Abstract
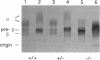
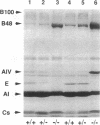
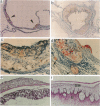
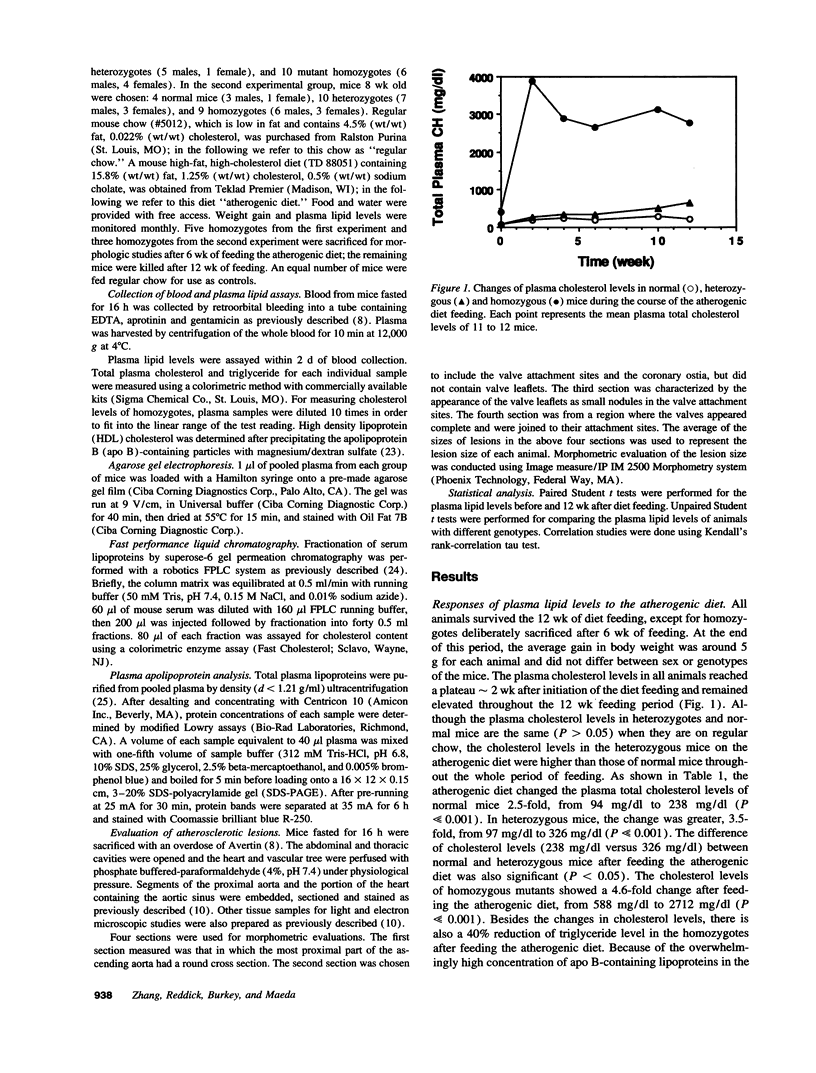
With the aim of establishing whether a genetically reduced capability of producing apolipoprotein E (apo E) can affect atherogenesis, we have compared the consequences of dietary stress on normal mice and on mice heterozygous or homozygous for a disrupted apo E gene. A dramatically accelerated development of lesions occurred in the vasculature of the homozygous mutants as a result of feeding an atherogenic diet for 12 wk, and extensive deposition of lipid-filled macrophages was found outside the cardiovascular system. In nine heterozygotes fed the atherogenic diet for 12 wk, the amount of apo E in their total plasma lipoproteins increased to a level comparable to normal, but all nine developed much larger foam cell lesions in their proximal aorta than those found in 3 of 9 normal mice fed the same diet. The other six normals had no lesions. Our study demonstrates that heterozygous mice with only one functional apo E gene are more susceptible to diet-induced atherosclerosis than are normal, two-copy mice. Genetically determined quantitative limitations of apo E could, therefore, have similar effects in humans when they are stressed by an atherogenic diet.
