Abstract
Patas were not reported to carry a species-specific SIV, but SIVagm cross-species transmission to patas occurred in the wild. We report that patas share immunophenotypic features with natural hosts of SIV, i.e., low levels of CD4+ T cells and low CCR5 expression on CD4+ T cells. In one patas with undetectable levels of CD4+ T cells, experimental exposure to SIVagm did not result in infection. Remaining experimentally-infected patas showed an infection pattern similar to SIV infection in natural hosts. Thus, downregulation of CD4 and of CCR5 expression on CD4+ T cells may effectively control HIV acquisition and result in SIV extinction.
Keywords: Simian Immunodeficiency Virus, African green monkeys, patas monkeys, cross-species transmission, effector memory cells, immune activation, virus extinction
Introduction
Over 40 different simian immunodeficiency viruses (SIVs) naturally infect African nonhuman primates (NHPs) [1]. In natural hosts, SIV infection is persistent nonprogressive, being characterized by (i) high peak and set-point viral replication, similar to, or higher than that reported in pathogenic infections; (ii) acute depletion of peripheral and mucosal CD4+ T cells, with rebound to preinfection levels in periphery and partial restoration at mucosal sites during chronic infection despite sustained viral replication; (iii) low levels of CD4+CCR5+ cells in blood and tissues, (iv) in vivo virus replication in short-lived cells, (v) virus-specific immune responses similar or lower than those observed in pathogenic HIV/SIV infections, only partially controlling virus replication, and (vi) transient and moderate increases in immune activation and T cell proliferation during acute infection, with return to baseline levels during the chronic phase [2]. In the vast majority of cases, this exquisite host adaptation to control the deleterious consequences of SIV infection prevents progression to AIDS in natural hosts [2–4]. This virus-host adaptation occurred through host-dependent evolution, as SIV ancestors were infecting African monkeys at the time of their speciation [1].
Patas monkeys (Erythrocebus patas) are widespread in SubSaharan Africa, their habitat overlapping that of African green monkeys (AGMs). They are not a known natural host of SIV, similar to other African NHP species, such as white crowned mangabeys or baboons [1]. In these species, cross-species transmissions of SIVagm from sympatric AGM species were reported to occur in the wild [1, 5]. The outcome of SIVagm.sab infection in patas is not known, as viral replication and its impact on immune cell populations have never been assessed in this species.
We report that; SIVagm.sab induce persistent nonprogressive infection of patas, with virus replication, CD4+ T cell depletion and immune activation patterns very similar to those of SIV infection in natural hosts; that patas share numerous immunophenotypic features with natural hosts; and that these immunophenotypic SIV imprints may have rendered patas resistant to SIV infection and led to the extinction of a hypothetical patas-specific SIV. This is the first evidence that host adaptation to lentiviral infection may result not only in the control of disease progression, but also in controlling virus acquisition.
Materials and Methods
Animals and infection
Immunophenotyping was performed in 12 SIV uninfected juvenile or adult patas and four baboons (Papio papio) housed at Bioqual and Tulane National Primate Research Center (TNPRC). Three patas and five Carribean AGMs (Chlorocebus sabaeus) were intravenously exposed to 300 TCID50 SIVagm.sab and monitored for 1.5 years. All experimentally-infected animals were housed at the TNPRC, an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International facility, according to regulations set forth by the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. Animal experiments were approved by the Tulane University Institutional Animal Care and Use Committee.
Sampling and sample processing
At least 20 ml of EDTA blood was collected from all the animals for immunophenotyping. From SIV infected animals, blood (4.9 ml EDTA) was collected at 2 time points preinfection, at the time of SIVagm.sab inoculation, twice per week for the first two weeks postinfection (p.i.), weekly for four weeks, every two weeks for two months and every three months, up to day 540 p.i. Intestinal endoscopies (10–15, 1–2 mm2 pieces of proximal jejunum) were obtained on days 0, 28, 42, 60, 90, 180, 240, 360, 540 p.i.
Within one hour of removal, blood was used for flow cytometry. Plasma was separated and stored at −80°C. Peripheral blood mononuclear cells (PBMCs) were extracted from whole blood and frozen at −80°C, as described [6–9]. Lymphocytes were separated from duodenal biopsies as described [6–9]. Within 30 minutes after separation, cells were stained for flow cytometry.
