Abstract
How living systems detect the presence of genotoxic damage embedded in a million-fold excess of undamaged DNA is an unresolved question in biology. Here we have captured and structurally elucidated a base-excision DNA repair enzyme, MutM, at the stage of initial encounter with a damaged nucleobase, 8-oxoguanine (oxoG), nested within a DNA duplex. Three structures of intrahelical oxoG-encounter complexes are compared with sequence-matched structures containing a normal G base in place of an oxoG lesion. Although the protein–DNA interfaces in the matched complexes differ by only two atoms—those that distinguish oxoG from G—their pronounced structural differences indicate that MutM can detect a lesion in DNA even at the earliest stages of encounter. All-atom computer simulations show the pathway by which encounter of the enzyme with the lesion causes extrusion from the DNA duplex, and they elucidate the critical free energy difference between oxoG and G along the extrusion pathway.
Damaged nucleobases in DNA, generated spontaneously through the attack of reactive species on the genome, are an important source of carcinogenic mutations1,2. Lesion-specific DNA glycosylases catalyse removal of most aberrant nucleobases and initiate the base-excision repair pathway3,4. Searching through the vast genome to locate the exceedingly rare lesions (1 per 106–107 base pairs) without energy infusion represents an especially challenging task for DNA glycosylases, because the covalent structures of the target lesions often closely resemble their normal counterparts.
One lesion of particular interest is 8-oxoguanine (oxoG, Fig. 1a), an oxidation product of guanine5, which causes G•C to T•A transversion mutations during replication6. Despite the obvious structural similarity of oxoG to the vastly more abundant G, and its rather innocuous effects on isolated DNA helices7–10, oxoG is recognized specifically and efficiently by 8-oxoguanine DNA glycosylases, namely bacterial MutM and eukaryotic OGG1. High-resolution structures of complexes comprising MutM11 and OGG112 bound to oxoG-containing DNA and poised for base excision (lesion-recognition complexes, or LRCs) have shown that both enzymes extrude oxoG completely from the DNA helical stack and insert the extrahelical lesion into a recognition pocket on the enzyme (Fig. 1b). Importantly, structures of LRCs depicting a diverse array of damaged nucleobases bound to DNA glycosylases have established that all enzymes acting on single-nucleobase lesions employ extrahelical base excision13. These findings have raised considerable interest in understanding the sequence of events that begins with a DNA glycosylase scanning DNA and encountering a lesion, and ends with insertion of the lesion into the active site followed by catalysis of base excision. Though much is known structurally about the end stage of this process, namely extrahelical lesion recognition and excision, virtually nothing is known about the nature of the initial encounter between the enzyme and an intrahelical lesion. Here we report the structures of three complexes representing the state of initial encounter between MutM and a fully intrahelical, base-paired oxoG lesion in DNA. The clearly discernible structural differences between the encounter complexes (ECs) versus sequence-matched complexes having a target G instead of oxoG (interrogation complexes, or ICs) indicate that MutM can discriminate an oxoG lesion from its normal counterpart even at the stage of intrahelical encounter. Computational simulations enabled by such closely matched structures indicate active participation of MutM in the early steps of lesion extrusion from DNA.
Figure 1. Generation and recognition of 8-oxoguanine.
a, Structural comparison of G versus oxoG, with differences highlighted. b, Overall structure of a lesion-recognition complex, LRC3, with an extrahelical oxoG lesion bound in the enzyme active site. c, Sequence-matched interrogation complex, IC3 (ref. 14), with a fully intrahelical target G•C base-pair. d, Close-up view of the oxoG-capping loop (OCL) and its contacts to oxoG in LRC3. The light pink region represents the residues deleted in EC3–5. Residues with grey side chains were mutated to prolines in the ECs bearing OCL point mutations. e, Sequence-matched encounter complex, EC3, bearing a fully intrahelical target oxoG•C base-pair.
Entrapment strategy
To trap the ordinarily fleeting state of intrahelical lesion encounter, we introduced two key modifications: (1) disulphide-crosslinking14 to limit the roaming range of MutM on DNA, and (2) mutation of the oxoG-capping loop (OCL), which interacts extensively with the extrahelical oxoG (Fig. 1d), so as to prevent the LRC from dominating the thermodynamic landscape of MutM–DNA interactions. Biochemically, the MutM OCL variants failed to excise oxoG from DNA, but remained competent in cleaving DNA at abasic sites (Supplementary Fig. 2a–b). This activity profile indicates that OCL mutation only disrupts oxoG-recognition dependent reaction step(s) in the base excision cascade.
