Abstract
The undifferentiated spermatogonia of adult mouse testes are composed of both true stem cells and committed progenitors. It is unclear what normally prevents these adult germ cells from manifesting multipotency. The critical elements of the spermatogonial stem cell niche, while poorly understood, are thought to be composed of Sertoli cells with several other somatic cell types in close proximity. We recently discovered a novel orphan G-protein coupled receptor (GPR125) that is restricted to undifferentiated spermatogonia within the testis. GPR125 expression was maintained when the progenitor cells were extracted from the in vivo niche and propagated under growth conditions that recapitulate key elements of the niche. Such conditions preserved the ability of the cells to generate multipotent derivatives, known as multipotent adult spermatogonial derived progenitor cells (MASCs). Upon differentiation, the latter produced a variety tissues including functional endothelium, illustrating the potential applications of such cells. Thus, GPR125 represents a novel target for purifying adult stem and progenitors from tissues, with the goal of developing autologous multipotent cell lines.
Keywords: GPR125, spermatogonia, testis, multipotent, angiogenesis, stem, MASC, SSC, progenitor, GPCR
Introduction
Mammalian spermatogonial stem cells (SSCs) in vivo reside in a niche comprised of Sertoli cells, peritubular cells, and a complex array of matrix proteins. Through signals provided by adhesion molecules and other cell surface receptors, the normal SSC pool is maintained throughout adulthood. The essential nature of such interactions is underscored by dramatic phenotypes observed in mouse gene knockout studies (e.g., for GDNF or ERM).1,2 It is a matter of speculation about whether cell types other than Sertoli cells, such as endothelial cells or tissue macrophages, can make transient direct contact with SSCs and influence the cell fate decision about whether to remain quiescent, to self-renew, or to begin the process of differentiation. However, the fact that self-renewing SSCs can be propagated in vitro implies that certain critical elements of the niche can be simulated using a culture system.
The stem cells and the most primitive type A spermatogonia in vivo are collectively referred to as undifferentiated spermatogonia. Historically, the hierarchy of germ cell types in the adult testis was delineated using morphological criteria.3,4 However, recent evidence obtained using genetic lineage tracing has suggested that the concept of the spermatogonial stem cell as the only long-term self-renewing cell type may be too rigid.5,6 We recently discovered that undifferentiated spermatogonia in vivo express a novel orphan G-protein coupled receptor known as GPR125.7 As discussed below, expression of GPR125 is maintained in long-term germ cell cultures and even after a remarkable transition—the conversion in vitro into multipotent adult spermatogonial derived stem cells (MASCs), which can form functional differentiated tissues.
The SSC Niche In Vivo
The number of SSCs in the adult mammalian testis has been estimated based on a combination of morphological studies and transplantation experiments.8–10 However, due to the indirect nature of these measurements, the exact number is unknown. Also, the utility of morphologic criteria, such as the significance of spermatogonia in chains,4 has recently been questioned.11,12 The most immature spermatogonial population, containing the putative stem cells, resides along the basement membrane of the seminiferous tubule,13 with a large fraction of the cell membrane in direct contact with the Sertoli cell.14 A smaller fraction of the spermatogonial cell membrane is in direct contact with extracellular matrix components (e.g., laminin) of the basement membrane or possibly the peritubular cells.14 While the role of peritubular cells in stem cell homeostasis is unclear, the important role of Sertoli cells is well documented.
