Abstract
Most individuals, adults and children alike, exposed to HIV-1 do not become infected. Understanding the basis of this protection depends on systematically and comprehensively defining factors that determine the infectiousness of the host and the susceptibility of the recipient. Successful transmission depends on the relative balance between infectiousness and susceptibility, both of which are influenced by biologic, behavioral and environmental factors. In this review, we discuss the advantages and disadvantages of mother-to-child HIV transmission as a model to in which to elucidate correlates of immune protection.
Keywords: Mother to child transmission of HIV, Breast milk, Innate immunity
Most persons exposed to HIV do not become infected. Despite over 25 years of intensive research, the biologic basis of this “natural protection” remains elusive and controversial. Is it simply luck, or do genetic, immunologic and environmental factors work alone or in concert to determine infection? The challenges to unraveling this mystery are many, but the need for novel approaches to prophylactic vaccine is even greater. Transmission is a complex biologic process reflecting the interplay between both members of the transmitting dyad with the outcome depending on the infectiousness of the transmitter and the susceptibility of the recipient. Host, viral, environmental and behavioral factors determine both infectiousness and susceptibility; protection is likely to be relative and multi-factorial. Therefore, progress in this field depends on accurate and comprehensive assessment of infectiousness and susceptibility. In this paper, we will review the strengths and limitations of mother-to-child HIV transmission (MTCT) as a model to identify biologic factors associated with protection from HIV infection.
In all forms of transmission, the major factor associated with successful transmission is the amount of HIV in the transmitter (viral dose). The most powerful factor mitigating HIV acquisition is a rare polymorphism in CCR5, which is largely irrelevant to African populations[1]. Defining correlates of natural protection, therefore critically depends on accurate quantification of HIV exposure. Entry of HIV into the transmitting fluid requires passage from the circulation across mucosal surfaces; the permeability of this epithelial barrier can vary over time influencing HIV levels and even viral phenotype. Transmission involves crossing the recipient’s mucosal barriers and susceptibility to infection also varies with normal (e.g. pregnancy) or abnormal (e.g. ulcerative sexually transmitted disease) physiological states, as well as behaviors (e.g. rectal vs. genital intercourse). Thus, relevant biologic samples and the collection of accurate behavioral data at the time of exposure must be obtained from both individuals. This can be extremely challenging in studies of humans engaging in intimate and/or taboo behaviors.
MTCT is an attractive model in which to study correlates of natural protection (see Table 1). Both members of the transmitting dyad are easily identified and followed—the degree of exposure and the period of risk are well defined, relatively easily quantified, and in the absence of antiretrovirals transmission rates are much higher than other forms of HIV acquisition. Children born to HIV-infected women can become infected at three discrete time points: in utero, intra-partum (at the time of delivery) and during breastfeeding. In chronically HIV-infected women who do not receive antiretroviral therapy, transmission rates are approximately 5–10% in utero, 20–30% intrapartum and 10–20% via breast milk. Despite many years of investigation, it is still not clear whether most children escape infection because they are not exposed to enough virus, or whether they are protected by intrinsic or acquired immune responses.
Table 1.
Advantages and disadvantages of Mother-to-Child HIV Transmission (MTCT) as a model to study protective immunity to HIV
| Advantages | Disadvantages |
|---|---|
| Transmission dyad easily identified and in a stable relationship | Use of antiretrovirals markedly reduces transmission rates and transmission during prophylaxis may not represent |
| Period of risk exposure relatively easily quantified and susceptibility can be studied before transmission event in the case of breast milk transmission | Factors that protect infants may not be relevant to other modes of transmission particularly since infection usually occurs in presence of maternal antibody |
| Exposure occurs in a consistent and predictable manner | Genetic relatedness between mother and infant may limit the generalizability of certain immune responses |
| Relatively little social stigma associated with risk behaviors and other predisposing factors inflammatory states (e.g. infection, mastitis) |
It is well established that during pregnancy there is bi-directional cellular trafficking of both maternal and fetal cells, resulting in variable but persistent levels of microchimerism in both the mother and child[2]. This would suggest that significant numbers of infants are exposed to HIV in utero and may account for the HIV-infected women’s increased risk of miscarriage[3]. Consistent with this model are numerous reports of cellular HIV-specific responses in exposed uninfected infants, as well as the protective association of certain HLA types (reviewed in [4, 5]). Neonatal protection from early HIV infection (in utero and intrapartum) has also been associated with apparently HIV-specific natural killer (NK) cells[6]. Interestingly, unlike NK responses detected in individuals exposed to HIV via sex or intravenous drug use, detection of these responses is dependent on the presence of non-IgG mediated plasma factor and targeted variable HIV proteins[6].