To isolate the effect of the lesion on DNA, we crystallized matched pairs of structures, with each pair differing only in whether the DNA component contains a G or an oxoG at the interrogation site by MutM. To establish the effect of sequence context on intrahelical lesion recognition, we structurally characterized matched EC/IC pairs and the corresponding LRCs with three different sequences (for example, EC3/IC3/LRC3, Supplementary Fig. 3).
Global structures of encounter complexes
The global structure of EC3 (1.89 Å resolution) closely resembles that of IC3 (Fig. 1c), with a sharp bend in the DNA localized to the binding site of MutM (Fig. 1e). The target oxoG•C base pair in EC3 is unambiguously intrahelical (Supplementary Fig. 4); EC3 is thus the first structure of a DNA glycosylase directly observed at the stage of its initial encounter with an intrahelical lesion. Importantly, EC3 yielded the same crystal form (Supplementary Table 1) that has captured diverse states of nucleobase extrusion by MutM, from unextruded to partially and fully extruded11,14. Therefore, the un-extruded recognition state in EC3 represents a preferred mode of MutM–DNA interaction when the fully extruded state is destabilized.
Three MutM residues serve critical roles in probing the target nucleobase. In LRC3, F114 and M77 intercalate into the DNA helix on the 3′-side of the broken oxoG•C pair, and R112 reaches into the space formerly occupied by the oxoG to form a hydrogen bond with the opposite C (Fig. 2a). In the intrahelical state observed in EC3, only F114 penetrates the kinked DNA helix and buckles the target base-pair, whereas M77 is retracted. Most significantly, insertion of R112 into the helical stack is prevented by the intrahelical oxoG•C base-pair. R112 instead curls under to form a hydrogen bond with the N3-atom of the 3′-side G neighbouring the target C (Fig. 2c and Supplementary Fig. 5a), and all four nucleobases can interact with R112 in the equivalent minor groove position. As in IC3 (Fig. 2b), this alternative conformation of R112 is stabilized through hydrogen bonding with E78.
Figure 2. Helix-penetration by MutM residues.
a, LRC3; b, IC3; c, EC3. Colour-coding is as in Fig. 1, except side chains of the key residues (M77, R112, F114 and E78) are shown in cyan.
Despite the differences in DNA sequences and the crosslinking positions on the DNA, the structures of EC4 (1.62 Å resolution) and EC5 (1.83 Å) bear all defining global structural characteristics described above for EC3 (Supplementary Fig. 5b–c). The pronounced similarity among the EC structures indicates that the global features of the encounter between MutM and an intrahelical lesion are relatively independent of local sequence context.
MutM distinguishes a target oxoG from G
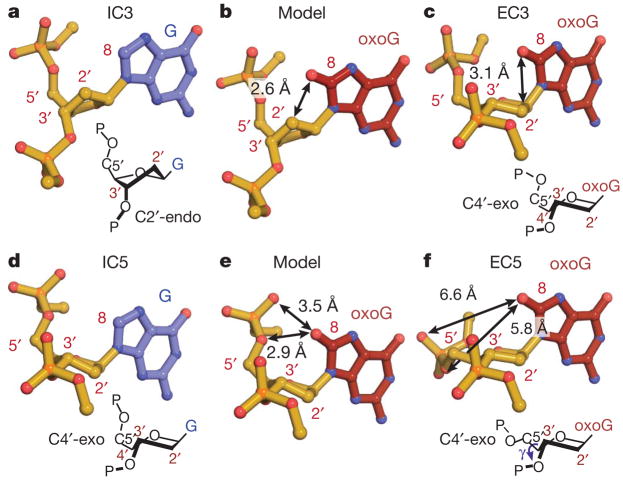
Each EC and its sequence-matched IC differ in the protein–DNA interface by only two atoms, those that distinguish oxoG from G (Fig. 1a). Despite several shared key features, each EC/IC pair bears significant elements of structural divergence in stacking and buckling in the DNA component, and in the protein–DNA interface (below and Supplementary Information). The most pronounced differences lie in the DNA backbone conformation. The target G in IC3 adopts the ground-state 2′-endo sugar pucker (Fig. 3a). Replacing G by oxoG results in pseudorotation of the sugar to an alternative pucker (C4′-exo) (Fig. 3c and Supplementary Fig. 9). An identical change in sugar pucker is observed in going from IC4 to EC4 (Supplementary Fig. 10a–c). This conformational reorganization of the DNA backbone is directly attributable to the 8-oxo substituent on oxoG, as modelling of an 8-oxo carbonyl onto the G in IC3 shows a steric clash with C2′ (2.6 Å, Fig. 3b). Importantly, oxoG in isolated B-form DNA adopts the 2′-endo sugar pucker, with an 8-O/C2′ distance (3.0 Å) beyond the repulsion range7,10. This indicates that the aforementioned steric strain results from conformational manipulation by MutM while inspecting a target base in DNA. The mechanism of this effect is readily apparent from the crystal structures. Buckling of the target base pair by MutM (Supplementary Fig. 6) causes glycosidic bond rotation that thrusts C8 towards C2′, making the C8/C2′ distance shorter than in naked DNA. Whereas the C8-H in G is small enough to avoid a clash with C2′, the much larger C8-O in oxoG jams into C2′, thereby introducing the clash that disfavours the 2′-endo sugar pucker. Interestingly, DNA polymerases employ a similar strategy to amplify the difference between oxoG-containing DNA and normal DNA15.