Several lines of genetic evidence reveal a crucial role of Sertoli cell derived factors. Glial cell line-derived neurotrophic factor (GDNF) is a key germ cell growth factor produced by Sertoli cells in vivo.1 Remarkably, the loss of even a single allele of GDNF results in a significant disruption of spermatogonial cell proliferation in mice,1 consistent with the dramatic effect of GDNF on SSCs grown in vitro (see below).15 In mice lacking the ets-related transcription factor ERM, the SSCs are rapidly lost at maturity.2 In that study, expression of ERM was found to be exclusively in the somatic (Sertoli) cell compartment,2 suggesting the germline stem cell loss was entirely dependent on a non-cell autonomous niche effect. However, these results were recently called into question when ERM was found not only in Sertoli cells but also in germ cells both in vitro and in vivo.16
While the functional borders of the SSC niche have been historically viewed as confined within the seminiferous tubule, recent data suggest that factors extrinsic to the individual tubule may be quite important. Using a genetic fluorescent reporter system, Yoshida et al. (2007) recently examined the distribution of ngn3-positive undifferentiated spermatogonia, comprising the SSCs, relative to interstitial components.17 They found a striking correlation between the location of intertubular blood vessels and undifferentiated spermatogonia, such that the latter preferentially localized adjacent to blood vessels. Although neither the mechanisms nor functional significance for these phenomena are known, the link between endothelial cells and SSCs is intriguing. This concept closely parallels the evolving notion that other adult stem cells, including hematopoietic and neural stem cells reside in what has been termed the “vascular niche.”18–20
In Vitro Culture of Germline Stem and Progenitor Cells
Recent technological advances combining different culture media, growth factors, and feeders cells have enabled the study of SSCs using in vitro culture systems.15,21 However, the fraction of spermatogonia in long-term culture that retain spermatogenesis reconstituting activity upon transplantation is very low.22 At the same time, it has been found that spermatogonial progenitor cells with transit-amplifying activity in vivo can change their fate and function as actual stem cells in situations in which stem cell loss is induced, such as after busulfan treatment.12,6 Based on these data, we employ the designation of spermatogonial progenitor cells (SPCs) to refer primarily to the pool of undifferentiated spermatogonia that retain long-term clonagenic self-renewal potential in vitro and transplantation ability in vivo. A key feature of this population is the maintenance of GPR125 expression in vitro (see below).
It was previously thought that testicular somatic cells had an unequivocally deleterious effect on SSC culture by inducing cell differentiation.23 Therefore, a large effort has been undertaken by various groups to develop means for isolating fresh spermatogonial stem cells from testes of different ages. These efforts have ranged from utilizing cell surface proteins, such as α6 integrin, CD9 or GFRα1, to functional properties of stem cells, such as side population activity.24–27 For the purposes of immediate analysis of freshly purified SSCs, it is abundantly clear that multiple surface markers are required to obtain a highly enriched population. However, the case for derivation of adult SPC lines is quite different.
While somatic cells contaminating juvenile testis cultures can easily out-compete germline stem cells, we have found, in contrast, that the use of mitomycin-inactivated testicular somatic feeder cells greatly facilitates the derivation of adult SPC lines. Whereas prior studies have found a 30–50% efficiency of deriving adult germline stem cell lines using either from unselected testicular cells28 or using CD9 selection29 by employing mouse embryo fibroblast (MEF) feeders, we were unable to initiate similar cultures using MEF in conjunction with a previously described highly-supplemented growth medium.15
We therefore tested the hypothesis that testicular somatic cell-derived factors supporting stem cell maintenance and proliferation would dominate over those driving differentiation or apoptosis. To this end, we developed primary adult mouse testicular stroma (mTS) cell cultures using high serum medium (Fig. 1). Under high serum conditions, the germ cell colony formation is suppressed.22 The outgrowth contains a mixed population of CD34+, α-smooth muscle actin+ and vimentin+ cells, although the true fraction of CD34+ cells is difficult to estimate due to the cyclic nature of CD34 expression in vitro.30 Prior to use as feeders, the mTS were then inactivated in a similar fashion to MEFs, using mitomycin-C, which inhibits cell proliferation. By plating fresh adult testicular cell suspensions, on the inactivated mTS, we were able to derive long-term SPC lines in >90% of attempts. Using ubiquitously GFP-labeled testicular cells to derive the SPCs, we determined that serial passaging resulted in near total elimination of contaminating somatic cells from the culture. While the functional significance of CD34 expression on cultured mTS is not currently known, we have confirmed the intriguing results of Kuroda et al. (2004), who found that CD34+ stromal cells exist in close association with peritubular myoid cells in vivo and that peritubular myoid cells may also express CD34.31
Figure 1.
