Abstract
The Banff scoring schema provides a common ground to analyze kidney transplant biopsies. Interstitial inflammation (i) and tubulitis (t) in areas of viable tissue are features in scoring acute rejection, but are excluded in areas of tubular atrophy. We studied inflammation and tubulitis in a cohort of kidney transplant recipients undergoing allograft biopsy for new-onset late graft dysfunction (N=337). We found inflammation (“iatr”) and tubulitis (“tatr”) in regions of fibrosis and atrophy to be strongly correlated with each other (p<0.0001). Moreover, iatr was strongly associated with death-censored graft failure when compared to recipients whose biopsies had no inflammation, even after adjusting for the presence of interstitial fibrosis (Hazard Ratio=2.31, [1.10-4.83]; p=0.0262) or tubular atrophy (Hazard Ratio=2.42, [1.16-5.08]; p=0.191), serum creatinine at the time of biopsy, time to biopsy, and i score. Further, these results did not qualitatively change after additional adjustments for C4d staining or donor specific antibody. Stepwise regression identified the most significant markers of graft failure which include iatr score. We propose that a more global assessment of inflammation in kidney allograft biopsies to include inflammation in atrophic areas may provide better prognostic information. Phenotypic characterization of these inflammatory cells and appropriate treatment may ameliorate late allograft failure.
Keywords: biopsy, inflammation, fibrosis, injury, graft failure, Banff schema
INTRODUCTION
The classification of kidney allograft pathology by the Banff criteria provides reproducible diagnostic categories of allograft injury (1). Common histological features of failing allografts are interstitial fibrosis (IF) and tubular atrophy (TA), the severity of which is graded semi-quantitatively as mild, moderate and severe (2). Recent studies have demonstrated that the simple quantification of IF/TA is insufficient to identify those at greatest risk for long-term graft loss (3). Protocol biopsies, including those from living donors, at one year post transplant nearly uniformly show IF/TA. In these studies, IF/TA was associated with a small decrease in allograft survival compared to normal histology; however, the combination of IF/TA with inflammation of any degree was associated with a worse prognosis than fibrosis alone (4-6).
In the current Banff schema, interstitial inflammation and tubulitis are scored only in areas of non-fibrotic interstitium and non-atrophic tubules, respectively. Subcapsular inflammation is also excluded. However, a protocol biopsy study performed in recipients of kidney–pancreas transplants showed that inflammation in areas of interstitial fibrosis and tubular atrophy (referred to as the “chronic damage index” or “cdi”) predicted the development of progressive tubulointerstitial injury in sequential biopsies (7), and although its association with allograft failure was not determined, the finding of inflammation in areas of chronic injury appeared to indicate “active” –i.e., progressive fibrosis. At the 2007 meeting of the Banff allograft pathology group, future study of the association between a total inflammation score (“total i”) including all areas of the renal parenchyma, and allograft survival, was proposed (8). Accordingly, Mengel et al have since noted the importance of infiltrates in areas of fibrosis and reported that total i correlates better than the i score with subsequent graft deterioration (9;10). Thus, understanding the role of ongoing inflammatory injury, both in areas of preserved architecture as well as areas of chronic injury is critically important to the prognosis and management of the failing kidney grafts.
The Long-Term Deterioration of Kidney Allograft Function (DeKAF) study is a multicenter study designed to identify the causes of late allograft dysfunction (11). To date, 337 renal transplant recipients with new onset, late graft dysfunction have undergone allograft biopsies that were read, using standard Banff criteria, by a central pathologist. Additionally, semi-quantitative scoring of inflammatory cell infiltrates (“iatr”) and tubulitis (“tatr”) in areas of tubular atrophy were obtained. We found that iatr was frequently present in this cohort; and that it was strongly associated with time to death-censored graft failure even after adjustment for serum creatinine, Banff i score, and extent of interstitial fibrosis. These results support a more comprehensive assessment of inflammatory cell infiltrates in kidney allografts than described in the current Banff system.
METHODS
Patients and enrollment
The DeKAF study consists of two cohorts of kidney transplant recipients enrolled at 7 transplant centers in the US and Canada: 1) a cross-sectional cohort transplanted prior to October, 2005 and developing new onset late graft dysfunction; and 2) a prospective cohort transplanted on or after January 1, 2006 (11). The study is registered at www.clinicaltrials.gov. Institutional Review Board approval was obtained at all participating sites.
For the current analysis, we studied biopsies done for new onset late graft function in the cross-sectional cohort. Recipients were eligible for enrollment if transplanted prior to October 1, 2005, having a baseline serum creatinine < 2.0 mg/dL as of January 1, 2006, and subsequently developing a ≥ 25% increase in serum creatinine, or new onset proteinuria [albumin/Cr ratio >0.2 or protein/Cr ratio >0.5]) leading to an allograft biopsy. Enrollment occurred at the time of the biopsy.