So if children are exposed to HIV, why is it that so few infants become infected? Most have attributed this to low dose exposures, defective virus and/or intrinsic factors that render HIV replication incompetent. Recent studies suggest an intriguing, albeit counterintuitive hypothesis. Mold et al reported that maternal alloantigens promote the development of tolerogenic fetal regulatory cells[7]. Since productive HIV infection requires immune activation, it is possible that HIV-specific T-regulatory cell abort productive infection. In other words, because babies don’t respond to HIV-infected maternal cells, immune activation necessary for viral replication is absent and infection is thereby aborted. Consistent with this hypothesis is the observation that HIV-exposed, uninfected infants have robust HIV-specific responses that are detected after removal of regulatory T cells[8]. Of note, high levels of regulatory T cells have been associated with resistance to HIV infection among commercial sex workers[9]. These data raise the provocative question of whether HIV vaccine approaches should promote HIV tolerance as a means of avoiding infection.
Breast milk transmission remains a major issue in resource-poor countries, particularly in Africa where most HIV-infected women reside. In many of these settings, the reduction in HIV transmission by the avoidance of breastfeeding is counter-balanced by increases in morbidity and mortality from other non-HIV related causes[10]. The inefficacy of breast milk transmission is remarkable. Despite immunologic immaturity, prolonged exposure (months to years) and multiple daily exposures (as often as every 2 hours), fewer than one in five infants become infected. The ability of breast milk to protect infants against a wide variety of infectious agents has recently been “rediscovered” by investigators in HIV[11]. Breast milk is not only food; it contains an extraordinary number of factors that comprise and modulate both the adaptive and innate immune systems[12]. Indeed, some evolutionary biologists believe the mammary gland first evolved from the innate immune system and its nutritional role developed later[13, 14]. Infant survival is a biologic imperative; therefore evolutionary selection over millions of years has produced a secretion that protects infants from a wide variety of infectious agents. Given human milk’s potent immunomodulatory and antimicrobial effects, it is likely that study of this complex fluid will reveal novel substances that protect infants from HIV. Identification of immune factors potent enough to mitigate infection of infants would clearly be important targets for vaccine development.