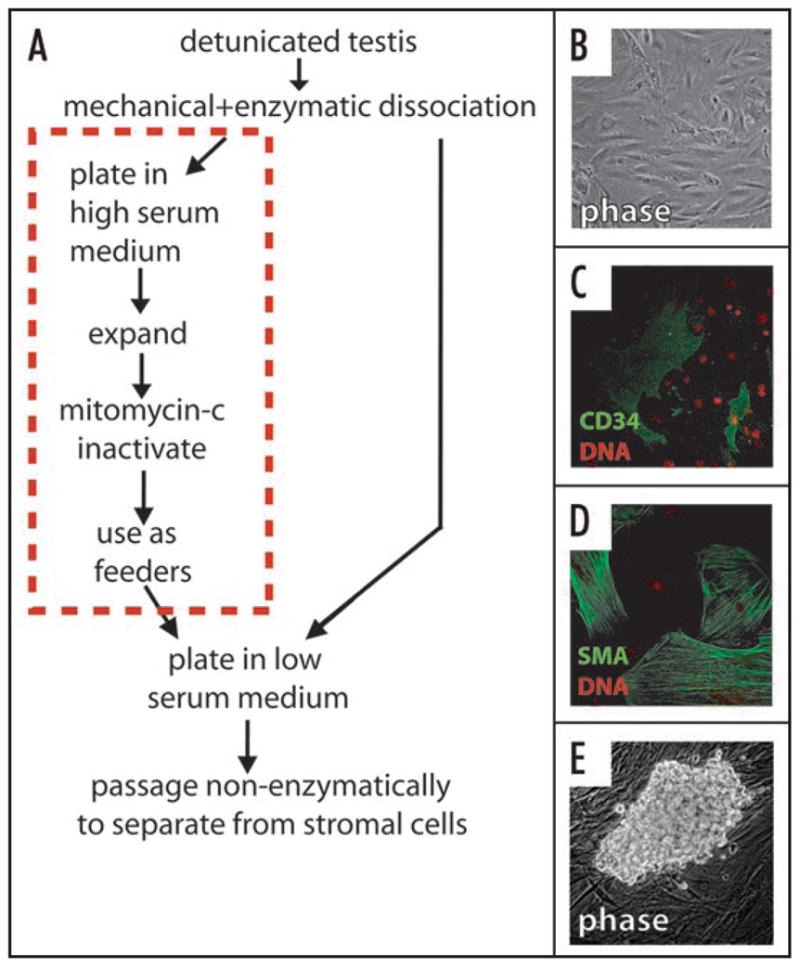
Derivation of spermatogonial progenitor cell (SPC) cultures using testicular stroma. (A) Testes are mechanically and enzymatically dissociated. Stromal cultures are prepared by expansion in high serum medium and inactivated with mitomycin-C before use as feeders. SPCs are derived on the inactivated stroma in low serum medium by serial non-enzymatic passaging to dilute out contaminating somatic cells. (B–D) Testicular stroma is characterized by morphologically heterogeneous adherent cells (B), containing CD34+ cells (C) and α-smooth muscle actin+ cells (D). Robust SPC colonies formed with in several weeks of first plating (E).
Our experience with deriving and propagating SPCs differs significantly from certain other published studies involving germline stem cell cultures. While others have found that SSCs can be propagated under feeder-free conditions,22 our cultures underwent massive apoptosis within 10–14 days of removal from feeders (unpublished data). Multiple studies have found GDNF to be important for SSC culture,15,32 although a recent study contradicted this.33 In the latter study, however, it is not clear whether long-term cultures of in vitro and in vivo repopulating germline stem cells were derived. In contrast, the in vitro self-renewal potential of SPCs in our hands was lost over 10–14 days if GDNF was omitted but SPCs could be propagated >1 year in the presence of GDNF (unpublished data). Therefore, SPC lines are dependent on both GDNF and somatic feeder cells for their propagation in vitro.
