in the preservation of human fat-free body mass during periods of reduced caloric intake.
In addition to its clinically relevant primary outcome measures, this study also examined an ancillary set of mostly research oriented metabolic endpoints that have been hypothesized to reflect important mechanistic relationships between sleep and human energy metabolism (6). The difference in RQ (Figure 3) between the two treatments suggests that sleep loss was accompanied by changes in substrate utilization, which is in good agreement with the observed sparing of body fat. Serum concentrations of acylated ghrelin also increased during the short-sleep condition (APPENDIX Table II), which resembled the changes in total ghrelin associated with caloric restriction and acute sleep deprivation (6). Acylated ghrelin has been shown to reduce energy expenditure, stimulate hunger and food intake, promote retention of fat, and increase hepatic glucose production to support the availability of fuel to glucose-dependent tissues (8–10, 16). In our experiment, sleep restriction was accompanied by a similar pattern of increased hunger and elevated fasting and postprandial RQ values consistent with relatively reduced oxidation of fat (Figure 3). Importantly, sleep restriction was not accompanied by higher 24-h concentrations of catabolic hormones such as serum cortisol, T3 and T4, and plasma catecholamines (APPENDIX Table II). Together, these results suggest that the loss of sleep at times of limited food intake amplifies the pattern of ghrelin-associated changes in human hunger, glucose and fat utilization, and energy metabolism. Thus, the increased loss of fat-free body mass during the short-sleep condition of our study may be due to increased conversion of body protein into glucose to support the more prolonged metabolic needs of the waking brain and other glucose-dependent tissues (17). Although this hypothesis is compatible with the energy-saving and restorative functions of mammalian sleep (1), it remains untested and requires more detailed human studies.
The reduced loss of fat during the short-sleep condition implies a difference in the balance of energy intake and expenditure between the two treatments. Based on energy conversion factors of 9.46 and 4.32 kcal/gram of lost fat and protein (11) and 21% protein content of fat-free body mass, the estimated energy deficit during the 5.5-h time-in-bed condition was ~520 kcal/day compared to ~920 kcal/day during the 8.5-h time-in-bed condition. Since caloric intake was nearly identical, these calculations suggest that the energy expenditure of the participants during the 5.5-h vs. 8.5-h time-in-bed condition was reduced by ~400 kcal/day. Using doubly labeled water, we were not able to detect a change in total energy expenditure as suggested by the differential loss of body fat between the two sleep conditions. Unfortunately, the larger than anticipated variance of the total energy expenditure data makes the interpretation of these results rather difficult. Our initial power calculations were based on previous work showing that the standard deviation for within-subject change in total energy expenditure by doubly labeled water was 140 kcal/day. This would have given us 80% power to detect a change in total energy expenditure between the two sleep conditions of 392 kcal/day. However, the observed standard deviation in our study was 340 kcal/day. As a result, the confidence intervals for total energy expenditure measured by doubly labeled water include the change that would be required to explain the differences in the composition of weight loss between the two treatments (~400 kcal/day). The high standard deviation may have resulted from the lower total energy expenditure during this inpatient weight loss study protocol and variation in physical activity between visits. It should also be noted that the strongest difference between treatments was the increased loss of fat-free mass during the 5.5-h time-in-bed condition (Table 1, Figure 2), which has the weakest relationship to energy balance because lean tissue is mostly water and has low energy density. Since there is also variation in the measurement of change in body composition, the actual difference in total energy expenditure between the two sleep conditions may lie somewhere between 400 kcal/day suggested by the difference in fat loss and the absence of change in total energy expenditure as measured with doubly labeled water.