Full text
PDF
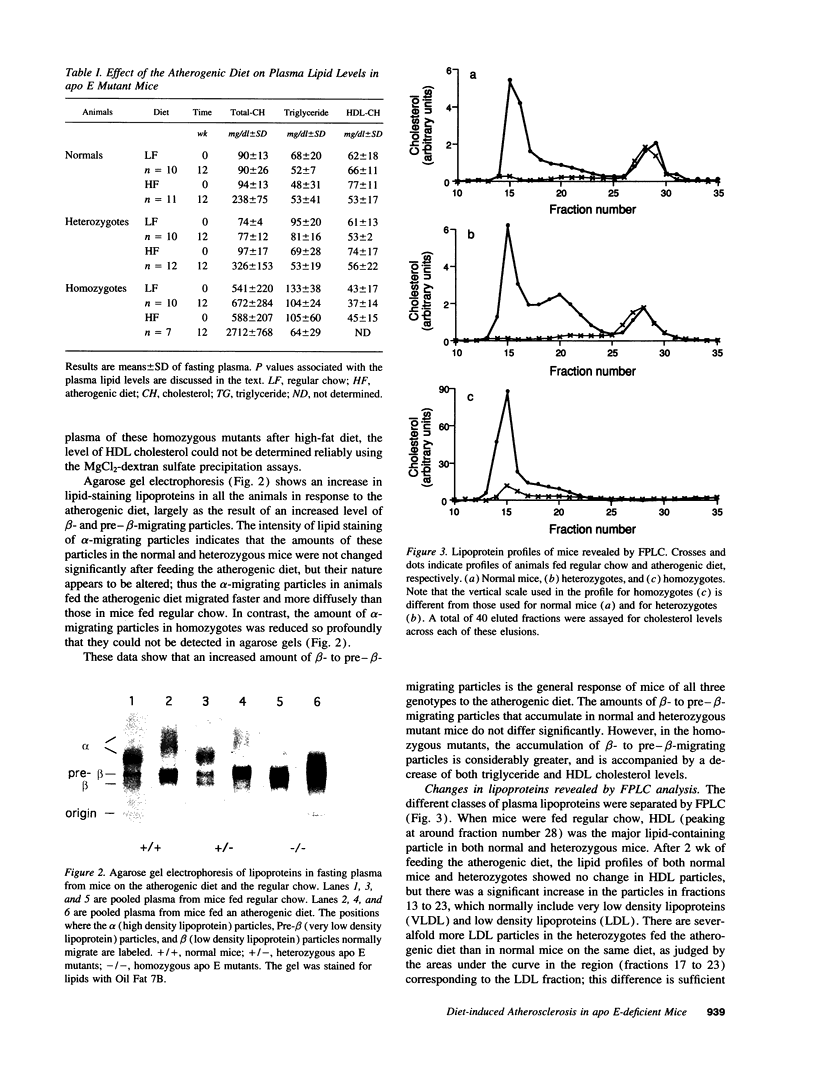

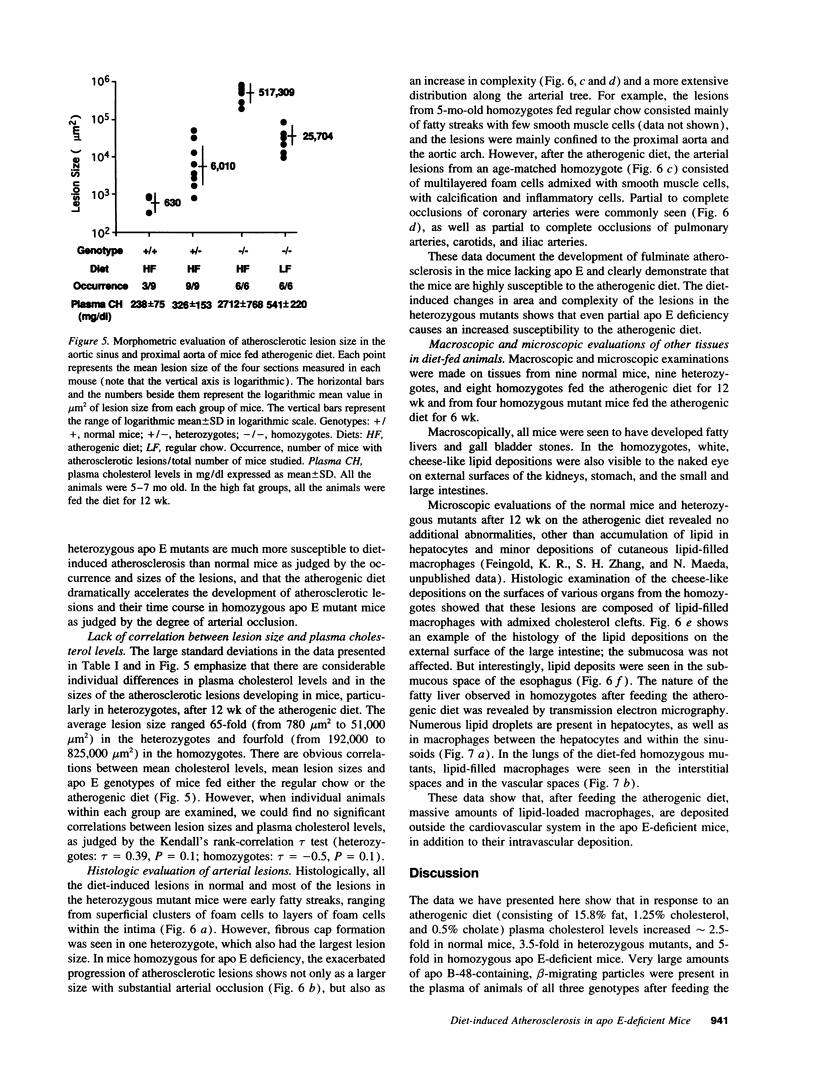
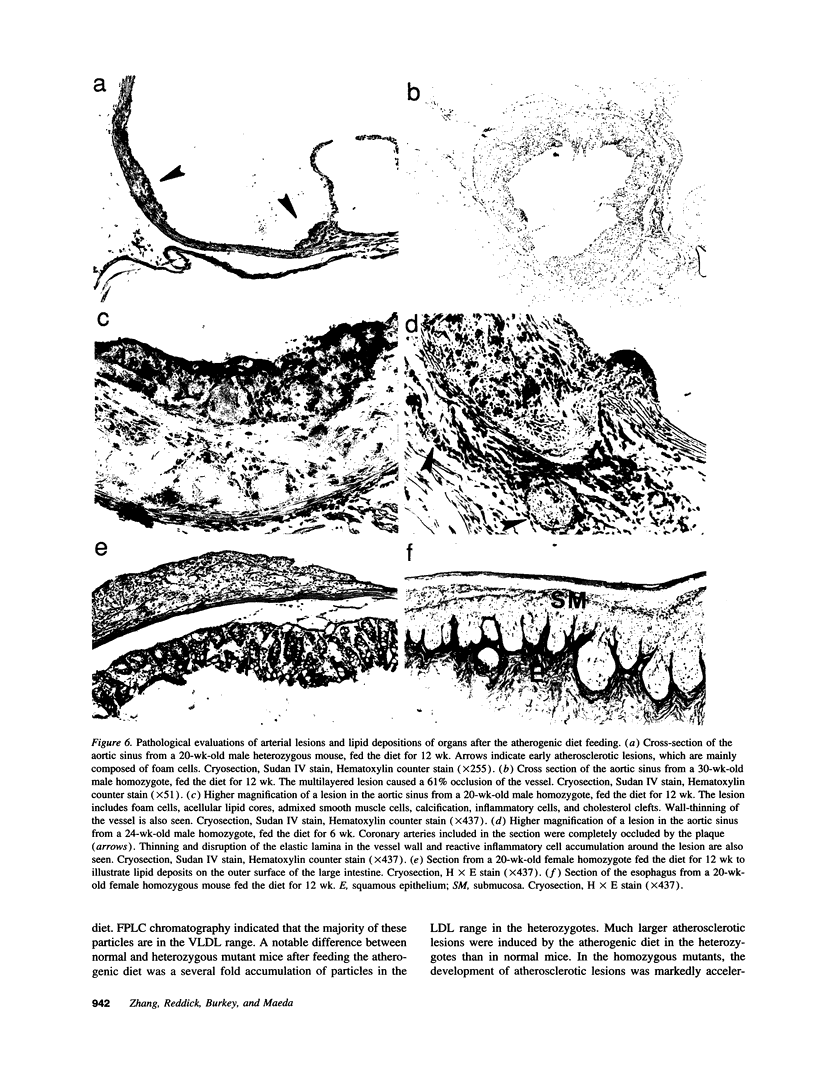



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. B., Hoover R. L., Berry K. K., Swift L. L. Cholesterol-fed heterozygous Watanabe heritable hyperlipidemic rabbits: a new model for atherosclerosis. Atherosclerosis. 1989 Aug;78(2-3):123–136. doi: 10.1016/0021-9150(89)90216-5. [DOI] [PubMed] [Google Scholar]
- Boerwinkle E., Utermann G. Simultaneous effects of the apolipoprotein E polymorphism on apolipoprotein E, apolipoprotein B, and cholesterol metabolism. Am J Hum Genet. 1988 Jan;42(1):104–112. [PMC free article] [PubMed] [Google Scholar]
- Brecher P., Chobanian A. V., Small D. M., Van Sickle W., Tercyak A., Lazzari A., Baler J. Relationship of an abnormal plasma lipoprotein to protection from atherosclerosis in the cholesterol-fed diabetic rabbit. J Clin Invest. 1983 Nov;72(5):1553–1562. doi: 10.1172/JCI111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow J. L. Transgenic mouse models of lipoprotein metabolism and atherosclerosis. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8314–8318. doi: 10.1073/pnas.90.18.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook D., Weisgraber K. H., Boyles J. K., Mahley R. W. Isolation and characterization of plasma lipoproteins of common marmoset monkey. Comparison of effects of control and atherogenic diets. Arteriosclerosis. 1990 Jul-Aug;10(4):633–647. doi: 10.1161/01.atv.10.4.633. [DOI] [PubMed] [Google Scholar]