G-Protein Coupled Receptor 125 (GPR125) in the Testis
As discussed above, the array of known cell surface markers for SSCs is quite limited. In the course of a larger screen to identify novel G-protein coupled receptors associated with stem cells and angiogenesis, we evaluated a potential marker for undifferentiated spermatogonia, known as GPR125. A member of the adhesion family of G-protein coupled receptors,34 little was previously known about GPR125 (Tem5-like/PGR21), except that it was identified as a binding partner in vitro for human Drosophila discs large homolog (hDlg).35 Additionally, gene expression studies have identified GPR125 as downregulated in colon cancer36 or in PTEN−/− MEFs37 and associated with the CD133+ fraction of umbilical cord blood cells.38
We employed highly sensitive means of detecting GPR125 expression in vivo, by knocking the E. coli LacZ gene into the mouse GPR125 locus, downstream and in-frame with respect to the putative first extracellular and first transmembrane domains, such that the full, endogenous complement of regulatory elements drive expression of the reporter (Fig. 2).7,39 In GPR125-LacZ testes, GPR125 was expressed only within the seminiferous tubules from the neonatal period to adulthood (Fig. 3). In pre-pubertal and adult tubules, expression was restricted to a zone along the basement membrane, in close proximity to peritubular cells and CD34+ stromal cells. This raised the possibility that GPR125 is expressed on SSCs. To assess this, we developed long term cultures of GPR125-LacZ SPCs and found that they maintained GPR125 expression even after months in culture and even after cloning of single cells. Importantly, these cells could reconstitute spermatogenesis when transplanted into busulfan-treated mice and also maintained GPR125 expression under those conditions.
Figure 2.
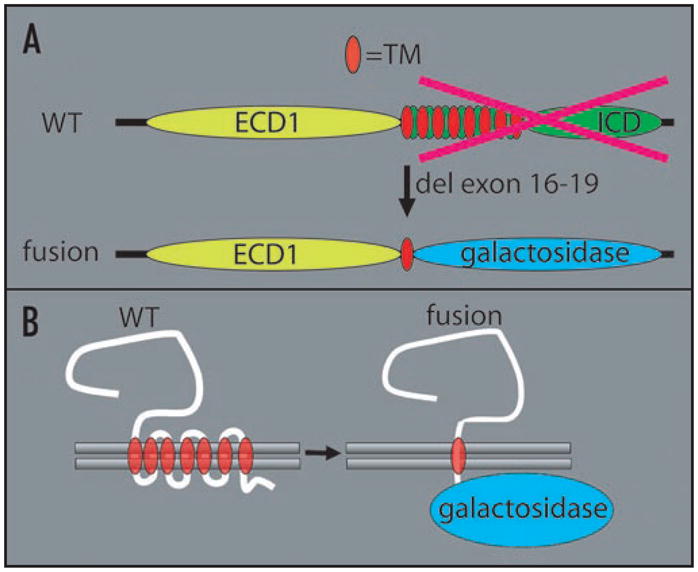
Structure of wild type GPR125 and engineered N-terminally truncated GPR125 fused to β-galactosidase in GPR125-LacZ mice. (A) Only the first extracellular domain (ECD1) and the first transmembrane domain (TM) of GPR125 are retained in the in-frame fusion to β-galactosidase, with exons 16–19 of the gene deleted. (B) Schematic of putative structure of WT GPR125 and GPR125-LacZ in the plasma membrane.
Figure 3.
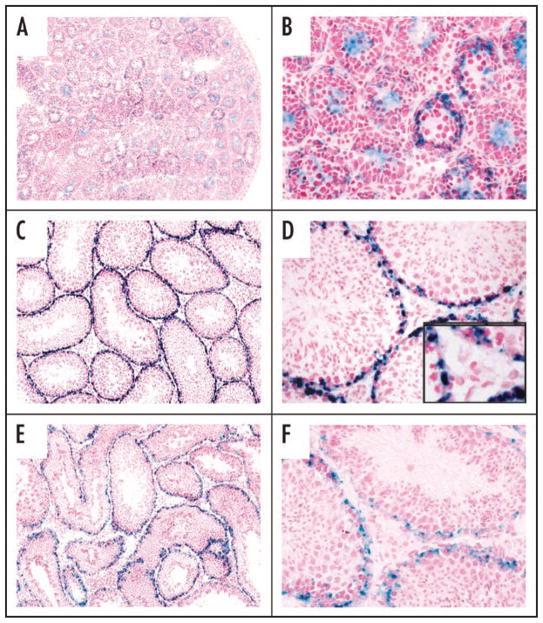
Restricted expression of GPR125 along the tubular basement membrane in GPR125-lacZ mouse testes. X-gal staining was employed for detection of β-galacto-sidase in cryosections take from post-natal day 7 (A and B), day 21 (C and D), and adult testes (E and F). Staining was seen only in areas occupied by undifferentiated spermatogonia. No staining was seen in the intertubular instititium or in WT negative controls (not shown).