Ongoing depletion of energy stores in humans is accompanied by metabolic, neuroendocrine, and behavioral compensations to produce opposing declines in RMR and non-resting energy expenditure (18–22). Notably, the resting metabolic rate was significantly lower at the end of the 5.5-h vs. 8.5-h time-in-bed condition. This decline in resting metabolic rate was greater than expected based on the observed loss of fat and fat-free body mass alone (Table 1), and could contribute to the decrease in estimated energy expenditure during the period of combined caloric and sleep restriction (18–20). A greater decline in adrenomedullary activity (APPENDIX Table II) (21) and energy expenditure in activities of daily living (7, 19, 22) could also enhance the development of a more thrifty phenotype in the presence of sleep loss. The fat-derived hormone, leptin, plays a key role in the metabolic and neuroendocrine adaptations to weight loss (20) and, in previous experiments, short-term sleep restriction was accompanied by lower 24-h concentrations of plasma leptin (6, 23). However, this and other controlled studies (5, 7, 24, 25) did not find a significant independent effect of sleep loss on 24-h leptin levels, suggesting that acuity of exposure to sleep and caloric restriction may be important determinants of any such change (3).
In a broader context, the results of this study shed new light on the paradoxical association of human obesity with the loss of the most energy-efficient and sedentary human behavior, which is sleep (26). Our data suggest that insufficient sleep may compromise the maintenance of fat-free body mass and promote the retention of fat when people aim to re-establish their usual weight following life events associated with excessive food intake and increased adiposity. The enhanced metabolic, neuroendocrine, and behavioral compensation in the form of increased hunger and reduced energy expenditure that develop in response to combined caloric and sleep restriction can disrupt their adherence to a lower-energy diet and promote efficient weight regain once it is discontinued. However, it should be noted that due to the high cost and technical difficulty of such experiments, our discussion is based on the detailed laboratory evaluation of a small number of subjects over a limited period of time. Additional studies will be needed to examine the longer-term effects of sleep loss on body composition, energy metabolism, and substrate utilization in weight-reduced individuals.
In summary, exposure of overweight middle-aged adults to 2 weeks of combined energy and sleep restriction produced a catabolic state characterized by reduced loss of body fat and increased loss of fat-free body mass, accompanied by increased hunger and changes in energy expenditure and the neuroendocrine control of substrate utilization. These results highlight the importance of human sleep for the maintenance of fat-free body mass during periods of reduced energy intake and raise the possibility that insufficient sleep may compromise multiple factors that contribute to the efficacy of and adherence to dietary energy-restriction strategies for metabolic risk reduction.
Acknowledgments
This study involved over 250 inpatient days in the University of Chicago Sleep Research Laboratory, which is directed by Eve Van Cauter. We thank Theodore Karrison for advice with the selection of an appropriate approach for statistical analysis, Eve Van Cauter for advice during the planning of this study, and the staff of the University of Chicago Clinical Resource Center, Sleep Research Laboratory, Endocrinology Clinic, and Diabetes Research and Training Center for their skilled technical assistance.
Primary Funding Source: This work was supported by NIH grants P01-AG11412, R01-HL089637, CTSA-RR 024999 and P60-DK020595.
Appendix
APPENDIX Table I.