Figure 3. OxoG-dependent local DNA structure alterations at the site of the target base in EC3 and EC5.
a and d, target G; c and f, target oxoG; b and e, models constructed by the addition of a carbonyl oxygen to the 8-position of the corresponding target G residue in a and d, respectively. The perspectives were chosen to facilitate examination of sugar puckers (diagrammed below a, c, d, and f). Steric clashes in the models are indicated by double-headed arrows.
The IC5/EC5 pair also shows clear evidence of oxoG-specific intra-helical recognition, but of a different form from that in sets 3 and 4. In IC5, the target G already adopts the alternative C4′-exo sugar pucker (Fig. 3d), hence conversion to oxoG engenders no 8-oxo/2′-CH2 steric clash. However, the close proximity between the C8-H and 5′-phosphate of the target G in IC5 would result in unfavourable steric and electronic interactions between the 8-oxo carbonyl of an oxoG in the same conformation and its own 5′-phosphate (Fig. 3e). This clash is avoided in EC5 (Fig. 3f) by rotations about the C4′-C5′ bond and the phosphodiester linkage to the 5′-neighbouring nucleoside (Supplementary Fig. 10d). Again, the above DNA backbone adjustments are absent in isolated oxoG-containing DNA7,10.
In conclusion, when MutM encounters an intrahelical oxoG, the protein can induce two distinct local modes of conformational change in the DNA backbone to avoid unfavourable interactions with the 8-oxo group: pseudorotation of the sugar as in EC3/4, and backbone rotations observed in all three ECs. The differences between EC3/4 and EC5 (Supplementary Figs 6, 7 and 11) might simply result from the non-identical constraints on helix architecture imposed by base-stacking with either C (EC3/4) or G (EC5) on the 5′-side of the lesion.
The structural changes on replacement of a normal target G by an oxoG lesion, although caused by local steric effects, are transmitted to the surrounding DNA backbone, causing consistent differences in the MutM–DNA interface across all three sets of structures. Most obviously, the DNA backbone around the lesion moves towards the LRC conformation in going from ICs to ECs (Supplementary Fig. 12); importantly, this takes place at positions distal from sites of crystal contacts (Supplementary Fig. 13). In general, more residues in the ECs directly contact the backbone of the lesion-containing strand than in the corresponding ICs, and the LRCs maintain these contacts (Supplementary Fig. 14). Although no single structural feature is absolutely diagnostic for the presence of an oxoG, an intrahelical lesion modifies the overall complex structure further towards the extruded state.