Flow cytometry analysis of lymphocyte populations
Functional characterization of patas and baboon lymphocytes was done as follows: for intracellular cytokine staining, PBMCs were incubated overnight at 37°C with medium alone, 1 μg of SEB (Sigma) in the presence of 0.5 μg each of monoclonal antibodies (mAbs) to CD28 (CD28.2, Beckman Coulter) and CD49d (9F10, BD Bioscience) and 1 μg/ml brefeldin A (Sigma). After stimulation, cells were washed twice and incubated with Live/Dead fixable aqua dead cell stain (Invitrogen). Cells were then stained for surface markers with mAbs to CD3 (SP34-2), CD4 (L200), CD8 (RPA-T8) and CD95 (DX2) (BD Bioscience). Cells were washed and permeabilized with Cytofix/Cytoperm buffer (BD), intracellularly stained with fluorescence-conjugated mAbs to IL-17 (eBio64DEC17, eBioscience), IL-2 (MQ1-17H12, BD), and CD40L (TRAP1, BD), incubated at 4°C for 20 min, washed and fixed with a 1% paraformaldehyde solution (Electron Microscopy Sciences).
Immunophenotyping of lymphocytes isolated from the blood and intestine of experimentally-infected animals was performed by using fluorescently conjugated mAbs in an eight-color staining technique. Samples were run using a LSR-2 flow cytometer (Becton Dickinson); data were analyzed using FlowJo (Tree Star, Inc). The mAbs were: CD3 (SP34), CD20 (L27), CD3 (SP34-2), HLA-DR (L243), CD8α (SK1), CCR5 (3A9), CD4 (L200), Ki-67 (B56) (BD Bioscience) and CD8αβ (2ST8.5H7) (Beckman Coulter). All of these mAbs were shown to be cross-reactive for AGMs and patas. Whole blood was lysed using FACS lysing solution (BD Biosciences) and stained as described [6, 7]. Mononuclear cells from the intestine were stained as described [6, 7]. The absolute number of peripheral lymphocytes was determined by performing cell blood counts.
Viral load quantification
Plasma VLs were quantified by real-time PCR, as described [10, 11]. Assay sensitivity was 100 RNA copies per 1 ml of plasma.
Statistical analysis of data
Statistical analyses were performed using Prism software (GraphPad). Statistical significance was based on P values <0.05.
Results
Patas but not baboons share immunophenotypic features with natural hosts of SIVs
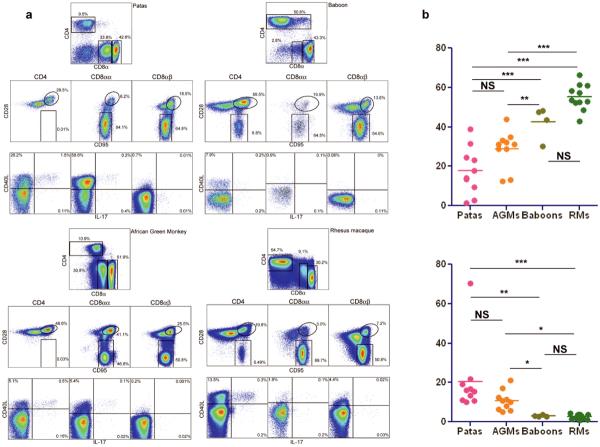
We previously reported that CD4 downregulation by memory CD4+ T cells in vivo renders AGMs resistant to progressive SIVagm infection [12] and that downregulation of CCR5 expression on CD4+ T cells may prevent SIV breastfeeding transmission in natural hosts [13, 14]. We performed a comparative immunophenotypic analysis of SIV uninfected patas and baboons, two African species not previously reported to be natural hosts of SIVs, and we report that, similar to AGMs, many CD4+ T cells from patas downregulate CD4 in vivo as they enter the memory pool (Figure 1a); these CD4neg or CD8αα memory T cells maintain functions normally attributed to CD4+ T cells, including production of interleukin-2 (IL-2), production of IL-17 and expression of CD40 ligand (Figure 1a); finally, these CD4neg T cells can maintain major histocompatibility complex class II restriction (data not shown). Conversely, the immunophenotyping of baboon and rhesus macaque samples did not reveal these adaptations to SIV infection. In fact, the CD8αα T cell population was not observed in baboons and the proportion of double negative CD4negCD8neg T cells was significantly lower in baboons (8.59±1.75%) versus patas (21.69±9.07%) (p=0.014) (Figure 1b). Also, the overall levels of CD4+ T cells were higher in baboons (46.96±7.56%) versus patas (22.95±16.66%, p=0.016), who had CD4+ T cell counts in range with those observed for AGMs (Figure 1b). The levels of CD4+ T cells decreased with age in patas, similar to natural hosts of SIVs [2, 12]. Finally, the CCR5 expression by CD4+ T cells in patas was lower than in baboons or rhesus macaques, being in the range of that observed in AGMs (data not shown). Therefore, patas share immunophenotypic features with natural hosts of SIVs, in spite of the fact that a species-specific SIV has not been described in this species. Conversely, baboons share immunophenotypic features with macaques and humans, which are not natural hosts of SIV/HIV.