Histological analysis
Allograft biopsies were read by the local pathologist and pathologic diagnosis was used to guide clinical care and immunosuppressive management per local protocols using Banff 1997 criteria (2) and the updated criteria additions of 2007 (8). Representative sections (H&E, silver, PAS, trichrome stains, and 11 unstained sections for additional studies) were submitted to a central laboratory where all biopsies were interpreted by the same pathologist in a masked fashion (N=337; JG).
Interstitial inflammation and tubulitis were scored separately in non-atrophic and atrophic regions of the renal cortex. Inflammation and tubulitis in non-atrophic regions of the cortex was scored according to the “standard” Banff classification scheme (2) for assessment of “i” and “t” scores, respectively. Inflammation in areas of atrophy—currently ignored in the “standard” Banff classification scheme---was assessed as the percentage of atrophic cortex with inflammatory infiltrates (“iatr”): 0 = inflammation in less than 10% of atrophic regions; 1 = inflammation in 10-25% of atrophic regions; 2 = inflammation in 26-50% of atrophic regions; and 3 = inflammation in >50% of atrophic regions. Similarly, tubulitis in atrophic tubules (“tatr”) was assessed in the same manner as for non-atrophic ones (0 = no mononuclear cells in tubules; 1 = foci with 1-4 cells/tubular cross section; 2 = foci with 5-10 cells/tubular cross section; and 3 = foci with >10 cells/tubular cross section). Illustrative examples of inflammation and tubulitis in regions of atrophy are shown in Figure 1. Total i score, as defined by the proportion of total cortical surface area involved by inflammation, whether atrophic or non-atrophic, was assessed as previously described by Mengel (9).
Figure 1.
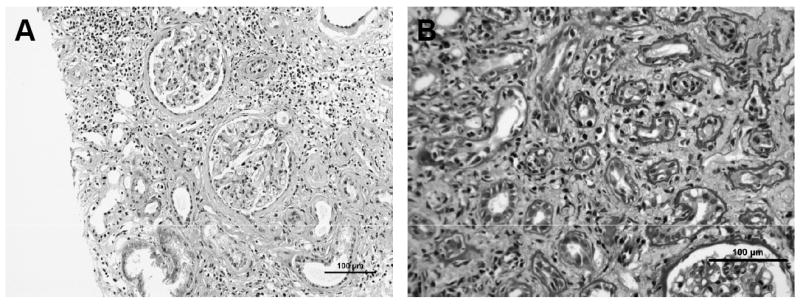
Representative photomicrographs showing (A) inflammation in region of atrophy, and (B) tubulitis in atrophic tubules.
C4d staining was performed using standard immunohistochemical methods. Briefly, antigen retrieval was carried out by heat treatment in EDTA for 30 min using a vegetable steamer. Endogenous biotin in the kidney was blocked by treating with 3% H2O2, followed by the Vector Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA). Anti-human C4d antibody (C4d pAb; Alpco Diagnostics, Salem, NH) was applied for 30 min, followed by rabbit EnVision+ HRP (Dako, Carpinteria, CA) for 30 min. NovaRED (Vector Labs) was used for color development, followed by hematoxylin staining. To facilitate consistency, slides were batched and stained on a Dako autostainer. C4d stains were read in a masked fashion, without clinical or pathologic information. The estimated percentage of peritubular capillaries staining positively for C4d was recorded as negative, ≥10%, ≥25%, or ≥50%, using the Banff classification scheme (12) and as described by Crary et al (manuscript submitted).
Donor Specific Antibody Testing
Serum samples (2 mL) were collected at the time of each biopsy and tested for anti-donor HLA antibodies (DSA) by a central laboratory (JMC) using microparticles with individual purified HLA antigens (Ag) covalently bound as targets (One Lambda, Inc, Canoga Park, CA) on the Luminex platform. DSA was considered positive (+) if antibodies were detected against donor HLA-A, B, DR, or DQ. DSA was negative (-) if no DSA was present as well as no antibody against HLA-Cw or DP (as donor typing at these loci was not reported).
Clinical Data Management
For all enrolled subjects data were collected every 6 months and at the time of allograft loss. These data included: serum creatinine, urine protein and creatinine, demographics, current immunosuppressive medications, other intervening illnesses, date and cause of graft loss (return to dialysis, re-transplant or recipient death).
Statistical Analysis
Associations of iatr, tatr, i, and total i were analyzed by Chi square testing. Tests of association between diagnoses of acute rejection and iatr were assessed by Fisher’s Exact Test. Strength of association between categorical variables was measured by Kendall’s Tau-b coefficient. Analysis of variance was used to compare mean time from transplant to biopsy by level of iatr. Non-parametric methods (Kaplan-Meier graphs, log-rank test) were used to analyze time to death-censored graft failure for biopsies with and without inflammation, in areas of fibrosis and with and without tubulitis, in regions of atrophy. To adjust for center specific effects, log-rank tests to compare these survival curves were also stratified by clinical center. To estimate relative hazards and adjust for the presence of other covariates, Cox proportional hazard models were developed for time to death-censored graft failure stratified by clinical center, and adjusting for time from transplant to biopsy, serum creatinine, inflammation (Banff i score), total i score, tubular atrophy (Banff ct score), allograft fibrosis (Banff ci score), C4d staining, donor specific antibody, and treatment for acute rejection. Stepwise regression methods were used to select an optimal subset of these covariates with an entry-criterion for variable selection of a p-value p < 0.15 and a retention criterion of a p<0.10. Because not all subjects had both C4d and donor specific antibody available for analysis, donor specific antibody was not included in the stepwise regression procedures for considerations of sample size.