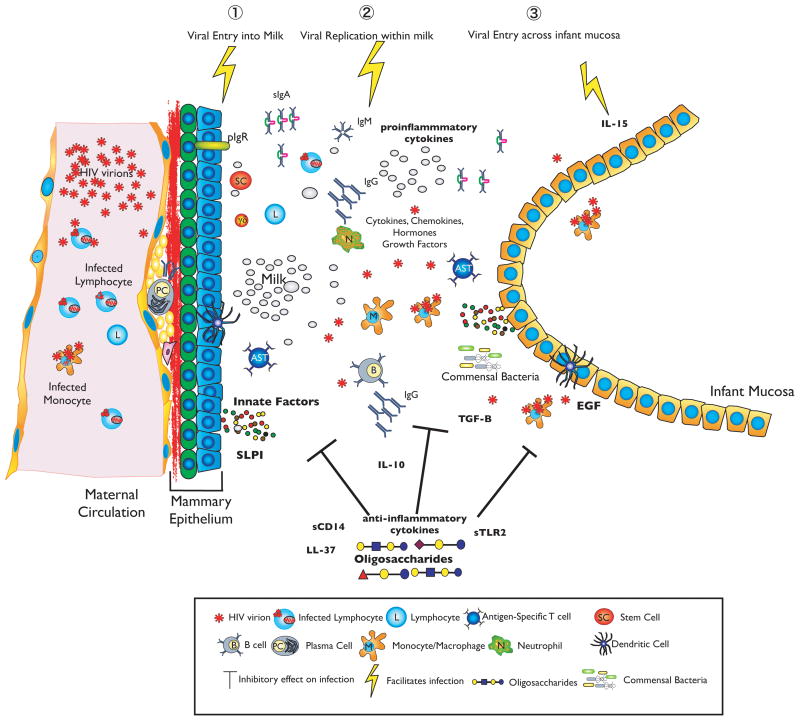
In many respects, breast milk transmission is similar to sexual transmission. Transmission depends on HIV: 1) breaching an epithelial barrier that usually significantly limits the amount of HIV in the transmitting fluid; 2) remaining in an infectious form within a secretion; 3) traversing another mucosal surface to infect a new host (Figure 1). At each of these steps, both local and systemic factors can facilitate or inhibit HIV infection. However, unlike sexual partnerships, the mother-infant bond is reliably identified and stable; the transmitting fluid is easy to collect and enters a single body cavity in a consistent fashion; and there is no stigma to quantifying exposures, behaviors or inflammatory states (e.g. infections, ulcers) that facilitate transmission. Although factors may be unique for infant protection, the mammary gland is part of a “common mucosal immune system” that is linked to other mucosal inductive sites, including the gastrointestinal and respiratory tracts.
Figure 1. Schematic of Breast Milk HIV transmission.
HIV can cross from maternal blood through the mammary epithelium into milk. Mucosal factors are likely to regulate the passage of HIV into milk by modulating mammary epithelia integrity. Within milk, substances may inactivate HIV and/or prevent binding to the infant mucosa and/or infant target cells. Milk also contains many factors anti-inflammatory factors that would limit viral replication within milk as well as maintaining the integrity of both the mammary and infant mucosal epithelium. Tropic substances (e.g. epithelial growth factor) are concentrated in breast milk and promote intestinal maturation and integrity. Inflammatory conditions within the infant mucosa such as thrush or gastroenteritis may facilitate infection by disrupting the epithelial barrier and/or activating HIV target cells. Many innate factors such as oligosaccharides, sTLR2 are concentrated in milk and appear to act at all 3 steps. These factors promote epithelial integrity both within the breast (reduce transmissibility) and in the infant oral or gut mucosa (reduce susceptibility) as well as interfere with HIV binding.
One of the reasons breast milk transmission is relatively rare is the remarkable ability of the mammary epithelium to curtail viral entry. HIV-1 RNA in breast milk levels are approximately two logs lower than those in the circulation and even in the setting of a breast abscess remain significantly below those in blood[15]. At least a third of HIV-infected women have breast milk HIV RNA levels below 50 copies/ml, while another third shed virus only intermittently[15]. The factors governing viral restriction are incompletely understood. There is increasing appreciation that epithelial cells are not merely passive barriers, but have dynamic and reciprocal interactions with adaptive and innate immune factors[16]. Although overall, viral shedding in milk is significantly related to plasma viral load, the correlation is weak and substantial discrepancies exist with some women having low levels in milk but high levels in plasma and vice versa. External, non-HIV related factors account for some of this variability, including sudden changes in the frequency of breastfeeding, parity and mastitis (which tends to be unilateral)[17]. This variability provides a highly informative model in which to study innate immune factors that may affect these patterns of discordant and discrepant shedding, relevant for transmission. To date, most studies of correlates of HIV protective immunity have focused on adaptive immune responses but innate responses especially at mucosal surfaces may be more important modulators of transmission.
