Abstract
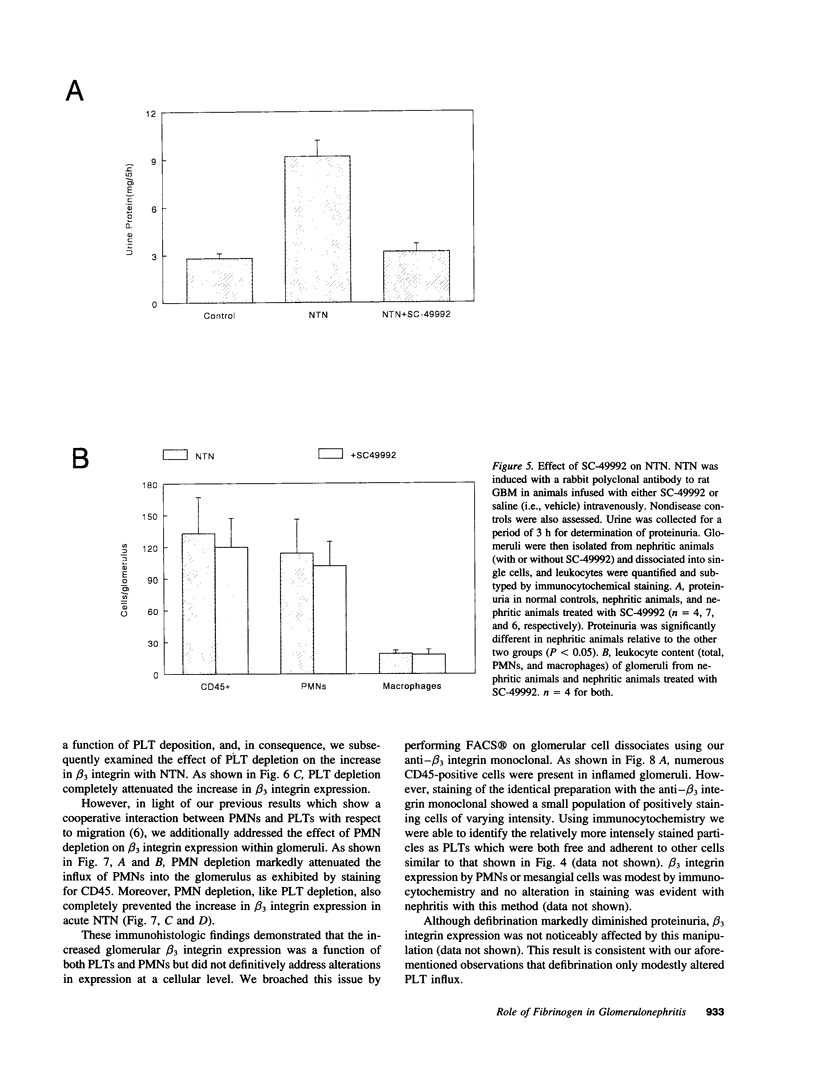
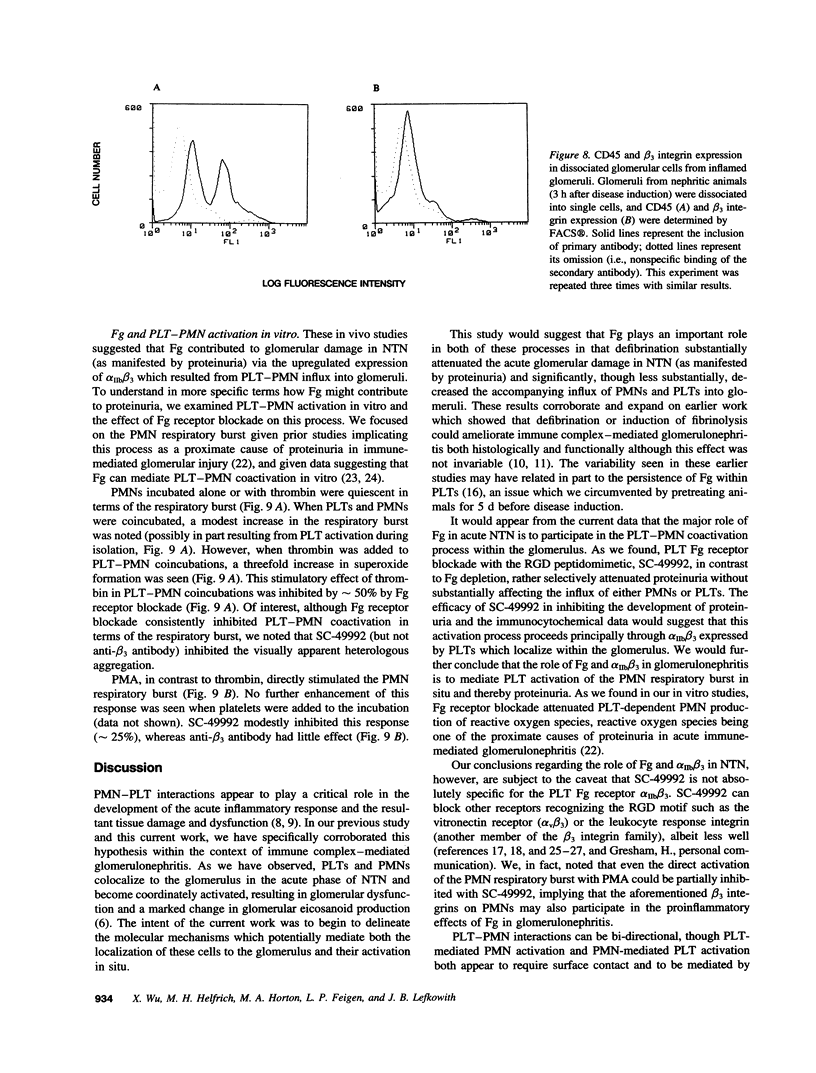
The metabolic and functional alterations which occur during the acute phase of nephrotoxic nephritis (NTN) in rats, a model of immune-mediated glomerulonephritis, result from a cooperative interaction between PMNs and platelets (PLTs). In consequence, we hypothesized that fibrinogen (Fg) might play a critical role in this process and, accordingly, we found that defibrination of animals decreased both the acute phase proteinuria in NTN (approximately 70%) as well as the influx of PLTs and PMNs into the glomerulus (approximately 40-50%). In contrast, blockade of the PLT Fg receptor, alpha IIb beta 3, with the RGD peptidomimetic SC-49992 decreased proteinuria (approximately 90%) without substantially altering the influx of PMNs or PLTs. Immunocytochemistry showed a marked increase in beta 3 integrin expression in inflamed glomeruli which was prevented either by PMN or PLT depletion before disease induction. FACS and immunocytochemical analysis of glomerular cell dissociates demonstrated that beta 3 integrin expression was predominantly on intraglomerular PLTs. In vitro, activated PLTs stimulated the PMN respiratory burst, an interaction which could be inhibited by Fg receptor blockade. In sum, acute NTN is accompanied by a marked increase in glomerular beta 3 integrin expression predominantly due to the influx of PLTs which localize to the glomerulus in a PMN-dependent fashion. Fg appears to serve a major role as a coactivating stimulus for PLT-PMNs in situ via alpha IIb beta 3, potentially mediating the PMN respiratory burst which contributes to proteinuria. Fg may also play a subsidiary role in PMN/PLT comigration.
Full text
PDF






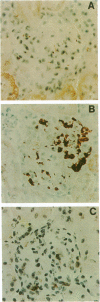
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C. Coagulation assembly on leukocytes in transmembrane signaling and cell adhesion. Blood. 1993 Feb 1;81(3):569–579. [PubMed] [Google Scholar]
- Border W. A., Noble N. A. From serum sickness to cytokines: advances in understanding the molecular pathogenesis of kidney disease. Lab Invest. 1993 Feb;68(2):125–128. [PubMed] [Google Scholar]
- Carreno M. P., Gresham H. D., Brown E. J. Isolation of leukocyte response integrin: a novel RGD-binding protein involved in regulation of phagocytic function. Clin Immunol Immunopathol. 1993 Oct;69(1):43–51. doi: 10.1006/clin.1993.1148. [DOI] [PubMed] [Google Scholar]
- Couser W. G. Mediation of immune glomerular injury. J Am Soc Nephrol. 1990 Jul;1(1):13–29. doi: 10.1681/ASN.V1113. [DOI] [PubMed] [Google Scholar]