Figure 1. Comparison of T cell phenotypes and functionality in patas, AGMs, baboons and rhesus macaques.
a. Flow cytometry staining panels Representative staining patterns of: CD4 and CD8□ by CD3 gated T cells (Top panels); Central memory (CD28+CD95+) and effector memory (CD28negCD95+) T cell subsets among CD4+ T cells, CD8αα+ T cells, and CD8αβ+ T cells (Middle panels); Expression of CD40L and IL-17 after polyclonal stimulation of CD4+ T cells, CD8αα+ T cells, and CD8αβ+ T cells in patas, AGMs, baboons and rhesus macaques (bottom panels). b. Comparison of percentages of CD3+ T cells that express CD4 (up) and of percentages of CD3+ T cells that express CD8aa (bottom) in peripheral blood in SIV uninfected patas monkeys, African green monkeys (AGMs), baboons and rhesus macaques.
Naturally-occurring low levels of CD4+ T cells prevent SIV infection in patas
Three patas were intravenously inoculated with SIVagm. Prior to infection, PBMCs and intestinal mononuclear cells were immunophenotyped. The levels of CD4+ T cells were low in uninfected patas both systemically (Figure 2b) and at mucosal sites (Figure 2c). In one animal, preinfection CD4+ T cell counts were negligible (4 CD4+ T cells/μl). Intravenous exposure to SIV did not result in productive SIV infection in this animal (Figure 2a). In the remaining two patas, SIV induced persistent infection.
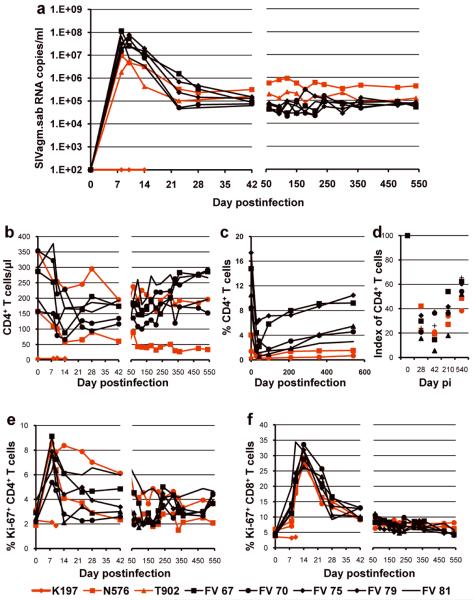
Figure 2. Comparative pathogenesis of SIVagm infection in AGMs and patas.