Energetics of lesion extrusion
To determine the thermodynamic and possible kinetic consequences of the structural perturbations described above, we performed molecular dynamics simulations to calculate the free energies for the extrusion of oxoG and G from DNA in the presence and absence of MutM. The availability of matched intrahelical and extrahelical structures (EC4/IC4/LRC4) enables computational studies of the nucleobase extrusion pathway, which consists of three successive events: (1) disruption of the target base pair and extrusion of the target base into the minor groove; (2) rotation about the glycosidic bond from anti to syn torsion angle; and (3) swivelling of the target nucleoside away from the helix to an extended, fully extrahelical state (Fig. 4b and Supplementary Figs 15–17, Supplementary Movies 1 and 2). Although the simulations encompass all three stages of base extrusion, event (1) is of primary interest for understanding the origin of lesion recognition by MutM. Figure 4a plots free energies along the extrusion pathway from the initial intrahelical state to the lowest free-energy state in which the target base is extrahelical but not yet rotated and extended. In the presence of MutM, the overall barrier to oxoG extrusion is only ~4 kcal mol−1 (Z =0.45), whereas that for G is ~11 kcal mol−1 (Z =0.63, Supplementary Table 2). Rates for nucleobase extrusion were estimated from these free energy barriers based on earlier work16: ~1 ×10−3 ps−1 for oxoG and 1 ×10−8 ps−1 for G (Supplementary Information). Simulations based on the EC5/IC5/LRC5 structures give similar trends (Supplementary Fig. 18, Supplementary Movies 3 and 4).
Figure 4. Free energy profiles of nucleobase extrusion imply active participation of MutM.
a, Free energies along the base extrusion pathways, as a function of the normalized arc length, Z, in b and Supplementary Fig. 17. Critical events associated with main energetic barriers are marked. b, Free energy landscape plots for extrusion of oxoG starting from EC4 (top) and G from IC4 (bottom). See Supplementary Fig. 16 for definitions of qext and qrotat. The black dotted lines denote the minimal free energy paths for base extrusion, which define Z. The lowest free energy points in the intrahelical states are set to zero.
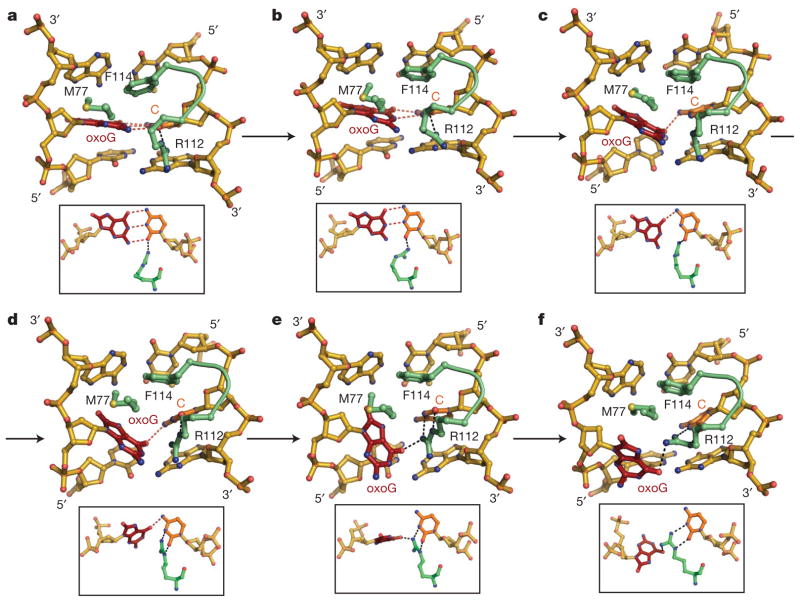
What roles does MutM have in base extrusion? In the absence of MutM, bent DNA, assuming the conformation in EC4/IC4, experiences considerably reduced overall free energy barriers to target base extrusion, as compared to naked unbent DNA (Fig. 4a); the values obtained here for unbent DNA are close to those reported previously17–19. Therefore, one mechanism by which MutM promotes nucleobase extrusion is by introducing a bend in the DNA near the enzyme active site, as the loss of stacking interactions for the target base20 destabilizes the intrahelical state. Furthermore, comparison of the free energy barriers for the naked bent DNA with those for MutM-bound DNA indicates that MutM actively facilitates the target nucleobase extrusion, and the facilitation effect is greater for oxoG than G. This additional reduction of the free energy for base extrusion involves several MutM residues (Fig. 5 and Supplementary Fig. 15). F114 and M77 buckle the target base pair to interrupt base-stacking interactions. Most striking are the effects of R112. At the initial MutM–DNA encounter stage, R112 remains close to the non-Watson–Crick (WC) lone pair on O2 of the C opposite the target base, and this serves as the wedge point for helix invasion (Fig. 5a). R112 moves from its interaction with the non-WC to the WC lone pair of O2-C, in direct competition with the target nucleobase (Fig. 5b, c). From this ‘anchored’ position, R112 invades the helix to establish bidentate hydrogen bonds with O2 and N3 of C, concomitant with breakage of the target base-pair (Fig. 5d–f). To confirm the role of R112 in promoting target base-pair disruption, free energy simulations for MutM-bound DNA were repeated with the interactions between the R112 side chain and the target oxoG•C or G•C turned off. R112 now fails to invade the DNA helix, the disruption of the target base pair is delayed, and both oxoG and G experience elevated free energies at Z =1.0 (Supplementary Fig. 19). A similar arginine residue (R128) in O6-alkylguanine-DNA alkyltransferase has been observed computationally to invade the DNA helix and disrupt the target base-pair following spontaneous base-pair fluctuations16.