RESULTS
The cross-sectional cohort began enrollment in February 2006; and to date, 496 recipients have been enrolled. Of these enrollees, 53% are female; 78% are Caucasian, and 14% African American. Serum creatinine for this cohort (mean ±SE) prior to January 2006 was 1.4 ± 0.3 mg/dL (median, 1.4 mg/dL) with mean time (±SD) from transplant to biopsy 7.1 ± 5.9 years (median, 5.7 years). Of 496 patients enrolled in this study to date, 337 consecutive patients have had biopsies reviewed by the central pathology lab. Of these 337, death-censored graft failure occurred in 77 recipients and 16 additional recipients died with graft function, consistent with the high risk nature of the population under study. Overall, the mean time to death-censored allograft failure after biopsy was 306 ± 262 days. Of the 337 consecutive biopsies reviewed, 291 were classified as IF/TA based on a definition of ci>0 or ci=0 (n=290) and ct≥2 (n=1). Conversely, there were 43 cases that were not counted as IF/TA with ci=0, ct=1 and 3 cases with ci=ct=0.
There was a strong association between iatr and tatr scores (Kendall’s Tau-b 0.59 ± 0.03; Table 1). The presence of both iatr and tatr was also strongly associated with the centrally evaluated i scores (p<0.0001). In spite of these associations, of 210 recipients with Banff i score of 0, 108 (51%) had iatr scores ≥ 1 and 151 (72%) had tatr scores ≥ 1, demonstrating the relatively moderate association of inflammatory cell infiltrates in areas of interstitial fibrosis and tubular atrophy with inflammation in areas of viable tubule-interstitial parenchyma which is part of the criteria for acute cellular rejection. As a measure of the strength of this association, the Kendall’s Tau-b statistic between iatr and tatr and the Banff i score was 0.54±0.04 and 0.41±0.04, respectively. By comparison, the Kendall Tau-b measuring the association of the Banff i and t scores was 0.73±0.03.
Table 1.
Two-way frequency distribution of “iatr” and “tatr” scores in allograft biopsies*
| “iatr” Scores | “tatr” Scores Number and % of Total N (337) |
Total | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| 0 | 53 (15.7%) | 42 (12.5%) | 8 (2.4%) | 2 (0.6%) | 105 |
| 1 | 11 (3.3%) | 47 (14.0%) | 50 (14.8%) | 5 (1.5%) | 113 |
| 2 | 1 (0.3%) | 13 (3.9%) | 65 (19.3%) | 14 (4.2%) | 93 |
| 3 | 0 (0.0%) | 1 (0.3%) | 18 (5.3%) | 7 (2.1%) | 26 |
| Total | 65 | 103 | 141 | 28 | 337 |
Chi Square 176.1761; p<0.0001
High iatr and tatr scores were significantly associated with decreased graft survival as demonstrated by log-rank tests (iatr Log-rank=43.91 3df, p<0.0001 and tatr Log-rank=9.0 3df, p=0.0293; figure 2). Similarly, log-rank tests comparing the presence of iatr (iatr≥1) versus no iatr (iatr=0), demonstrated that allograft failure was significantly more common when iatr was detected, regardless of its severity (Log-rank 11.73 1df; p=0.0006; figure 3). Similar results were obtained for tatr (not shown) but the intensity of this relationship was less strong (Log-rank=4.88 1df; p=0.0272).
Figure 2.
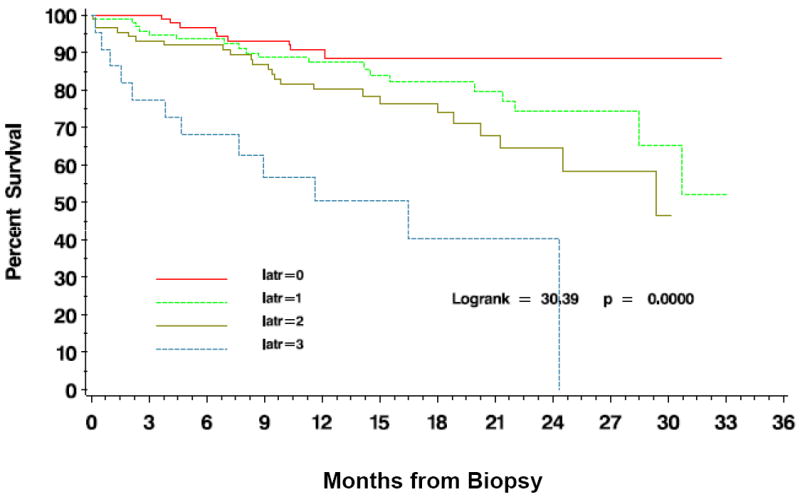
Time to death censored graft failure after allograft biopsy is dependent on the severity of scoring for iatr.
