Abstract
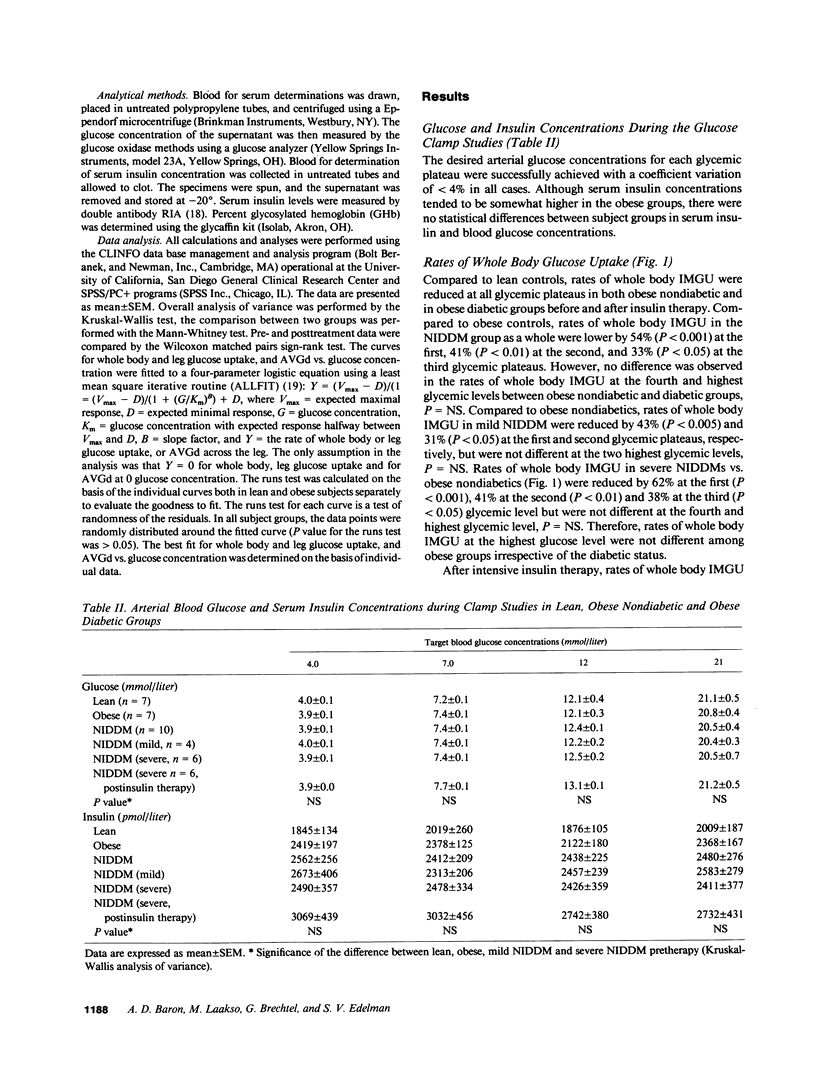
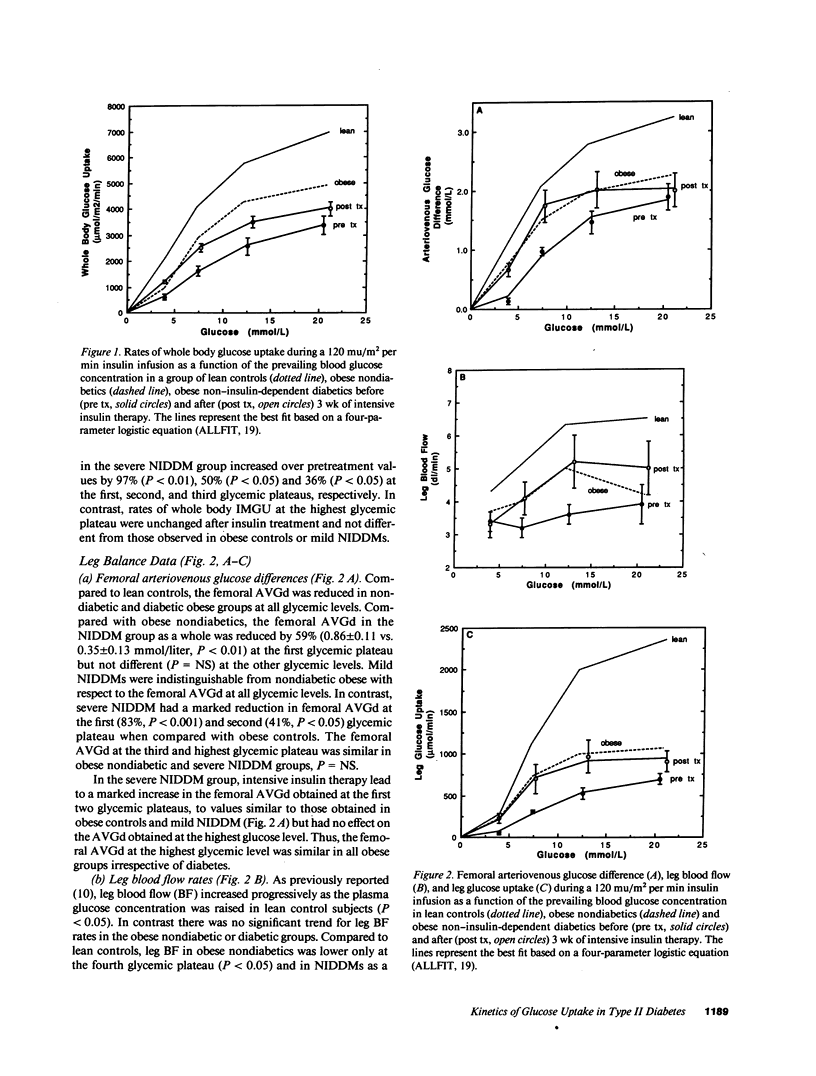
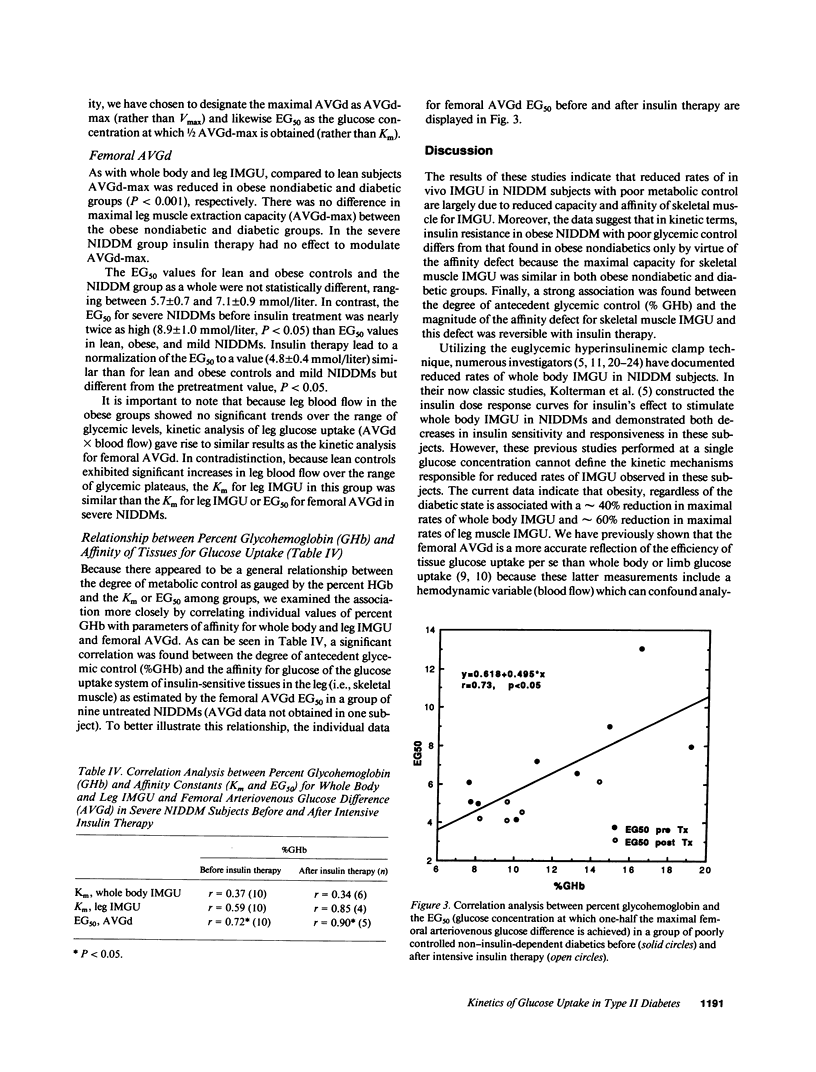
We have estimated the capacity and affinity of insulin-mediated glucose uptake (IMGU) in whole body and in leg muscle of obese non-insulin-dependent diabetics (NIDDM, n = 6) with severe hyperglycemia, glycohemoglobin (GHb 14.4 +/- 1.2%), lean controls (ln, n = 7) and obese nondiabetic controls (ob, n = 7). Mean +/- SEM weight (kg) was 67 +/- 2 (ln), 100 +/- 7 (ob), and 114 +/- 11 (NIDDM), P = NS between obese groups. NIDDM were also studied after 3 wk of intensive insulin therapy, GHb post therapy was 10.1 +/- 0.9, P less than 0.01 vs. pretherapy. Insulin (120 mu/m2 per min) was infused and the arterial blood glucose (G) sequentially maintained at approximately 4, 7, 12, and 21 mmol/liter utilizing the G clamp technique. Leg glucose uptake (LGU) was calculated as the product of the femoral arteriovenous glucose difference (FAVGd) and leg blood flow measured by thermodilution. Compared to ln, ob and NIDDM had significantly lower rates of whole body IMGU and LGU at all G levels. Compared to ob, the NIDDM exhibited approximately 50% and approximately 40% lower rates of whole body IMGU over the first two G levels (P less than 0.02) but did not differ at the highest G, P = NS. LGU was 83% lower in NIDDM vs. ob, P less than 0.05 at the first G level only. After insulin therapy NIDDM were indistinguishable from ob with respect to whole body IMGU or LGU at all G levels. A significant correlation was noted between the percent GHb and the EG50 (G at which 1/2 maximal FAVGd occurs) r = 0.73, P less than 0.05. Thus, (a) insulin resistance in NIDDM and obese subjects are characterized by similar decreases in capacity for skeletal muscle IMGU, but differs in that poorly controlled NIDDM display a decrease in affinity for skeletal muscle IMGU, and (b) this affinity defect is related to the degree of antecedent glycemic control and is reversible with insulin therapy, suggesting that it is an acquired defect.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. J., Vasquez B., Nagulesparan M., Klimes I., Foley J., Unger R., Reaven G. M. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984 Jul;33(7):634–642. doi: 10.2337/diab.33.7.634. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Brechtel G., Wallace P., Edelman S. V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988 Dec;255(6 Pt 1):E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Kolterman O. G., Bell J., Mandarino L. J., Olefsky J. M. Rates of noninsulin-mediated glucose uptake are elevated in type II diabetic subjects. J Clin Invest. 1985 Nov;76(5):1782–1788. doi: 10.1172/JCI112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J. D., Judzewitsch R. G., Pfeifer M. A., Beard J. C., Halter J. B., Porte D., Jr The effect of chronic sulfonylurea therapy on hepatic glucose production in non-insulin-dependent diabetes. Diabetes. 1982 Apr;31(4 Pt 1):333–338. doi: 10.2337/diab.31.4.333. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Scarlett J. A., Kao M., Olefsky J. M. Role of glucose transport in the postreceptor defect of non-insulin-dependent diabetes mellitus. Diabetes. 1982 Nov;31(11):1016–1022. doi: 10.2337/diacare.31.11.1016. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman S. V., Laakso M., Wallace P., Brechtel G., Olefsky J. M., Baron A. D. Kinetics of insulin-mediated and non-insulin-mediated glucose uptake in humans. Diabetes. 1990 Aug;39(8):955–964. doi: 10.2337/diab.39.8.955. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Matthaei S., Olefsky J. M. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. J Clin Invest. 1988 May;81(5):1528–1536. doi: 10.1172/JCI113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Griffin J., Hamman R. F., Kolterman O. G. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985 Mar;34(3):222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- Henry R. R., Wallace P., Olefsky J. M. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986 Sep;35(9):990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Barzilai A., Rafaeloff R., Armoni M. Distribution of glucose transporters in membrane fractions isolated from human adipose cells. Relation to cell size. J Clin Invest. 1986 Oct;78(4):1051–1055. doi: 10.1172/JCI112660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Nyomba B. L., Bogardus C. No accumulation of glucose in human skeletal muscle during euglycemic hyperinsulinemia. Am J Physiol. 