(a) Dynamics of SIVagm.sab replication in plasma. Peak VL are slightly lower in patas than in AGMs, but chronic viral replication is slightly higher in patas. During chronic infection, viral replication is remarkably stable in both species. (b) Changes in CD4+ T cells in blood: an initial CD4+ T cell depletion of similar magnitude in both species is partially/completely restored in AGMs. The levels of CD4+ T cell restoration are lower in patas. (c) Massive acute mucosal CD4+ T cell depletion occurs in both species, followed by a modest restoration during the chronic infection. When percents of mucosal CD4+ T cells are compared, restoration appears to be more limited in patas. (d) Comparison of indexes of mucosal CD4+ T cells demonstrate that the magnitude of mucosal CD4+ T cell depletion is similar between the two species and that, although the restoration is indeed more limited in patas, the levels of mucosal CD4+ T cells are relatively similar during the chronic infection between the two species. Dynamics of CD4+ (e) and CD8+ T cell (f) immune activation (as defined by changes in the expression of Ki-67) in peripheral blood of SIVagm-infected AGMs and patas. Red symbols and lines denote patas. Black symbols and lines denote the control AGMs.
Patterns of viral replication, CD4+ T cell depletion and immune activation in SIVagm-infected patas are similar to those observed in SIVagm-infected AGMs
In the two productively infected patas, peak VLs of 107 and 5×106 copies/ml occurred between days 8–10 p.i. VLs reached set-point by day 42 p.i. and were maintained between 105 and 106 copies/ml during the follow-up (Figure 2a). This overall pattern of viral replication was similar to that observed in naturally-infected AGMs, however the peak of VL was 1 log lower in patas than in AGMs (Figure 2a), probably due to a lower availability of CD4+ T cells (Figures 2b and 2c). Interestingly, during the chronic infection, SIVagm VLs were slightly higher in patas than in AGMs.
Similar to SIVagm-infected AGMs, viral replication resulted in CD4+ T cells depletion at both systemic and mucosal sites (Figures 2b and 2c). During chronic infection, CD4+ T cells were partially restored at both sites in AGMs. In one patas, there was no restoration of systemic CD4+ T cells, in keeping with the high VLs (Figure 2b). The restoration of mucosal CD4+ T cells was limited in patas (Figure 2c). One should however note that the very low levels of mucosal CD4+ T cells observed in patas masked the restoration observed during the chronic infection, when CD4+ T cell baseline indexes were considered (Figure 2d).
The dynamics of immune activation during SIVagm infection of patas, as assessed by Ki-67 expression on CD4+ and CD8+ T cell (Figures 2e and 2f), was characterized by transient increases during acute infection and return to near baseline levels during chronic infection. This pattern is very similar to those observed in natural infections, where immune activation is controlled through various mechanisms [2,3].
Altogether, these pathogenic features demonstrate that the pattern of SIV infection in patas monkeys is very similar to those observed in natural hosts.
Discussion
We report here that, in spite of not being a known natural host of SIVs, patas have immunological imprints of SIV infection that they share with natural hosts, most notably low CCR5 expression on CD4+ T cells and an in vivo downregulation of CD4 expression by CD4+ T cells. Both these features result in a low availability of SIV target cells at mucosal sites, a common feature among SIV natural hosts that may impact the efficacy of SIV mucosal transmission in the wild [12–14].
We also report experimental infection of patas with SIVagm.sab, a virus that naturally infects AGMs and reportedly transmitted to patas in the wild [5]. Surprisingly, intravenous exposure to SIVagm.sab doses that always resulted in infection of AGMs and macaques, did not result in productive SIV infection in a patas with no detectable levels of CD4+ T cells at both systemic and mucosal sites prior to SIV exposure.
Finally, we report that the pathogenic pattern of SIVagm.sab infection in patas is similar to those observed in natural hosts of SIVs [2], being characterized by lack of disease progression in the context of persistent, robust viral replication, partial recovery of CD4+ T cells and control of chronic immune activation.
What is the significance of these results? Previous studies reported that SIV cross-species transmission to nonnatural hosts, such as macaques or humans lead to disease progression if chronic viral replication is not controlled [1]. In our study, patas did not control SIVagm replication, nevertheless they did not show clinical or biological signs of disease progression. Moreover, in spite of robust viral replication during chronic infection, SIV-infected patas showed a partial CD4+ T cell restoration at mucosal sites in the context of controlling chronic immune activation, which is one of the most important mechanisms of nonpathogenicity in natural infections [2, 6]. Patas ability of controlling SIV disease progression, similar to natural hosts, may appear paradoxical, as patas are not a known natural host of SIV [1]. This paradox can be explained if considering the case of the patas that was exposed intravenously to large amounts of SIVagm and did not get infected, which demonstrates that downregulation of the CD4 molecule and of CCR5 expression from the surface of CD4+ T lymphocytes may prevent SIV acquisition. In this context, it is conceivable that during the evolutionary history, exquisite adaptation of patas monkeys to lentiviral infection might have resulted in the extinction of their hypothetical species-specific SIV.