Figure 3.
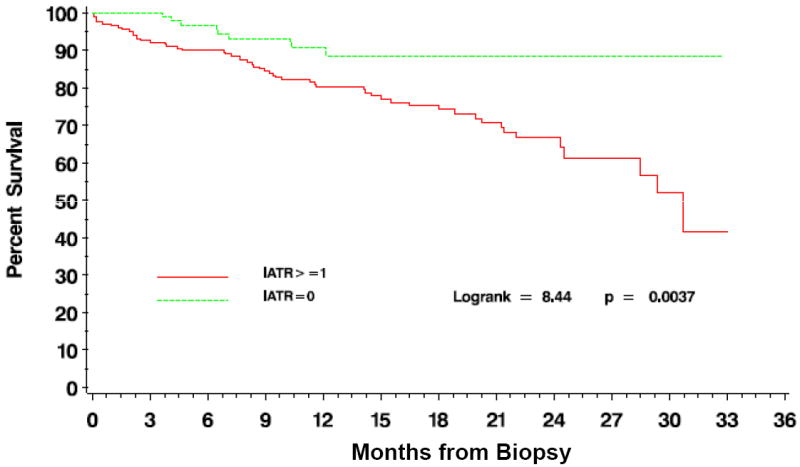
Time to death censored graft failure after allograft biopsy based on the presence or absence of iatr.
In multivariate Cox proportional hazards models stratified by clinical center and adjusted for creatinine at time of biopsy, scores of iatr or tatr of 1 (mild) were not associated with allograft outcome (p=0.075, p=0.066 respectively). However, with iatr score = 2, the risk of allograft failure was 2.52 (p=0.009), compared to biopsies with iatr = 0 (Table 2A). This risk increased dramatically as iatr score increased to 3 (HR= 6.35 p<0.001). When further adjusted for inflammation (i score) within the biopsy, iatr score was associated with an even greater risk of allograft loss (Table 2A), suggesting that the effect of iatr is independent of the extent of inflammation in viable areas of the kidney. While time to allograft biopsy could confound the analysis of survival and iatr, time to allograft biopsy was not statistically significant when added to the models in Table 2A (data not shown). There was no difference in mean time from transplant to biopsy between grades of iatr, with the shortest period of time occurring in the iatr=3 group (6.6±5.4 years) and the longest in the iatr = 0 (7.4±5.6 years; p=0.76).
Table 2.
A. Proportional hazards regression of time to death-censored graft failure based on inflammation in areas of interstitial fibrosis (iatr) adjusted for serum creatinine at time of biopsy, and extent of inflammation (i), tubular atrophy (ct), and fibrosis (ci), C4d positivity (C4d+) and donor specific antibody positivity (DSA+).
| Hazard Ratio [95% Confidence Interval]; P-value | ||||||
|---|---|---|---|---|---|---|
| Group | Model 1 Adjusted for creatinine | Model 2 Adjusted for i and creatinine | Model 3 Adjusted for ci and creatinine | Model 4 Adjusted for ct and creatinine | Model 5 Adjusted for ci and ct and creatinine | Model 6 Adjusted for i, ci, ct, C4d+, DSA+ and creatinine |
| iatr=0 | REF* | REF | REF | REF | REF | REF |
| iatr=1 | 1.91 [0.95,3.90]; 0.075 | 2.47 [1.17,5.20]; 0.018 | 1.59 [0.77,3.30]; 0.212 | 1.68 [0.81,3.48]; 0.161 | 1.60 [0.77,3.32]; 0.207 | 3.36 [1.05,10.68]; 0.0403 |
| iatr=2 | 2.52 [1.26,5.02]; 0.009 | 4.38 [1.95,9.82] <0.001 | 2.12 [1.02,4.38]; 0.043 | 2.00 [0.96,4.16]; 0.065 | 2.07 [0.99,4.35]; 0.053 | 5.11 [1.44,18.07]; 0.0114 |
| iatr=3 | 6.35 [2.91,13.85]; <0.001 | 12.0 [4.4,32.61]; <0.001 | 3.36 [1.39,8.13]; 0.007 | 3.44 [1.42,8.33]; 0.006 | 3.23 [1.29,8.06]; 0.012 | 8.07 [1.71,38.07]; 0.0083 |
| Overall p-value for iatr | <0.0001 | <0.0001 | 0.0441 | 0.0543 | 0.0756 | 0.0450 |
| B. Proportional hazards regression of time to death-censored graft failure based on tubulitis in areas of tubular atrophy (tatr) adjusted for serum creatinine at time of biopsy, and extent of inflammation (i), tubular atrophy (ct), interstitial fibrosis (ci), C4d positivity (C4d+) and donor specific antibody positivity (DSA+). | ||||||
| Hazard Ratio [95% Confidence Interval]; P-value | ||||||
| Group | Model 1 Adjusted for serum creatinine | Model 2 Adjusted for i and serum creatinine | Model 3 Adjusted for ci and serum creatinine | Model 4 Adjusted for ct and serum creatinine | Model 5 Adjusted for ci and ct and serum creatinine | Model 6 Adjusted for i, ci, ci, C4d+, DSA+ and serum creatinine |
| tatr=0 | REF* | REF | REF | REF | REF | REF |
| tatr=1 | 2.26 [0.95,5.38]; 0.0656 | 2.44 [1.01,5.92]; 0.0482 | 2.09 [0.87,5.02]; 0.1013 | 2.20 [0.91,5.28]; 0.0787 | 2.09 [0.87,5.05]; 0.0997 | 5.76 [1.28,25.91]; 0.0224 |
| tatr=2 | 3.17 [1.46,6.85]; 0.0034 | 4.05 [1.69,9.70]; 0.0017 | 2.42 [1.08,5.41]; 0.0310 | 2.30 [1.03,5.14]; 0.0417 | 2.30 [1.02,5.16]; 0.0445 | 6.84 [1.51,20.94]; 0.0125 |
| tatr=3 | 2.56 [0.96,6.85]; 0.0605 | 3.13 [0.99,9.90]; 0.525 | 2.01 [0.73,5.49]; 0.1750 | 1.64 [0.59;4.53]; 0.3436 | 1.71 [0.61,4.79]; 0.3047 | 7.31 [1.39,38.37]; 0.0187 |