Multipotency in Adult Mammalian Germ Line Stem Cells
The ability of normal post-natal germline stem cells to exhibit pluripotency was first identified in neonatal cultures.29 It was subsequently speculated that the failure of previous efforts to obtain pluripotent cells from cultured germ cells was due to the use of heterogeneous starting material.40 We found that oct4+ MASCs arose in culture among SPCs after several months of passaging. MASCs were competent in formation of classical teratomas in NOD-SCID mice and could contribute to embryonic chimeras when microinjected into early blastocysts (see Fig. 4). The appearance of MASCs in SPC cultures occurred in spite of the heterogeneous nature of the testicular cells plated at the initiation of the culture (passage zero). Furthermore, as noted above, the MASCs, which also express GPR125, appeared in the course of expanding SPCs on mTS, which itself is composed of somatic testicular cells. Taken together, these data suggest that the prior failure to obtain multipotent cell lines from germline stem cell cultures is not due to the presence of inhibitory somatic cell-derived factors. On the contrary, our data suggest that the presence of contaminating somatic cells in early passages of SSCs preserves the inherent cellular potential for multipotency.
Figure 4.
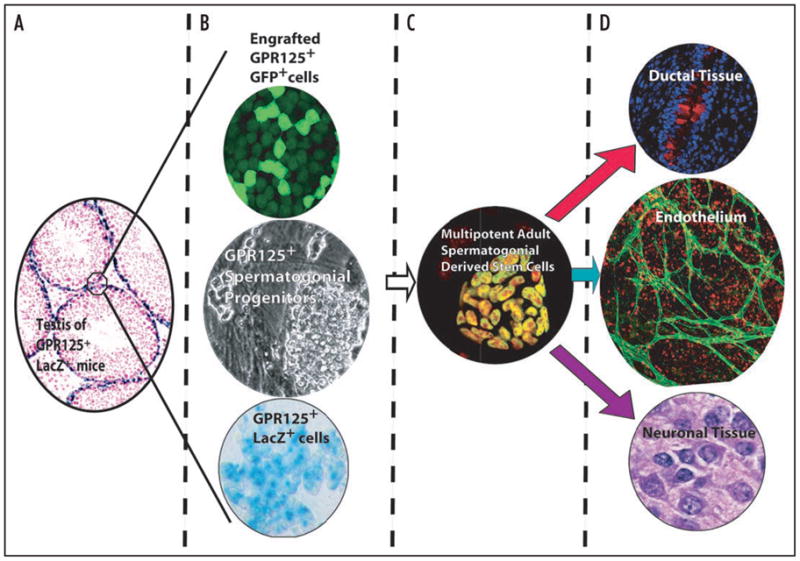
Schematic showing origin and multipotency of multipotent spermatogonial derived stem cells (MASCs) from GPR125+ SPCs (adapted from Seandel et al. [Nature, 2007])7. GPR125 expression (blue-green) staining is maintained when SPCs are extracted from the niche in vivo (A) and expanded in vitro (B, middle and bottom). Transplanted GFP-labeled GPR125+ SPCs repopulated busulfan-treated WT mouse testes (B, top). (C) MASCs formed spontaneously in vitro after several months of SPC culture and exhibited nuclear oct4 protein (green stain with red nuclear counterstain). (D) MASCs differentiated into derivates of all three germ layers in vitro and in vivo. Bronchial epithelium stained for mucin (red) in upper panel. Endothelium stained with anti-VE-cadherin (green, middle). Morphologically neuronal-type tissue stained with H&E is depicted in the lower panel and contained GFAP+ and NeuN+ cells (not shown).