Figure 5. R112-catalysed oxoG extrusion.
a –f, Snapshots from targeted molecular dynamics (TMD) simulations in the vicinity of the target base, showing helix invasion by R112 and concomitant extrusion of the target oxoG. The three DNA helix-invading MutM residues (M77, R112 and F114) are coloured in green. Nucleosides around the target oxoG•C pair are shown in gold. The inset figure of each panel is an orthogonal view. See the text for the progression of events a–f. Refer to the Supplementary Movies for the complete base extrusion trajectories of oxoG and G by MutM.
How does MutM locate oxoG in the genome?
Recent single-molecule tracking studies have established that MutM translocates along DNA with a nearly negligible free energy barrier (~2 kcal mol−1 per base pair, ref. 21). This effectively rules out the possibility that MutM must inspect every nucleobase in its extrahelical lesion recognition pocket to locate oxoG. MutM must therefore possess a mechanism for discriminating intrahelical lesions from normal bases. The absence of a thermodynamic preference for the MutM OCL variants to bind oxoG•C-containing DNA (<twofold, Supplementary Fig. 2c) indicates that intrahelical lesion recognition by MutM must have a kinetic origin. This conclusion is supported by biochemical experiments on wild-type MutM22–24. The matched sets of structures reported here indicate that encounter of a lesion by MutM promotes an ‘extrudogenic’ DNA backbone conformation that is conducive to nucleobase extrusion. Molecular dynamics simulations of the base extrusion pathway based on these structures clearly show that, in the presence of MutM, an oxoG lesion encounters a much lower and flatter energy barrier for initial extrusion from the helical stack than does G. In both ECs and ICs, a cherry-picker residue in MutM, R112, promotes the disruption of the target pair by competing with Watson–Crick base-pairing. This active intrahelical interrogation and extrusion mechanism differs from the passive mechanism proposed for uracil DNA glycosylase (UNG), in which the protein is envisaged to preferentially capture a spontaneously extruded lesion25.
The massively redundant Brownian search mechanism employed by MutM to inspect DNA requires minimization of the kinetically expensive extrusion of normal bases. MutM initially encounters a fully intrahelical, base-paired oxoG lesion. Through a combination of DNA bending, unstacking, buckling of the target pair and helix invasion by R112, MutM can discriminate a lesion from an undamaged base and preferentially promotes oxoG extrusion. This mechanism indicated by the structures and computational studies here provides clues for the kinetic efficiency of MutM in lesion search and recognition.
METHODS SUMMARY
Geobacillus stearothermophilus MutM with crosslinking mutation Q166C and OCL mutation (Δ220–235, V222P or T224P) was overexpressed in Escherichia coli and purified as described before11. DNA oligomers were synthesized with standard phosphoramidite chemistry and the disulphide crosslinker was introduced using H-phosphonate chemistry14. Purified crosslinker-containing DNA duplex (10 μM) was mixed with 20 μM MutM in 20 mM Tris pH 7.4 and 50 mM NaCl for 2–3 days. The crosslinked complex was purified from the mixture with a Mono Q column, concentrated to 225–250 μM in 50 mM NaCl, 20 mM Tris pH 7.4, and crystallized in reservoir solutions containing 12–18% PEG 8K, 100 mM sodium cacodylate pH 7.0 and 5% glycerol. Diffraction data were collected on synchrotron light sources with cryo-protected crystals. Structures were solved by molecular replacement using coordinates of the protein part from isomorphous structures of MutM crosslinked to undamaged DNA.