| Overall p-value for tatr | 0.0308 | 0.0160 | 0.1976 | 0.1937 | 0.2281 | 0.0889 |
REF=reference group
Moderate to severe allograft fibrosis is associated with worsened outcomes compared to non-fibrotic kidneys and may thus confound the results of either iatr or tatr (7). However, we found that when adjusted for the extent of interstitial fibrosis (“ci”) within the biopsy, iatr scores of 2 or 3 remained significantly associated with the risk of allograft failure (hazard ratios 2.12 and 3.36, respectively) (Table 2a), suggesting that extent of inflammation which is normally not accounted for by conventional Banff scoring, contributes to the demise of allografts with fibrosis present. When adjusting for all biopsy fibrosis, atrophy, and inflammation, iatr scores of ≥ 2 demonstrated a 3.4 to over 5-fold increase in allograft loss (Table 2A), demonstrating that iatr is strongly predictive of allograft loss even when holding other relevant Banff score variables constant.
One recent report emphasizes the assessment of inflammation present throughout the biopsy using a total inflammation score (“total i”; (9) and its relationship to predicting graft failure, particularly in biopsies with IF/TA. To address whether this variable would affect our models, we also scored biopsies using the total i schema. Not surprisingly, total i score and iatr are strongly associated (Kendal Tau-b 0.78±0.024; p<0.0001) as iatr is included in the total i assessment. Regression analysis which included of any level of total i score was not significantly associated with graft loss (data not shown, P=0.8766), although this model contained an additional 4 degrees of freedom. Inclusion of total i score did reduce the level of hazard ratio and significance of iatr with graft failure to 1.83 for iatr = 1 (p=0.195) and 2.55 for iatr = 2 (p=0.0919). However, hazard ratio for iatr = 3 remained strong and significant (5.19; p=0.0098). When i score was also included in the analysis, resulting in 11 degrees of freedom, significance for the prediction of graft failure was not observed for total i, iatr or i score, possibly due to correlation between these covariates. Our data suggest that inflammation in atrophic areas of the allograft biopsy is an important component of the total i score as a marker of allograft failure.
To address the differential impact of inflammation in various compartments on cases with IF/TA, we have performed additional subgroup analyses on the 291 biopsies classified as IF/TA, assessing the effect of Banff i and iatr on graft-survival while adjusting for serum creatinine. In these models, iatr was a significant predictor of outcome with hazard ratios ranging from 1.78-12.35 (p<0.0001) when ct was not adjusted and 1.7-7.1 with ct adjustment (p=0.0093). However, i was of marginal significance regardless of the adjustment of ct scores (p=0.061 without and p=0.085 with adjustment, respectively). The corresponding models for total i are significant without adjustment for ct (p=0.004) but are of marginal significance after adjusting for ct (p=0.058). Thus, after adjusting for serum creatinine at the time of biopsy, time to allograft failure in biopsies with IF/TA is strongly associated with iatr while marginally associated with other inflammation.
In contrast to iatr, tatr was less strongly associated with allograft loss. When controlled for serum creatinine, tatr was associated with graft failure (p=0.0308) (Table 2B). However, when the extent of tubular atrophy or interstitial fibrosis was controlled, tatr was not significantly associated with graft failure (Table 2B).
As late allograft failure has been linked to donor specific antibody and endothelial injury (9, 10), we also assessed the effects of the presence of central C4d staining and donor specific antibody (Table 2A and 2B). Adjustment for these factors did not weaken the ability for iatr to predict allograft failure.