1988 Dec;255(6 Pt 1):E942–E945. doi: 10.1152/ajpendo.1988.255.6.E942. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun;85(6):1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Olefsky J. M., Brechtel G., Wallace P., Baron A. D. Kinetics of in vivo muscle insulin-mediated glucose uptake in human obesity. Diabetes. 1990 Aug;39(8):965–974. doi: 10.2337/diab.39.8.965. [DOI] [PubMed] [Google Scholar]
- Prager R., Wallace P., Olefsky J. M. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Invest. 1986 Aug;78(2):472–481. doi: 10.1172/JCI112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revers R. R., Kolterman O. G., Olefsky J. M. Relationship between serum glucose level and the metabolic clearance rate of glucose in non-insulin-dependent diabetes mellitus. Diabetes. 1983 Jul;32(7):627–632. doi: 10.2337/diab.32.7.627. [DOI] [PubMed] [Google Scholar]
- Robertson R. P. Type II diabetes, glucose "non-sense," and islet desensitization. Diabetes. 1989 Dec;38(12):1501–1505. doi: 10.2337/diab.38.12.1501. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett J. A., Gray R. S., Griffin J., Olefsky J. M., Kolterman O. G. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982 Jul-Aug;5(4):353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- Simonson D. C., Ferrannini E., Bevilacqua S., Smith D., Barrett E., Carlson R., DeFronzo R. A. Mechanism of improvement in glucose metabolism after chronic glyburide therapy. Diabetes. 1984 Sep;33(9):838–845. doi: 10.2337/diab.33.9.838. [DOI] [PubMed] [Google Scholar]
- Zawadzki J. K., Bogardus C., Foley J. E. Insulin action in obese non-insulin-dependent diabetics and in their isolated adipocytes before and after weight loss. Diabetes. 1987 Feb;36(2):227–236. doi: 10.2337/diab.36.2.227. [DOI] [PubMed] [Google Scholar]
- Ziel F. H., Venkatesan N., Davidson M. B. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988 Jul;37(7):885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]
- Zierler K. L. THEORY OF THE USE OF ARTERIOVENOUS CONCENTRATION DIFFERENCES FOR MEASURING METABOLISM IN STEADY AND NON-STEADY STATES. J Clin Invest. 1961 Dec;40(12):2111–2125. doi: 10.1172/JCI104437. [DOI] [PMC free article] [PubMed] [Google Scholar]