- Couser W. G. Pathogenesis of glomerulonephritis. Kidney Int Suppl. 1993 Jul;42:S19–S26. [PubMed] [Google Scholar]
- Faint R. W. Platelet-neutrophil interactions: their significance. Blood Rev. 1992 Jun;6(2):83–91. doi: 10.1016/0268-960x(92)90010-n. [DOI] [PubMed] [Google Scholar]
- Feigen L. P., Nicholson N. S., King L. W., Campion J. G., Tjoeng F. S., Panzer-Knodle S. G. SC-49992, a mimetic of the peptide arginine-glycine-aspartic acid-phenylalanine that blocks platelet aggregation, enhances recombinant tissue plasminogen activator-induced thrombolysis and prevents reocclusion in a canine model of coronary artery thrombosis. J Pharmacol Exp Ther. 1993 Dec;267(3):1191–1197. [PubMed] [Google Scholar]
- Gresham H. D., Goodwin J. L., Allen P. M., Anderson D. C., Brown E. J. A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J Cell Biol. 1989 May;108(5):1935–1943. doi: 10.1083/jcb.108.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger S. A., McEver R. P. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 1990 Feb 1;75(3):550–554. [PubMed] [Google Scholar]
- Handagama P. J., Shuman M. A., Bainton D. F. In vivo defibrination results in markedly decreased amounts of fibrinogen in rat megakaryocytes and platelets. Am J Pathol. 1990 Dec;137(6):1393–1399. [PMC free article] [PubMed] [Google Scholar]
- Helfrich M. H., Nesbitt S. A., Horton M. A. Integrins on rat osteoclasts: characterization of two monoclonal antibodies (F4 and F11) to rat beta 3. J Bone Miner Res. 1992 Mar;7(3):345–351. doi: 10.1002/jbmr.5650070315. [DOI] [PubMed] [Google Scholar]
- Henson P. M. Interactions between neutrophils and platelets. Lab Invest. 1990 Apr;62(4):391–393. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Floege J., Couser W. G., Alpers C. E. Role of platelet-derived growth factor in glomerular disease. J Am Soc Nephrol. 1993 Aug;4(2):119–128. doi: 10.1681/ASN.V42119. [DOI] [PubMed] [Google Scholar]
- Lefkowith J. B., Nagamatsu T., Pippin J., Schreiner G. F. Role of leukocytes in metabolic and functional derangements of experimental glomerulonephritis. Am J Physiol. 1991 Aug;261(2 Pt 2):F213–F220. doi: 10.1152/ajprenal.1991.261.2.F213. [DOI] [PubMed] [Google Scholar]
- Lianos E. A., Andres G. A., Dunn M. J. Glomerular prostaglandin and thromboxane synthesis in rat nephrotoxic serum nephritis. Effects on renal hemodynamics. J Clin Invest. 1983 Oct;72(4):1439–1448. doi: 10.1172/JCI111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianos E. A., Zanglis A. Glomerular platelet-activating factor levels and origin in experimental glomerulonephritis. Kidney Int. 1990 Feb;37(2):736–740. doi: 10.1038/ki.1990.40. [DOI] [PubMed] [Google Scholar]