We also analyzed the relationship between iatr and acute cellular rejection, a diagnosis made in non-atrophic portions of cortex. While the association of iatr with a central diagnosis of cellular rejection is statistically significant (p<0.0001), 94.8% of biopsies with a central diagnosis of acute cellular rejection had iatr ≥ 1 compared with 63.4% of biopsies without rejection. Correspondingly, the strength of association as measured by Kendall’s Tau-b is low (0.34±0.04). In multivariate proportional hazards regression for time to death-censored graft failure, there was no significant impact of treatment of concomitant acute rejection adjusted for tubular atrophy on graft outcome [HR 0.90 (0.49-1.67), p=0.74], after adjustment for iatr, central Banff ct, and creatinine at biopsy. Thus, while acute rejection may be associated with iatr, the impact of iatr on graft failure is not primarily dependent on the presence of a diagnosis of acute rejection and its treatment.
To further assess the independent effect of iatr from the Banff i-score on survival, biopsies were categorized into four groups: 1) those with no inflammation (iatr=i=0, n=102), 2) those with inflammation solely in regions of atrophy (i=0 and iatr≥1, n=108), and 3) those with inflammation in both atrophic and non-atrophic interstitium (i≥1, iatr≥1; n=124), and 4) those with inflammation but not in regions of atrophy (i≥1, iatr=0; n=3) (Table 3). Because of the small case number in group 4, we combined groups 3 and 4 for analyses. By combining the 2 groups, we eliminated an underpowered estimate without discarding cases. Analysis of this model versus the 4 groups with the interaction provided similar results to those results are presented here. The time to death-censored graft failure was significantly different between groups (Figure 4; Log-rank=10.07 2df; p=0.0065). In multivariate proportional hazards regression stratified by clinical center and adjusted for creatinine at biopsy, the iatr-only group (2) was associated with a 3-fold increase in hazard over the no inflammation group (1) (Table 4). Although the hazard ratio decreased when adjusted for Banff ci or ct scores, the results remained statistically significant with hazard ratios in excess of 2.0 (Table 4). Additional adjustment of the models for the contribution of treatment for acute rejection was not significant (data not shown) and did not qualitatively alter these results.
Table 3.
Two Way Frequency of iatr and the presence of interstitial inflammation.
| Banff i score | “iatr” Scores Number and % of Total N (337) |
Total | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| 0 | 102 (29.6%) | 75 (22.5%) | 26 (7.8%) | 7 (2.1%) | 210 |
| 1 | 0 (0%) | 25 (7.5%) | 23 (6.9%) | 4 (1.2%) | 113 |
| 2 | 2 (0.6%) | 9 (2.7%) | 39 (11.7%) | 2 (0.6%) | 93 |
| 3 | 1 (0.3%) | 4 (1.2%) | 5 (1.5%) | 13 (3.9%) | 26 |
| Total | 105 | 113 | 93 | 26 | 337 |
Figure 4.
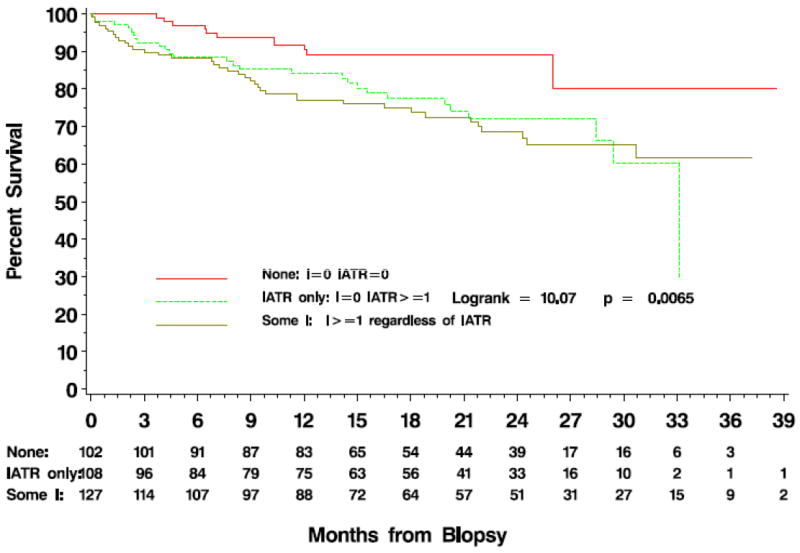
Time to death censored graft failure after allograft biopsy comparing biopsies with no inflammation (i=0, iatr=0; n=102), inflammation only in areas of fibrosis (i=0 and iatr≥1; n=108) and those with inflammation in both fibrotic and non-fibrotic areas (i≥1, iatr≥1; n=124 and i>0 and iatr=0; n=3) demonstrating the independent effect of iatr from Banff i score on allograft failure.
Table 4.