Individual changes in body weight and composition
| Body weight (kg) | Body fat (kg) | Fat-free mass (kg) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | T | TIB-8.5h | TIB-5.5h | TIB-8.5h | TIB-5.5h | TIB-8.5h | TIB-5.5h | ||||||||||||
| Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | ||
| 1, M | 8.5 | 83.0 | 81.1 | −1.9 | 81.4 | 78.7 | −2.7 | 21.7 | 20.3 | −1.5 | 20.7 | 19.0 | −1.7 | 61.3 | 60.8 | −0.4 | 60.7 | 59.7 | −1.0 |
| 2, M | 5.5 | 87.2 | 85.0 | −2.2 | 87.4 | 84.2 | −3.2 | 20.8 | 19.3 | −1.5 | 20.8 | 20.0 | −0.8 | 66.4 | 65.7 | −0.7 | 66.6 | 64.2 | −2.4 |
| 3, M | 8.5 | 76.9 | 73.5 | −3.4 | 74.4 | 71.9 | −2.5 | 17.0 | 15.5 | −1.5 | 14.9 | 14.7 | −0.2 | 59.9 | 58.0 | −1.9 | 59.5 | 57.2 | −2.3 |
| 4, F | 5.5 | 73.8 | 72.1 | −1.7 | 74.0 | 72.3 | −1.7 | 34.3 | 32.7 | −1.6 | 34.5 | 33.5 | −1.0 | 39.5 | 39.4 | −0.1 | 39.5 | 38.8 | −0.7 |
| 5, M | 8.5 | 75.5 | 73.1 | −2.4 | 72.6 | 70.4 | −2.2 | 25.9 | 24.4 | −1.5 | 23.9 | 23.0 | −0.9 | 49.6 | 48.7 | −0.9 | 48.7 | 47.4 | −1.3 |
| 6, M | 8.5 | 102.1 | 95.8 | −6.3 | 97.5 | 92.2 | −5.3 | 31.9 | 28.7 | −3.3 | 27.5 | 27.5 | 0.0 | 70.2 | 67.1 | −3.0 | 70.0 | 64.7 | −5.3 |
| 7, M | 5.5 | 84.7 | 81.4 | −3.3 | 85.2 | 81.3 | −3.9 | 22.1 | 21.2 | −0.9 | 22.2 | 21.7 | −0.4 | 62.7 | 60.2 | −2.4 | 63.0 | 59.6 | −3.5 |
| 8, M | 5.5 | 97.4 | 94.0 | −3.4 | 94.4 | 91.2 | −3.2 | 35.2 | 35.6 | 0.4 | 32.9 | 32.9 | 0.1 | 62.2 | 58.4 | −3.8 | 61.5 | 58.3 | −3.3 |
| 9, F | 8.5 | 67.2 | 65.3 | −1.9 | 69.4 | 67.3 | −2.1 | 30.6 | 28.5 | −2.1 | 31.0 | 30.7 | −0.3 | 36.6 | 36.8 | 0.2 | 38.4 | 36.6 | −1.8 |
| 10, F | 8.5 | 72.4 | 69.7 | −2.7 | 69.1 | 65.6 | −3.5 | 24.9 | 23.9 | −0.9 | 21.8 | 20.6 | −1.2 | 47.5 | 45.7 | −1.8 | 47.3 | 45.0 | −2.3 |
ID – subject number and gender (F: female; M: male); TIB-8.5h – 8.5-h time-in-bed condition; TIB-5.5h – 5.5-h time-in-bed condition; T – treatment order (8.5: subject completed TIB-8.5h condition first; 5.5: subject completed TIB-5.5h condition first); Pre – measurement taken before treatment; Post – measurement taken at the end of the treatment period; Δ – change during corresponding treatment calculated as the difference of the Post and Pre measurement.
APPENDIX Table II.
Metabolic hormone measurements
| Pre-treatment | End of treatment | |||
|---|---|---|---|---|
| TIB-8.5h | TIB-5.5h | TIB-8.5 | TIB-5.5h | |
| 24-h serum leptin (µg/L) | 13.1 (10.2) | 12.4 (9.5) | 9.7 (7.2) | 9.1 (9.2) |
| 24-h acylated ghrelin (ng/L) | 81 (50) | 73 (38) | 75 (40) | 84 (47)* |
| 24-h serum growth hormone (µg/L) | 0.88 (0.49) | 0.79 (0.34) | 0.81 (0.38) | 0.95 (0.47) |
| 24-h plasma epinephrine (pmol/L) | 129 (38) | 136 (58) | 140 (45) | 114 (30)** |
| 24-h plasma norepinephrine (pmol/L) | 1171 (589) | 1291 (829) | 1161 (512) | 1104 (481) |
| 24-h serum cortisol (nmol/L) | 196 (21) | 198 (25) | 190 (22) | 193 (23) |
| 24-h serum TSH (mU/L) | 1.2 (0.6) | 1.2 (0.5) | ||
| 24-h serum free T4 (pmol/L) | 16.1 (1.2) | 16.5 (1.2) | ||
| (ng/dL) | 1.25 (0.10) | 1.28 (0.10) | ||
| Serum T3 (nmol/L, n=7) | 1.89 (0.18) | 1.95 (0.24) | ||
Data are mean (SD) values (n=10 except for T3 where n=7).