Gene expression microarray analysis and subsequent quantitative PCR revealed that MASCs strongly express certain canonical embryonic stem cell-associated multipotency genes, such as oct4, nanog and sox2 but not others (e.g., rex1, esg1 and gdf3). These data suggest that MASCs have both significant molecular similarities and differences compared to embryonic stem cells. Nonetheless, the mechanisms by which SPC cultures give rise to MASCs and by which the latter maintain their multipotent phenotype are not known.
A recent study observed the appearance of multipotent embryonic-like stem cells following short term culture of Stra8-GFP selected testicular cells.33 While our data, too, support the concept that adult male germ cells, like neonatal germ cells, are predisposed to multipotency, there are several major differences between the prior study and ours, complicating the direct comparison of MASCs and maGSCs. (1) We employed a reporter driven by the full endogenous promoter (for GPR125) and all of its associated regulatory factors, as opposed to a transgenic promoter fragment. (2) The GDNF-dependant SPCs had been in long-term culture (greater than or equal to three months) prior to the appearance of MASCs. (3) Unlike the maGSCs described in the prior study, MASCs contain nuclear (not cytoplasmic) oct4. Given these differences, it is difficult to make a direct comparison between MASCs and maGSCs, although both are multipotent and could be applied toward studies of tissue/organ regeneration. However, future studies of human SSCs or SPCs and MASCs will necessitate use of molecular markers that are present on the cell surface (as opposed to transcription factors), since surface molecules are amenable for cell sorting. Hence, GPR125 is an attractive target molecule, especially for species like humans for which transgenic strategies are not available.
Therapeutic Implementation of MASCs
Recent proof-of-principle for the therapeutic use of germ cell-derived multipotent cells has been published. Baba et al. (2007) utilized an OP9 co-culture system to compare mGS, ES and embryonic germ cells and found a comparable yield in vitro of mesodermal derivatives including endothelial and cardiac cells from mGS, although the derivative cell types were not assessed for function in vivo.41 Another study found that maGSCs could form functional cardiac cells in vitro but the fate of these cells in vivo is less clear since only a transient fluorescent cell label (CM-DiI) was employed.42 We similarly found that MASCs spontaneously differentiate into mesodermal derivatives, including contractile cardiac tissue. To assess endothelial differentiation, we produced a lentiviral construct in which GFP expression is driven by a fragment of the endothelial-specific VE-cadherin promoter (VE-cad-GFP). By transducing MASCs with the VE-cad-GFP lentivirus, emergence of endothelial cells during differentiation could be easily visualized (see Fig. 4). To demonstrate functionality of donor-derived endothelial cells in vivo, we formed teratomas with VE-cad-GFP MASCs in NOD-SCID mice and, prior to sacrifice of the animals, labeled the circulatory system by perfusion with a red fluorophore-conjugated lectin. Confocal analysis of teratoma tissue revealed vessels that were GFP+ and bound by lectin, confirming the presence of functional MASC-derived blood vessels.
Conclusions and Open Questions
Spermatogonial stem cells in the testis are localized at the border between the tubular lumen and the interstitial space and are therefore exposed to signals coming from both sides of the basement membrane. Inside the tubule, Sertoli cells have a major role in regulating SSC fate by production of factors such as GDNF and ERM.1,2 In parallel, evidence has emerged that intertubular blood vessels or surrounding Leydig cells also have a role. For example, it has been shown by live imaging that undifferentiated spermatogonia start proliferating in close proximity to the blood vessels but then move away from them when differentiation takes place.17
We partially reproduced in vitro the spermatogonial stem cell niche, by using a mixed culture of testicular somatic cells in which cells expressing α-actin, CD34 and vimentin are present (see Fig. 4). Such feeders induce proliferation of undifferentiated (c-kit-negative) spermatogonia (including SSCs) that, in these conditions, can self-renew and reconstitute spermatogenesis when transplanted into sterile recipients. Employing the same feeders, we also observed spontaneous formation of multipotent cells from SPC cultures. Most likely, these multipotent cells (MASCs) originate by conversion from SPCs (which are, by definition, of germline origin), since the appearance of MASCs occurred after several passages, when virtually all contaminating somatic cells had disappeared from the cultures. Moreover, all the colonies show the typical appearance of SPCs before conversion, and we observed a morphologic change in some SPC colonies during the conversion (transitional colonies) and both SPCs and MASCs express GPR125. However, a final proof of the origin of MASCs will require their derivation from single-cell subcloned SPCs with concomitant lineage tracing, to rule out the possibility that contaminating somatic cells, which theoretically persist as dormant/quiescent cells for many months in culture—and after serial passaging—are the actual source of MASCs, an explanation that we believe is very unlikely.