Proportional hazards regression estimates of time to death-censored graft failure based on inflammation status: iatr only (i=0, iatr>0), and iatr or i ≥0 relative to no inflammation (i = 0 iatr = 0) adjusted for serum creatinine at time of biopsy, and extent of inflammation (i), atrophy (ct), fibrosis (ci), C4d positivity (C4d+) and Donor Specific Antibody positivity (DSA+).
| Hazard Ratio [95% Confidence Interval]; P-value | ||||||
|---|---|---|---|---|---|---|
| Group | Model 1 Adjusted for serum creatinine | Model 2 Adjusted for ci and serum creatinine | Model 3 Adjusted for ct and serum creatinine | Model 4 Adjusted for C4d+, DSA+ and serum creatinine | Model 5 Adjusted for C4d+, DSA+, ci and serum creatinine | Model 6 Adjusted for C4d+, DSA+, ct and serum creatinine |
| No inflammation [i=0, iatr=0] | REF* | REF | REF | REF | REF | REF |
| iatr only [i=0, iatr>0] | 3.06 [1.51,6.19]; 0.0018 | 2.31 [1.10,4.83]; 0.0262 | 2.42 [1.16,5.08]; 0.0191 | 5.06 [1.71,14.97]; 0.0034 | 3.70 [1.21,11.34]; 0.0221 | 4.02 [1.28,12.60]; 0.0170 |
| Inflammation [i>0, iatr≥0] | 2.10 [1.09,4.06]; 0.0268 | 1.60 [0.81,3.17]; 0.1780 | 1.51 [0.76,3.01]; 0.2367 | 2.88 [1.02,8.07]; 0.0448 | 2.35 [0.84,6.63]; 0.1052 | 2.35 [0.82,6.70]; 0.1105 |
REF= reference group
Due to the large number of correlated covariates of interest, we performed model selection through stepwise regression of time to graft failure utilizing all Banff subscores, as well as iatr, tatr, total i score, C4d, creatinine at the time of biopsy, time to biopsy from transplant, and treated acute rejection. In this model, 5 variables were selected as significantly associated with graft outcome: creatinine at time of biopsy (p<0.0001), iatr (p<0.0001), central ct score (p=0.075), central mm score (p<0.0001) and peritubular capillaritis (p=0.0097) while total i score, C4d presence and other inputted variables were not selected to fit this model. As shown in table 5, iatr remained a strong predictor of graft failure, even after controlling for numerous variables that might affect graft outcome. This supports the notion that iatr is a strong and important predictor of allograft failure in late kidney biopsies for graft dysfunction.
Table 5.
Stepwise regression analysis model estimates of time to death-censored graft failure of levels of iatr. The model selected iatr, and creatinine at time of biopsy, central mm, ct, and ptc scores as predictors of graft failure.
| iatr | Hazard Ratio [95% Confidence Interval]; P-value |
|---|---|
| 0 | REF* |
| 1 | 2.27 [0.891, 5.77]; 0.0860 |
| 2 | 2.98 [1.07, 8.34]; 0.0371 |
| 3 | 4.75 [1.58, 14.27]; p=0.0055 |
REF= reference group
DISCUSSION
Identifying pathological correlates of late kidney allograft loss is critical for guiding potential therapies, designing clinical trials, and developing biomarkers that may be used to identify recipients at risk. In this regard, the DeKAF study, with central pathological analysis of biopsies obtained for rising creatinine or significant proteinuria, provides a unique chance for correlating Banff schema with outcomes, an opportunity that has not previously existed in such a large scale.
In this study, we examined the extent of inflammation in areas of fibrosis and tubulitis in areas of tubular atrophy of the kidney allograft biopsied at late timepoints post transplantation. These are currently not included in the Banff schema (1,2), which focuses exclusively on inflammation in viable tissue, because inflammation in areas of atrophy and fibrosis was considered to be indicative of repair of previous injury. However, recent investigations into the role of cellular and antibody mediated inflammation in later allograft loss demonstrate a far reaching extent of response (4;9;13;14). Compartmentalizing the kidney into separate areas, those of atrophy and those without, while for pathological coding may seem sound, does not make biological sense, as inflammatory cell infiltration can have more far reaching effects in the microenvironment, through both secretory signals, as well as direct cell-cell contact (15).
While tubulitis in these atrophic areas had a significant relationship to graft failure, this relationship was not as strong as the presence of inflammation. We found iatr scores to be strongly associated with graft failure in biopsies of kidneys with new onset, late dysfunction. Moreover, this relationship remained independent of the extent of overall allograft fibrosis and atrophy, or of inflammation in areas of viable tubulo-interstitium (“i”) and acute cellular rejection. Of those cases with iatr≥1, only 62 (27%) had a primary or secondary diagnosis of rejection while remaining 168 cases did not have reported rejection. Thus acute cellular rejection occurred in the minority of biopsies classified with iatr. Similarly, only 19/230 (8.2%) of iatr biopsies had a primary or secondary diagnosis of antibody mediated rejection. Perhaps the strength of the association of the iatr-only group with outcome may be due to the presence of undiagnosed acute rejection, which, if left untreated, would naturally result in a worse outcome. This hypothesis is not directly testable in this dataset due to detection bias in the assignment of treatment. Our findings suggest not only that iatr may be critical to the promulgation of ongoing injury, but also should be included in the Banff analyses of allograft biopsies. This could lead to new classifications and ultimately the testing of treatment for this finding to provide direct evidence of the impact of inflammation in the failing allograft.