Understanding the mechanisms of lentiviral extinction in primate hosts would be of major interest in the context of the expanding HIV-1 pandemic. Current views are that blocking transmission through a reduction of chronic HIV replication below the threshold needed for human-to-human transmission can be achieved through an effective vaccine [15]. Our results show for the first time that reducing mucosal target cells [12–14] may be an effective strategy of natural hosts to control HIV acquisition, of which the extreme result may be virus eradication. Such an observation opens large avenues for developing alternative strategies to block the spread of HIV and to control AIDS pandemics.
Acknowledgements
We thank Drs. Marion Ratterree, Rudolf Bohm, Daniel Douek and Preston Marx for helpful discussion, Julie Bruhn and Calvin Lanclos for technical assistance, Division of Veterinary Medicine of Tulane National Primate Research Center for animal care. We would like to dedicate this manuscript to the memory of Dr. Jonathan S. Allan.
Financial support: This work was supported by NIH/NIAID/NCRR grants RO1 AI064066 and R21AI069935 (IP), R01 AI065325 (CA), R01 RR025781 (CA/IP) and RR-00168 (TNPRC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no conflict of interests.
Presented in part: International Symposium on Natural Immunity to HIV, Nov 2009, Winnipeg, Manitoba, Canada.
References
- 1.VandeWoude S, Apetrei C. Going wild: Lessons from T-lymphotropic naturally occurring lentiviruses. Clin Microbiol Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandrea I, Silvestri G, Apetrei C. AIDS in African nonhuman primate hosts of SIVs: A new paradigm of SIV infection. Curr HIV Res. 2009;6:57–72. doi: 10.2174/157016209787048456. [DOI] [PubMed] [Google Scholar]
- 4.Silvestri G. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J Med Primatol. 2005;34:243–52. doi: 10.1111/j.1600-0684.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 5.Bibollet-Ruche F, Galat-Luong A, Cuny G, et al. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopithecus aethiops sabaeus) in the wild. J Gen Virol. 1996;77:773–81. doi: 10.1099/0022-1317-77-4-773. [DOI] [PubMed] [Google Scholar]
- 6.Pandrea I, Gautam R, Ribeiro R, et al. Acute loss of intestinal CD4+ T cells is not predictive of SIV virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaufin T, Gautam R, Kasheta M, et al. Limited ability of humoral immune responses in control of viremia during infection with SIVsmmD215 strain. Blood. 2009;113:4250–4261. doi: 10.1182/blood-2008-09-177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandrea I, Gaufin T, Brenchley JM, et al. Experimentally-induced immune activation in natural hosts of SIV induces significant increases in viral replication and CD4+ T cell depletion. J Immunol. 2008;181:6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandrea I, Ribeiro RM, Gautam R, et al. Simian immunodeficiency virus SIVagm dynamics in African green monkeys. J Virol. 2008;82:3713–3724. doi: 10.1128/JVI.02402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandrea I, Apetrei C, Dufour J, et al. Simian immunodeficiency virus (SIV) SIVagm.sab infection of Caribbean African green monkeys: New model of the study of SIV pathogenesis in natural hosts. J Virol. 2006;80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandrea I, Kornfeld C, Ploquin MJ-I, et al. Impact of viral factors on very early in vivo replication profiles in SIVagm-infected African green monkeys. J Virol. 2005;79:6249–6259. doi: 10.1128/JVI.79.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaumier CM, Harris LD, Goldstein S, et al. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med. 2009;15:879–85. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandrea I, Apetrei C, Gordon S, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandrea I, Onanga R, Souquiere S, et al. Paucity of CD4+CCR5+ T-cells may prevent breastfeeding transmission of SIV in natural non-human primate hosts. J Virol. 2008;82:5501–5509. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–21. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]