P = 0.039,
P = 0.005: effect of sleep restriction at the end of the 5.5-h vs. 8.5-h time in bed condition based on a mixed linear model controlling for crossover study design (treatment period) and final body composition.
TSH: thyroid stimulating hormone; T4: thyroxine; T3: triiodothyronin
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
References
- 1.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437(7063):1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everson CA, Wehr TA. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol. 1993;264(2 Pt 2):R376–R387. doi: 10.1152/ajpregu.1993.264.2.R376. [DOI] [PubMed] [Google Scholar]
- 3.Everson CA, Szabo A. Recurrent restriction of sleep and inadequate recuperation induce both adaptive changes and pathological outcomes. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1430–R1440. doi: 10.1152/ajpregu.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 7.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90(6):1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 8.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 9.Nogueiras R, Tschop MH, Zigman JM. Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann N Y Acad Sci. 2008;1126:14–19. doi: 10.1196/annals.1433.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez A, Gomez-Ambrosi J, Catalan V, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009;33(5):541–552. doi: 10.1038/ijo.2009.40. [DOI] [PubMed] [Google Scholar]
- 11.Black AE, Prentice AM, Coward WA. Use of food quotients to predict respiratory quotients for the doubly-labelled water method of measuring energy expenditure. Hum Nutr Clin Nutr. 1986;40(5):381–391. [PubMed] [Google Scholar]
- 12.Hill AJ, Blundell JE. Nutrients and behaviour: research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. J Psychiatr Res. 1982;17(2):203–212. doi: 10.1016/0022-3956(82)90023-1. [DOI] [PubMed] [Google Scholar]
- 13.Penev P, Spiegel K, Marcinkowski T, Van Cauter E. Impact of carbohydrate-rich meals on plasma epinephrine levels: dysregulation with aging. J Clin Endocrinol Metab. 2005;90(11):6198–6206. doi: 10.1210/jc.2005-0415. [DOI] [PubMed] [Google Scholar]
- 14.Krotkiewski M, Landin K, Mellstrom D, Tolli J. Loss of total body potassium during rapid weight loss does not depend on the decrease of potassium concentration in muscles. Different methods to evaluate body composition during a low energy diet. Int J Obes Relat Metab Disord. 2000;24(1):101–107. doi: 10.1038/sj.ijo.0801092. [DOI] [PubMed] [Google Scholar]
- 15.Scrimshaw NS, Habicht JP, Pellet P, Piche ML, Cholakos B. Effects of sleep deprivation and reversal of diurnal activity on protein metabolism of young men. Am J Clin Nutr. 1966;19(5):313–319. doi: 10.1093/ajcn/19.5.313. [DOI] [PubMed] [Google Scholar]
- 16.Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther. 2008;118(2):239–249. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Boyle PJ, Scott JC, Krentz AJ, Nagy RJ, Comstock E, Hoffman C. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. J Clin Invest. 1994;93(2):529–535. doi: 10.1172/JCI117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravussin E, Burnand B, Schutz Y, Jequier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr. 1985;41(4):753–759. doi: 10.1093/ajcn/41.4.753. [DOI] [PubMed] [Google Scholar]
- 19.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115(12):3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landsberg L. Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26(4–6):497–508. doi: 10.1007/s10571-006-9010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redman LM, Heilbronn LK, Martin CK, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4(2):e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 24.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. Journal of Clinical Endocrinology & Metabolism. 2005;90(5):2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Littman AJ, Vitiello MV, Foster-Schubert K, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007;31(3):466–475. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 26.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]