Our data complements and adds to the studies of Mengel et al. who reported the association of inflammation in areas of fibrosis with decreased allograft survival. In their studies of late posttransplant biopsies for cause, 77 recipients had IF/TA. Of these, 46 biopsies had ≥ 50% of the fibrosis area showing infiltrates with 31 having <50% of fibrotic areas with infiltrates. Those with increased infiltrates in fibrotic areas had significantly decreased graft survival (p=.02) (10). In a subsequent study, Mengel et al showed that the total inflammation score was a better predictor of post-biopsy graft survival than the Banff “i” score (9). In our multicenter study of late posttransplant biopsies for cause (n = 337), we show that inflammation in areas of atrophy (iatr) can be scored in a similar manner to inflammation in viable tissue (Banff i score). In a Cox model - controlled for transplant center, inflammation in viable tissue (Banff i score), the extent of interstitial fibrosis (“ci”) within the biopsy, C4d positivity, the presence of DSA, and serum creatinine level at the time of biopsy, we found iatr to be associated with increased graft loss.
In summary, semi-quantitative analysis of inflammation in areas of interstitial fibrosis provides a powerful measure of allograft injury and is a strong predictor of graft loss. Even after adjusting for interstitial fibrosis and tubular atrophy, and renal function at the time of biopsy, iatr remains a strong marker of graft failure. We suggest that Banff schema be updated to include more global assessments of inflammation within the biopsy to enhance the descriptive and predictive value of allograft biopsy when obtained in the setting of clinical concern.
Acknowledgments
We would like to thank our local pathologists (William Cook, Lynn Cornell, Gretchen Crary, Ian Gibson, Donna Lager, Ramesh Nair, Behzad Najafian, Kim Solez) who are playing a critical role in this study and Stephanie Daily and Wendy Bailey for their help in the preparation of the manuscript. This work was supported by NIH funding 5U01AI058013.
References
- 1.Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44(2):411–22. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- 2.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 3.Cosio FG, Grande JP, Larson TS, Gloor JM, Velosa JA, Textor SC, et al. Kidney allograft fibrosis and atrophy early after living donor transplantation. Am J Transplant. 2005;5(5):1130–6. doi: 10.1111/j.1600-6143.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 4.Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5(10):2464–72. doi: 10.1111/j.1600-6143.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 5.Moreso F, Ibernon M, Goma M, Carrera M, Fulladosa X, Hueso M, et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant. 2006;6(4):747–52. doi: 10.1111/j.1600-6143.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 6.Shishido S, Asanuma H, Nakai H, Mori Y, Satoh H, Kamimaki I, et al. The Impact of Repeated Subclinical Acute Rejection on the Progression of Chronic Allograft Nephropathy. J Am Soc Nephrol. 2003 Apr 1;14(4):1046–52. doi: 10.1097/01.asn.0000056189.02819.32. [DOI] [PubMed] [Google Scholar]
- 7.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR, Allen RD. Delta analysis of posttransplantation tubulointerstitial damage. Transplantation. 2004;78(3):434–41. doi: 10.1097/01.tp.0000128613.74683.d9. [DOI] [PubMed] [Google Scholar]
- 8.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 9.Mengel M, Reeve J, Bunnag S, Einecke G, Jhangri GS, Sis B, et al. Scoring total inflammation is superior to the current Banff inflammation score in predicting outcome and the degree of molecular disturbance in renal allografts. Am J Transplant. 2009;9(8):1859–67. doi: 10.1111/j.1600-6143.2009.02727.x. [DOI] [PubMed] [Google Scholar]
- 10.Mengel M, Reeve J, Bunnag S, Einecke G, Sis B, Mueller T, et al. Molecular correlates of scarring in kidney transplants: the emergence of mast cell transcripts. Am J Transplant. 2009;9(1):169–78. doi: 10.1111/j.1600-6143.2008.02462.x. [DOI] [PubMed] [Google Scholar]
- 11.Gourishankar S, Leduc R, Connett J, Cecka JM, Cosio F, Fieberg AM, et al. Pathological and Clinical Characterization of the “Troubled Transplant”: Data from the DeKAF Study. American Journal of Transplantation. 2010;10(3):315–323. doi: 10.1111/j.1600-6143.2009.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3(6):708–14. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 13.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009 Nov;9(11):2520–31. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo LG, Campbell PM, Sis B, Einecke G, Mengel M, Chang J, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9(11):2532–41. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 15.Saadi S, Wrenshall LE, Platt JL. Regional manifestations and control of the immune system. FASEB J. 2002;16(8):849–56. doi: 10.1096/fj.01-0690hyp. [DOI] [PubMed] [Google Scholar]