Postnatal HIV transmission rates are surprisingly low even among infants with significant exposure to HIV-containing breast milk. In the Zambia Exclusive Breastfeeding study, only 36.9% of infants born to women with CD4 counts <200 and plasma HIV levels of greater 100,000 copies/ml acquired HIV via breastfeeding. In a recent study of HIV-exposed breastfeeding infants, HIV-specific responses (as measured by a gamma-interferon ELISPOT assay) were detected in almost half of the 200 infants studied[18]. Moreover, the magnitude of early HIV-specific responses was associated with protection from HIV infection[18]. These data, as well as the studies cited above in perinatally exposed children, provide support for the protective role of cellular immune responses. They also highlight another advantage of the mother-child model whereby factors in mothers and children can be studied using a prospective design and before transmission occurs. Whether these children will be protected from HIV upon later exposures is unknown. Identification of protective immune responses would provide targets for HIV vaccine targets.
A unique feature of mother-to-child transmission is the presence of maternal antibody. Although data are inconsistent, more recent studies suggest that transmitted variants are neutralization escape variants[19, 20]. This suggests that humoral immunity plays an important role in protection. There is now broad consensus that neutralizing antibody responses should be an important component of prophylactic vaccines. Studies of these transmissible neutralization escape variants may provide important insights into optimal protective antibody responses, and pathways used by HIV to evade these responses (see [5, 21] for a review).
A number of factors in milk including chemokines, cytokines, and alpha-defensins have been consistently associated with protection, while data are conflicting for others factor such as HIV-specific antibody responses[5, 21]. Breast milk also contains substances that interfere with HIV binding and infection in vitro including Lewis X factor, oligosaccharides, glycans, and MUC1[5, 21–24]. An interesting study reported protection associated with breast milk levels of long-chain n-6 polyunsaturated fatty acids[25]. Unfortunately, most of these studies have been limited by small sample size and a focus on a single or few factors. Many these factors are highly correlated, and each possesses pleiotrophic, redundant, and overlapping functions. Since protection is most likely multi-factorial, dissecting the precise role of any factor will require comprehensive approaches and large sample sizes. Nevertheless, identification of substances that interfere with HIV infection and yet are “safe enough to be in mothers’ milk” would represent a significant advance.
Although we have made considerable progress in treating HIV-infected persons, our efforts to develop a preventive vaccine or microbicide have lagged. As the epidemic continues to spread, novel preventative strategies and products are urgently needed. Although mother-to-child HIV transmission has a number of advantages as a model to investigate correlates of transmission, fortunately, transmission has become increasingly rare. In non-breastfeeding populations, the use of antiretroviral drugs during pregnancy and delivery has reduced transmission rates to <2%, making this a less robust model system for prospective studies. Nevertheless, samples from large cohorts of women and children who were not fortunate enough to benefit from the new interventions are available in repositories, and are a rich resource. Given the complexity of transmission, multiple model systems will need to be investigated. The study of MTCT may offer important and unique insights into correlates of protection.
Acknowledgments
The study was supported in part by grants from the Eunice Shriver National Institutes of Child Health and Human Development (NICHD) HD 57161, HD 39611, and HD 40777 and the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (UO1 AI068632-01). GMA is an Elizabeth Glaser Pediatric AIDS Foundation Scientist. We offer our apologies to colleagues whose work could not be adequately discussed or cited owing to space limitations.