On the basis of the EC4/IC4/LRC4 crystal structures, four end-state systems with MutM and oxoG-containing or normal DNA were prepared, energy minimized, and equilibrated using molecular dynamics simulations. The all-atom CHARMM force fields26–28 and TIP3P water model29 were used. All simulations were performed with orthorhombic periodic boundary conditions, and electrostatic interactions were evaluated using the particle mesh Ewald summation method30. The base extrusion pathways for oxoG and G were determined by employing steered and targeted molecular dynamics simulations31–33, and the free energies were evaluated using umbrella sampling free energy simulations34. Similar procedures were applied for simulations with naked bent and unbent DNA duplexes.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH: GM044853, GM047467, CA100742 (G.L.V.) and GM030804 (M.K.). Y.Q. is supported by a predoctoral fellowship from the National Science Foundation; M.C.S. by a predoctoral fellowship from the Howard Hughes Medical Institute; K.N. by a postdoctoral fellowship from National Cancer Center. We thank the staff of the NSLS, APS and CHESS synchrotron facilities. These experiments made use of the computing facilities at NERSC and Harvard FAS. We are grateful to J. Jin for experimental assistance and J. Pu, V. Ovchinnikov and other members of the Karplus and Verdine groups for helpful advice.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions Y.Q., M.C.S., K.N. and A.B. contributed equally to the study. A.B., S.J. and M.C.S. cloned the constructs. A.B., M.C.S. and S.J. performed the biochemical and FP assays. A.B., S.J., M.C.S. and Y.Q. purified, crystallized and collected X-ray diffraction data and solved structures (A.B., S.J.: EC4, EC5; M.C.S.: EC3, EC3V222P, EC3T224P, IC4, LRC5; Y.Q.: LRC3, EC3T224P, EC5, IC5, LRC5). A.B. and G.L.V. designed the trapping strategy and crystallographic studies. K.N. and M.K. designed the computational studies, which K.N. then performed. A.B., M.C.S., Y.Q., K.N., G.L.V. and M.K. analysed data and wrote the paper. G.L.V. and M.K. directed the research. All authors discussed the results and commented on the manuscript.
Author Information Atomic coordinates and structure factors for the reported crystal structures have been deposited with the Protein Data Bank under accession codes 3GPY (LRC3), 3GO8 (EC3), 3GP1 (EC3V222P), 3GPP (EC3T224P), 3GPU (EC4), 3GPX (IC4), 3GQ4 (LRC5), 3GQ3(EC5) and 3GQ5 (IC5). Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 2.Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230–3239. [PubMed] [Google Scholar]
- 3.Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet. 2004;38:445–476. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- 4.Fromme JC, Verdine GL. Base excision repair. Adv Protein Chem. 2004;69:1–41. doi: 10.1016/S0065-3233(04)69001-2. [DOI] [PubMed] [Google Scholar]
- 5.Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 6.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 7.Lipscomb LA, et al. X-ray structure of a DNA decamer containing 7,8-dihydro-8-oxoguanine. Proc Natl Acad Sci USA. 1995;92:719–723. doi: 10.1073/pnas.92.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oda Y, et al. NMR studies of a DNA containing 8-hydroxydeoxyguanosine. Nucleic Acids Res. 1991;19:1407–1412. doi: 10.1093/nar/19.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plum GE, Grollman AP, Johnson F, Breslauer KJ. Influence of the oxidatively damaged adduct 8-oxodeoxyguanosine on the conformation, energetics, and thermodynamic stability of a DNA duplex. Biochemistry. 1995;34:16148–16160. doi: 10.1021/bi00049a030. [DOI] [PubMed] [Google Scholar]
- 10.Bowman BR, Lee S, Wang S, Verdine GL. Structure of the E. coli DNA glycosylase AlkA bound to the ends of duplex DNA: a system for the structure determination of lesion-containing DNA. Structure. 2008;16:1166–1174. doi: 10.1016/j.str.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fromme JC, Verdine GL. DNA lesion recognition by the bacterial repair enzyme MutM. J Biol Chem. 2003;278:51543–51548. doi: 10.1074/jbc.M307768200. [DOI] [PubMed] [Google Scholar]
- 12.Bruner SD, Norman DPG, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature. 2000;403:859–866. doi: 10.1038/35002510. [DOI] [PubMed] [Google Scholar]