- Cumming A. M., Robertson F. W. Polymorphism at the apoprotein-E locus in relation to risk of coronary disease. Clin Genet. 1984 Apr;25(4):310–313. doi: 10.1111/j.1399-0004.1984.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Oida K., Sobel B. E., Schonfeld G. Dependence of metabolic and structural heterogeneity of cholesterol ester-rich very low density lipoproteins on the duration of cholesterol feeding in rabbits. J Clin Invest. 1988 Aug;82(2):562–570. doi: 10.1172/JCI113633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainaru M., Mahley R. W., Hamilton R. L., Innerarity T. L. Structural and metabolic heterogeneity of beta-very low density lipoproteins from cholesterol-fed dogs and from humans with type III hyperlipoproteinemia. J Lipid Res. 1982 Jul;23(5):702–714. [PubMed] [Google Scholar]
- Flynn M. A., Nolph G. B., Sun G. Y., Navidi M., Krause G. Effects of cholesterol and fat modification of self-selected diets on serum lipids and their specific fatty acids in normocholesterolemic and hypercholesterolemic humans. J Am Coll Nutr. 1991 Apr;10(2):93–106. doi: 10.1080/07315724.1991.10718132. [DOI] [PubMed] [Google Scholar]
- Gabelli C., Gregg R. E., Zech L. A., Manzato E., Brewer H. B., Jr Abnormal low density lipoprotein metabolism in apolipoprotein E deficiency. J Lipid Res. 1986 Mar;27(3):326–333. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele R. A. Gene-environment interactions in atherosclerosis. Mol Cell Biochem. 1992 Aug 18;113(2):177–186. doi: 10.1007/BF00231537. [DOI] [PubMed] [Google Scholar]