It is important to highlight that although MASCs share some pluripotency-related genes with ESC, certain others (e.g., rex1, esg1 and gdf3) are either low or undetectable in MASCs. This finding distinguishes MASCs also from neonatal GS cells and adult maGSCs.29,33 The significance of these differences is not known, although the data suggest that MASCs constitute an unique category of multipotent cell. It is possible that maGSCs and MASC are actually derived from different cells. This hypothesis is supported by the fact that the culture conditions used for deriving maGSCs are quite different and that maGSC precursors do not seem to require GDNF for their in vitro maintenance, while SPCs are completely dependent on GDNF and undergo cell death when this cytokine is withdrawn. An alternative hypothesis is that the differences in the culture conditions used to generate maGSCs and MASCs have influenced the outcome of the conversion process, even though they originate from a common precursor. If pluripotent or multipotent cells derived from the testis really reflect the consequence of reprogramming of SSCs to an embryonic phenotype, one can hypothesize that this conversion involves a multi-step process that can be either partial or complete. In other words, MASCs could represent an intermediate form between SSCs and ESCs, and therefore only some ESC genes may have been transcriptionally activated. Even if the precise origin of testis-derived pluri/multipotent cells is not fully established yet, recent evidence suggests that they offer a viable alternative to ESCs for regenerative medicine. In this respect, GPR125 represents a good candidate for the prospective isolation of SPCs from human testis.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute, the Ansary Center for Stem Cell Therapeutics, a Memorial Sloan Kettering Cancer Center T32 grant (M.S.), an AACR—Genentech BioOncology Fellowship for Cancer Research on Angiogenesis (M.S.), and National Heart, Lung and Blood Institute grants (S.R.).
Footnotes
Financial disclosure
Authors M.S. and S.R. have filed a patent application related to elements of this work.
References
- 1.Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 2.Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM, Hassell JA, Chandrashekar V, Hofmann MC, Hess RA, Murphy KM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–4. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huckins C, Clermont Y. Evolution of gonocytes in the rat testis during late embryonic and early post-natal life. Arch Anat Histol Embryol. 1968;51:341–54. [PubMed] [Google Scholar]
- 4.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–98. [PubMed] [Google Scholar]
- 5.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Simon A, Frisen J. From stem cell to progenitor and back again. Cell. 2007;128:825–6. doi: 10.1016/j.cell.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, Yancopoulos GD, Murphy A, Valenzuela DM, Hobbs RM, Pandolfi PP, Rafii S. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–50. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod. 2003;69:701–7. doi: 10.1095/biolreprod.103.016352. [DOI] [PubMed] [Google Scholar]
- 9.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 10.Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec. 1971;169:533–57. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 11.Seydoux G, Braun RE. Pathway to totipotency: lessons from germ cells. Cell. 2006;127:891–904. doi: 10.1016/j.cell.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Ohta H, Yomogida K, Dohmae K, Nishimune Y. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127:2125–31. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- 14.Hess RA, Cooke PS, Hofmann MC, Murphy KM. Mechanistic insights into the regulation of the spermatogonial stem cell niche. Cell Cycle. 2006;5:1164–70. doi: 10.4161/cc.5.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 16.Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842–51. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–6. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 18.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda ) 2005;20:349–56. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71:722–31. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 22.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–91. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 23.Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68:2207–14. doi: 10.1095/biolreprod.102.014050. [DOI] [PubMed] [Google Scholar]