- 13.Zharkov DO. Base excision DNA repair. Cell Mol Life Sci. 2008;65:1544–1565. doi: 10.1007/s00018-008-7543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee A, Santos WL, Verdine GL. Structure of a DNA glycosylase searching for lesions. Science. 2006;311:1153–1157. doi: 10.1126/science.1120288. [DOI] [PubMed] [Google Scholar]
- 15.Hsu GW, Ober M, Carell T, Beese LS. Error-prone replication of oxidatively damaged DNA by a high-fidelity DNA polymerase. Nature. 2004;431:217–221. doi: 10.1038/nature02908. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Ma A, Dinner AR. A two-step nucleotide-flipping mechanism enables kinetic discrimination of DNA lesions by AGT. Proc Natl Acad Sci USA. 2008;105:4615–4620. doi: 10.1073/pnas.0708058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Priyakumar UD, Mackerell AD., Jr NMR imino proton exchange experiments on duplex DNA primarily monitor the opening of purine bases. J Am Chem Soc. 2006;128:678–679. doi: 10.1021/ja056445a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banavali NK, MacKerell AD., Jr Free energy and structural pathways of base flipping in a DNA GCGC containing sequence. J Mol Biol. 2002;319:141–160. doi: 10.1016/S0022-2836(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 19.Cheng X, et al. Dynamic behavior of DNA base pairs containing 8-oxoguanine. J Am Chem Soc. 2005;127:13906–13918. doi: 10.1021/ja052542s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W. Poor base stacking at DNA lesions may initiate recognition by many repair proteins. DNA Repair (Amst) 2006;5:654–666. doi: 10.1016/j.dnarep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Blainey PC, van Oijen AM, Banerjee A, Verdine GL, Xie XS. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. Proc Natl Acad Sci USA. 2006;103:5752–5757. doi: 10.1073/pnas.0509723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minetti CA, et al. Energetics of lesion recognition by a DNA repair protein: thermodynamic characterization of formamidopyrimidine-glycosylase (Fpg) interactions with damaged DNA duplexes. J Mol Biol. 2003;328:1047–1060. doi: 10.1016/s0022-2836(03)00365-6. [DOI] [PubMed] [Google Scholar]
- 23.Fedorova OS, et al. Stopped-flow kinetic studies of the interaction between Escherichia coli Fpg protein and DNA substrates. Biochemistry. 2002;41:1520–1528. doi: 10.1021/bi011524u. [DOI] [PubMed] [Google Scholar]
- 24.Ishchenko AA, et al. Thermodynamic, kinetic, and structural basis for recognition and repair of 8-oxoguanine in DNA by Fpg protein from Escherichia coli. Biochemistry. 2002;41:7540–7548. doi: 10.1021/bi0121297. [DOI] [PubMed] [Google Scholar]
- 25.Parker JB, et al. Enzymatic capture of an extrahelical thymine in the search for uracil in DNA. Nature. 2007;449:433–437. doi: 10.1038/nature06131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacKerell AD, Jr, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 27.Mackerell AD, Jr, Banavali NK. All-atom empirical force field for nucleic acids: II Application to molecular dynamics simulations of DNA and RNA in solution. J Comput Chem. 2000;21:105–120. [Google Scholar]
- 28.Foloppe N, Mackerell AD. All-atom empirical force field for nucleic acids: I. Parameter optimization based on small molecule and condensed phase macromolecular target data. J Comput Chem. 2000;21:86–104. [Google Scholar]
- 29.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 30.Darden T, York D, Pedersen L. Particle mesh Ewald: an N•log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089. [Google Scholar]
- 31.van der Vaart A, Karplus M. Simulation of conformational transitions by the restricted perturbation-targeted molecular dynamics method. J Chem Phys. 2005;122:114903. doi: 10.1063/1.1861885. [DOI] [PubMed] [Google Scholar]
- 32.Paci E, Karplus M. Forced unfolding of fibronectin type 3 modules: an analysis by biased molecular dynamics simulations. J Mol Biol. 1999;288:441–459. doi: 10.1006/jmbi.1999.2670. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Ma A, Dinner AR. Bias annealing: A method for obtaining transition paths de novo. J Chem Phys. 2006;125:114101. doi: 10.1063/1.2335640. [DOI] [PubMed] [Google Scholar]
- 34.Torrie GM, Valleau JP. Nonphysical sampling distributions in Monte Carlo free-energy estimation: umbrella sampling. J Comput Phys. 1977;23:187. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.