- Hixson J. E. Apolipoprotein E polymorphisms affect atherosclerosis in young males. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb. 1991 Sep-Oct;11(5):1237–1244. doi: 10.1161/01.atv.11.5.1237. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Brown M. S., Goldstein J. L., Gerard R. D., Hammer R. E., Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993 Aug;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida B. Y., Blanche P. J., Nichols A. V., Yashar M., Paigen B. Effects of atherogenic diet consumption on lipoproteins in mouse strains C57BL/6 and C3H. J Lipid Res. 1991 Apr;32(4):559–568. [PubMed] [Google Scholar]
- Keidar S., Kaplan M., Rosenblat M., Brook G. J., Aviram M. Apolipoprotein E and lipoprotein lipase reduce macrophage degradation of oxidized very-low-density lipoprotein (VLDL), but increase cellular degradation of native VLDL. Metabolism. 1992 Nov;41(11):1185–1192. doi: 10.1016/0026-0495(92)90007-w. [DOI] [PubMed] [Google Scholar]
- Kurosaka D., Teramoto T., Matsushima T., Yokoyama T., Yamada A., Aikawa T., Miyamoto Y., Kurokawa K. Apolipoprotein E deficiency with a depressed mRNA of normal size. Atherosclerosis. 1991 May;88(1):15–20. doi: 10.1016/0021-9150(91)90252-x. [DOI] [PubMed] [Google Scholar]
- Mabuchi H., Itoh H., Takeda M., Kajinami K., Wakasugi T., Koizumi J., Takeda R., Asagami C. A young type III hyperlipoproteinemic patient associated with apolipoprotein E deficiency. Metabolism. 1989 Feb;38(2):115–119. doi: 10.1016/0026-0495(89)90249-7. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A., Gylling H., Vanhanen H. Serum cholesterol response to dietary cholesterol and apoprotein E phenotype. Lancet. 1988 Nov 26;2(8622):1261–1261. doi: 10.1016/s0140-6736(88)90862-8. [DOI] [PubMed] [Google Scholar]