- Mathieson P. W., Thiru S., Peters D. K., Oliveira D. B. Effects of ancrod and rtPA on fibrin accumulation, glomerular inflammation and renal function in nephrotoxic nephritis. Int J Exp Pathol. 1991 Dec;72(6):679–693. [PMC free article] [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Johnson K. J., Todd R. F., 3rd, Issekutz T. B., Miyasaka M., Tamatani T., Smith C. W., Anderson D. C., Ward P. A. Requirements for leukocyte adhesion molecules in nephrotoxic nephritis. J Clin Invest. 1993 Feb;91(2):577–587. doi: 10.1172/JCI116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Polley M. J., Bayer R. J., Nunn M. F., Paulson J. C., Ward P. A. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest. 1992 Oct;90(4):1600–1607. doi: 10.1172/JCI116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu T., Pippin J., Schreiner G. F., Lefkowith J. B. Paradoxical exacerbation of leukocyte-mediated glomerulonephritis with cyclooxygenase inhibition. Am J Physiol. 1992 Aug;263(2 Pt 2):F228–F236. doi: 10.1152/ajprenal.1992.263.2.F228. [DOI] [PubMed] [Google Scholar]
- Nicholson N. S., Panzer-Knodle S. G., King L. W., Taite B. B., Keller B. T., Tjoeng F. S., Engleman V. W., Giorgio T. D., Feigen L. P. SC-49992--a potent and specific inhibitor of platelet aggregation. Thromb Res. 1994 Jun 1;74(5):523–535. doi: 10.1016/0049-3848(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Nicholson N. S., Panzer-Knodle S. G., Salyers A. K., Taite B. B., King L. W., Miyano M., Gorczynski R. J., Williams M. H., Zupec M. E., Tjoeng F. S. Antiplatelet and antithrombotic effects of platelet glycoprotein IIb/IIIa (GPIIb/IIIa) inhibition by arginine-glycine-aspartic acid-serine (RGDS) and arginine-glycine-aspartic acid (RGD) (O-me)Y (SC-46749). J Pharmacol Exp Ther. 1991 Mar;256(3):876–882. [PubMed] [Google Scholar]
- Nicholson N. S., Panzer-Knodle S. G., Salyers A. K., Taite B. B., King L. W., Miyano M., Gorczynski R. J., Williams M. H., Zupec M. E., Tjoeng F. S. In vitro and in vivo effects of a peptide mimetic (SC-47643) of RGD as an antiplatelet and antithrombotic agent. Thromb Res. 1991 Jun 1;62(5):567–578. doi: 10.1016/0049-3848(91)90029-v. [DOI] [PubMed] [Google Scholar]
- Rovin B. H., Lefkowith J. B., Schreiner G. F. Mechanisms underlying the anti-inflammatory effects of essential fatty acid deficiency in experimental glomerulonephritis. Inhibited release of a monocyte chemoattractant by glomeruli. J Immunol. 1990 Aug 15;145(4):1238–1245. [PubMed] [Google Scholar]
- Ruf A., Schlenk R. F., Maras A., Morgenstern E., Patscheke H. Contact-induced neutrophil activation by platelets in human cell suspensions and whole blood. Blood. 1992 Sep 1;80(5):1238–1246. [PubMed] [Google Scholar]