- 24.Falciatori I, Borsellino G, Haliassos N, Boitani C, Corallini S, Battistini L, Bernardi G, Stefanini M, Vicini E. Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. FASEB J. 2004;18:376–8. doi: 10.1096/fj.03-0744fje. [DOI] [PubMed] [Google Scholar]
- 25.Buageaw A, Sukhwani M, Ben Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011–6. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- 26.Kanatsu-Shinohara M, Toyokuni S, Shinohara T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol Reprod. 2004;70:70–5. doi: 10.1095/biolreprod.103.020867. [DOI] [PubMed] [Google Scholar]
- 27.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96:5504–9. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa T, Ohmura M, Tamura Y, Kita K, Ohbo K, Suda T, Kubota Y. Derivation and morphological characterization of mouse spermatogonial stem cell lines. Arch Histol Cytol. 2004;67:297–306. doi: 10.1679/aohc.67.297. [DOI] [PubMed] [Google Scholar]
- 29.Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–12. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Dooley DC, Oppenlander BK, Xiao M. Analysis of primitive. Stem Cells. 2004;22:556–69. doi: 10.1634/stemcells.22-4-556. [DOI] [PubMed] [Google Scholar]
- 31.Kuroda N, Nakayama H, Miyazaki E, Hayashi Y, Toi M, Hiroi M, Enzan H. Distribution and role of CD34-positive stromal cells and myofibroblasts in human normal testicular stroma. Histol Histopathol. 2004;19:743–51. doi: 10.14670/HH-19.743. [DOI] [PubMed] [Google Scholar]
- 32.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–94. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 34.Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Irie K, Asada M, Mino A, Mandai K, Takai Y. Direct binding of the human homologue of the Drosophila disc large tumor suppressor gene to seven-pass transmembrane proteins, tumor endothelial marker 5 (TEM5), and a novel TEM5-like protein. Oncogene. 2004;23:3889–97. doi: 10.1038/sj.onc.1207495. [DOI] [PubMed] [Google Scholar]
- 36.Staub E, Grone J, Mennerich D, Ropcke S, Klamann I, Hinzmann B, Castanos-Velez E, Mann B, Pilarsky C, Brummendorf T, Weber B, Buhr HJ, Rosenthal A. A genome-wide map of aberrantly expressed chromosomal islands in colorectal cancer. Mol Cancer. 2006;5:37. doi: 10.1186/1476-4598-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G, Hu Y, Huo Y, Liu M, Freeman D, Gao J, Liu X, Wu DC, Wu H. PTEN deletion leads to up-regulation of a secreted growth factor pleiotrophin. J Biol Chem. 2006;281:10663–8. doi: 10.1074/jbc.M512509200. [DOI] [PubMed] [Google Scholar]
- 38.Jaatinen T, Hemmoranta H, Hautaniemi S, Niemi J, Nicorici D, Laine J, Yli-Harja O, Partanen J. Global gene expression profile of human cord blood-derived CD133+ cells. Stem Cells. 2006;24:631–41. doi: 10.1634/stemcells.2005-0185. [DOI] [PubMed] [Google Scholar]
- 39.Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, Chernomorsky R, Boucher M, Elsasser AL, Esau L, Zheng J, Griffiths JA, Wang X, Su H, Xue Y, Dominguez MG, Noguera I, Torres R, Macdonald LE, Stewart AF, DeChiara TM, Yancopoulos GD. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21:652–9. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 40.Kanatsu-Shinohara M, Shinohara T. The germ of pluripotency. Nat Biotechnol. 2006;24:663–4. doi: 10.1038/nbt0606-663. [DOI] [PubMed] [Google Scholar]
- 41.Baba S, Heike T, Umeda K, Iwasa T, Kaichi S, Hiraumi Y, Doi H, Yoshimoto M, Kanatsu-Shinohara M, Shinohara T, Nakahata T. Generation of Cardiac and Endothelial Cells from Neonatal Mouse Testis-derived Multipotent Germline Stem Cells. Stem Cells. 2007 doi: 10.1634/stemcells.2006-0574. [DOI] [PubMed] [Google Scholar]
- 42.Guan K, Wagner S, Unsold B, Maier LS, Kaiser D, Hemmerlein B, Nayernia K, Engel W, Hasenfuss G. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–25. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]