- Mänttäri M., Koskinen P., Ehnholm C., Huttunen J. K., Manninen V. Apolipoprotein E polymorphism influences the serum cholesterol response to dietary intervention. Metabolism. 1991 Feb;40(2):217–221. doi: 10.1016/0026-0495(91)90179-z. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994 Jan;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Reddick R. L., Zhang S. H., Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994 Jan;14(1):141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Savolainen M. J., Rantala M., Kervinen K., Järvi L., Suvanto K., Rantala T., Kesäniemi Y. A. Magnitude of dietary effects on plasma cholesterol concentration: role of sex and apolipoprotein E phenotype. Atherosclerosis. 1991 Feb;86(2-3):145–152. doi: 10.1016/0021-9150(91)90210-t. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Witztum J. L. Lipoproteins and atherogenesis. Current concepts. JAMA. 1990 Dec 19;264(23):3047–3052. [PubMed] [Google Scholar]
- THOMAS W. A., HARTROFT W. S. Myocardial infarction in rats fed diets containing high fat, cholesterol, thiouracil, and sodium cholate. Circulation. 1959 Jan;19(1):65–72. doi: 10.1161/01.cir.19.1.65. [DOI] [PubMed] [Google Scholar]
- Tikkanen M. J., Huttunen J. K., Ehnholm C., Pietinen P. Apolipoprotein E4 homozygosity predisposes to serum cholesterol elevation during high fat diet. Arteriosclerosis. 1990 Mar-Apr;10(2):285–288. doi: 10.1161/01.atv.10.2.285. [DOI] [PubMed] [Google Scholar]
- Warnick G. R., Benderson J., Albers J. J. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982 Jun;28(6):1379–1388. [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zilversmit D. B. Atherogenesis: a postprandial phenomenon. Circulation. 1979 Sep;60(3):473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]