- Shah S. V. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney Int. 1989 May;35(5):1093–1106. doi: 10.1038/ki.1989.96. [DOI] [PubMed] [Google Scholar]
- Tang L., Eaton J. W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J Exp Med. 1993 Dec 1;178(6):2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping P. G., Thomson N. M., Holdsworth S. R. A comparison of fibrinolytic and defibrinating agents in established experimental glomerulonephritis. Br J Exp Pathol. 1986 Aug;67(4):481–491. [PMC free article] [PubMed] [Google Scholar]
- Winn R. K., Liggitt D., Vedder N. B., Paulson J. C., Harlan J. M. Anti-P-selectin monoclonal antibody attenuates reperfusion injury to the rabbit ear. J Clin Invest. 1993 Oct;92(4):2042–2047. doi: 10.1172/JCI116799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. H., Bresnahan B. A., Lianos E. A. Hemodynamic role of arachidonate 12- and 5-lipoxygenases in nephrotoxic serum nephritis. Kidney Int. 1993 Jun;43(6):1280–1285. doi: 10.1038/ki.1993.180. [DOI] [PubMed] [Google Scholar]
- Wu X., Pippin J., Lefkowith J. B. Attenuation of immune-mediated glomerulonephritis with an anti-CD11b monoclonal antibody. Am J Physiol. 1993 Apr;264(4 Pt 2):F715–F721. doi: 10.1152/ajprenal.1993.264.4.F715. [DOI] [PubMed] [Google Scholar]
- Wu X., Pippin J., Lefkowith J. B. Platelets and neutrophils are critical to the enhanced glomerular arachidonate metabolism in acute nephrotoxic nephritis in rats. J Clin Invest. 1993 Mar;91(3):766–773. doi: 10.1172/JCI116295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Wittwer A. J., Carr L. S., Crippes B. A., DeLarco J. E., Lefkowith J. B. Cytokine-induced neutrophil chemoattractant mediates neutrophil influx in immune complex glomerulonephritis in rat. J Clin Invest. 1994 Jul;94(1):337–344. doi: 10.1172/JCI117326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yared A., Albrightson-Winslow C., Griswold D., Takahashi K., Fogo A., Badr K. F. Functional significance of leukotriene B4 in normal and glomerulonephritic kidneys. J Am Soc Nephrol. 1991 Jul;2(1):45–56. doi: 10.1681/ASN.V2145. [DOI] [PubMed] [Google Scholar]
- Zhou M., Brown E. J. Leukocyte response integrin and integrin-associated protein act as a signal transduction unit in generation of a phagocyte respiratory burst. J Exp Med. 1993 Oct 1;178(4):1165–1174. doi: 10.1084/jem.178.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Javors M. A., Olson M. S. Platelet-activating factor as an intercellular signal in neutrophil-dependent platelet activation. J Immunol. 1992 Sep 1;149(5):1763–1769. [PubMed] [Google Scholar]