Footnotes
Presented in part at the International Symposium on Natural Immunity to HIV, November 15–17, 2009, Winnipeg Manitoba Canada
Conflicts: No conflicts
References
- 1.Lederman MM, Penn-Nicholson A, Cho M, Mosier D. Biology of CCR5 and its role in HIV infection and treatment. JAMA. 2006;296:815–26. doi: 10.1001/jama.296.7.815. [DOI] [PubMed] [Google Scholar]
- 2.Adams KM, Nelson JL. Microchimerism: an investigative frontier in autoimmunity and transplantation. JAMA. 2004;291:1127–31. doi: 10.1001/jama.291.9.1127. [DOI] [PubMed] [Google Scholar]
- 3.Langston C, Lewis DE, Hammill HA, et al. Excess intrauterine fetal demise associated with maternal human immunodeficiency virus infection. J Infect Dis. 1995;172:1451–60. doi: 10.1093/infdis/172.6.1451. [DOI] [PubMed] [Google Scholar]
- 4.Farquhar C, John-Stewart G. The role of infant immune responses and genetic factors in preventing HIV-1 acquisition and disease progression. Clin Exp Immunol. 2003;134:367–77. doi: 10.1111/j.1365-2249.2003.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter J, Kuhn L, Aldrovandi GM. Advances in basic science understanding of mother-to-child HIV-1 transmission. Curr Opin HIV AIDS. 2008;3:146–50. doi: 10.1097/COH.0b013e3282f50bb2. [DOI] [PubMed] [Google Scholar]
- 6.Tiemessen CT, Shalekoff S, Meddows-Taylor S, et al. Cutting Edge: Unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J Immunol. 2009;182:5914–8. doi: 10.4049/jimmunol.0900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legrand FA, Nixon DF, Loo CP, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One. 2006;1:e102. doi: 10.1371/journal.pone.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis. 2009;199:1318–22. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn L, Aldrovandi GM, Sinkala M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–41. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn L, Sinkala M, Semrau K, et al. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;50:437–44. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol. 2004;4:565–72. doi: 10.1038/nri1393. [DOI] [PubMed] [Google Scholar]
- 13.Vorbach C, Capecchi MR, Penninger JM. Evolution of the mammary gland from the innate immune system? Bioessays. 2006;28:606–16. doi: 10.1002/bies.20423. [DOI] [PubMed] [Google Scholar]
- 14.McClellan HL, Miller SJ, Hartmann PE. Evolution of lactation: nutrition v. protection with special reference to five mammalian species. Nutr Res Rev. 2008;21:97–116. doi: 10.1017/S0954422408100749. [DOI] [PubMed] [Google Scholar]
- 15.Semrau K, Ghosh M, Kankasa C, et al. Temporal and lateral dynamics of HIV shedding and elevated sodium in breast milk among HIV-positive mothers during the first 4 months of breast-feeding. J Acquir Immune Defic Syndr. 2008;47:320–8. doi: 10.1097/qai.0b013e31815e7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010 doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 17.Thea DM, Aldrovandi G, Kankasa C, et al. Post-weaning breast milk HIV-1 viral load, blood prolactin levels and breast milk volume. AIDS. 2006;20:1539–47. doi: 10.1097/01.aids.0000237370.49241.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John-Stewart GC, Mbori-Ngacha D, Payne BL, et al. HIV-1-Specific Cytotoxic T Lymphocytes and Breast Milk HIV-1 Transmission. J Infect Dis. 2009 doi: 10.1086/597120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Parast AB, Richardson BA, et al. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol. 2006;80:835–44. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickover R, Garratty E, Yusim K, Miller C, Korber B, Bryson Y. Role of Maternal Autologous Neutralizing Antibody in Selective Perinatal Transmission of Human Immunodeficiency Virus Type 1 Escape Variants. J Virol. 2006;80:6525–6533. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehman DA, Farquhar C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev Med Virol. 2007;17:381–403. doi: 10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- 22.Naarding MA, Ludwig IS, Groot F, et al. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest. 2005;115:3256–64. doi: 10.1172/JCI25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeland E, de Jong MA, Nabatov AA, Kalay H, Geijtenbeek TB, van Kooyk Y. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol Immunol. 2009;46:2309–16. doi: 10.1016/j.molimm.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) Br J Nutr. 2009;101:482–6. doi: 10.1017/s0007114508025804. [DOI] [PubMed] [Google Scholar]
- 25.Villamor E, Koulinska IN, Furtado J, et al. Long-chain n-6 polyunsaturated fatty acids in breast milk decrease the risk of HIV transmission through breastfeeding. Am J Clin Nutr. 2007;86:682–9. doi: 10.1093/ajcn/86.3.682. [DOI] [PubMed] [Google Scholar]