Abstract
BACKGROUND
We conducted a trial of prophylactic platelet transfusions to evaluate the effect of platelet dose on bleeding in patients with hypoproliferative thrombocytopenia.
METHODS
We randomly assigned hospitalized patients undergoing hematopoietic stem-cell transplantation or chemotherapy for hematologic cancers or solid tumors to receive prophylactic platelet transfusions at a low dose, a medium dose, or a high dose (1.1×1011, 2.2×1011, or 4.4×1011 platelets per square meter of body-surface area, respectively), when morning platelet counts were 10,000 per cubic millimeter or lower. Clinical signs of bleeding were assessed daily. The primary end point was bleeding of grade 2 or higher (as defined on the basis of World Health Organization criteria).
RESULTS
In the 1272 patients who received at least one platelet transfusion, the primary end point was observed in 71%, 69%, and 70% of the patients in the low-dose group, the medium-dose group, and the high-dose group, respectively (differences were not significant). The incidences of higher grades of bleeding, and other adverse events, were similar among the three groups. The median number of platelets transfused was significantly lower in the low-dose group (9.25×1011) than in the medium-dose group (11.25×1011) or the high-dose group (19.63×1011) (P = 0.002 for low vs. medium, P<0.001 for high vs. low and high vs. medium), but the median number of platelet transfusions given was significantly higher in the low-dose group (five, vs. three in the medium-dose and three in the high-dose group; P<0.001 for low vs. medium and low vs. high). Bleeding occurred on 25% of the study days on which morning platelet counts were 5000 per cubic millimeter or lower, as compared with 17% of study days on which platelet counts were 6000 to 80,000 per cubic millimeter (P<0.001).
CONCLUSIONS
Low doses of platelets administered as a prophylactic transfusion led to a decreased number of platelets transfused per patient but an increased number of transfusions given. At doses between 1.1×1011 and 4.4×1011 platelets per square meter, the number of platelets in the prophylactic transfusion had no effect on the incidence of bleeding. (ClinicalTrials.gov number, NCT00128713.)
The optimal number of platelets in a prophylactic platelet transfusion is controversial.1,2 A standard dose for adults is considered to be approximately 3×1011 to 6×1011 platelets.3 Higher doses than these could potentially result in superior hemostasis,4 but a lower dose might be equally effective while conserving the platelet supply. Two randomized trials with limited enrollment — one of 111 patients5 and the other of 119 patients6 — have compared a low dose of platelets to the standard dose. In both trials, the two doses prevented bleeding to a similar degree. We conducted a randomized trial of prophylactic platelet transfusions to determine the effects of the dose of platelets on clinical signs of bleeding, the use of platelet and red-cell transfusions, changes in the recipient’s post-transfusion platelet count, days to next transfusion, and adverse events.7
METHODS
A subcommittee of the Transfusion Medicine/Hemostasis Clinical Trials Network investigators designed the study and wrote the manuscript, which was reviewed by all authors. Site coordinators gathered the data and submitted the results electronically. (The study investigators and staff are listed in the Appendix.) The data were analyzed with the use of SAS software (version 9.2).8 The lead author wrote the first draft of the manuscript and vouches for the completeness and accuracy of the data. Institutional review boards approved the study. Adults provided written informed consent; and for children, a parent or legal guardian provided written informed consent. Children provided assent if required by local site policy. A data and safety monitoring board reviewed the data twice a year. Stopping boundaries for comparison of the primary end point between each pair of treatment groups were calculated with the use of an alpha spending function similar to O’Brien–Fleming boundaries.
ELIGIBILITY CRITERIA
Patients were eligible for the study if they were inpatients of any age undergoing hematopoietic stem-cell transplantation or chemotherapy for hematologic cancers or solid tumors and it was expected that they would have platelet counts of 10,000 per cubic millimeter or lower for 5 days or more. Additional criteria were a weight of 10 to 135 kg, prothrombin and partial-thromboplastin times 1.3 times the upper limit of the normal range or less, a fibrinogen level of 100 mg per deciliter or more, and no previous platelet transfusions for thrombocytopenia during the current period of hospitalization.
Exclusion criteria were as follows: bleeding of grade 2 or higher, according to the World Health Organization (WHO) bleeding scale9 (ranging from grades 1 through 4, with higher grades indicating worse bleeding; see below and the Supplementary Appendix, available with the full text of this article at NEJM.org) before or at the time of assessment; performance of bedside platelet leukoreduction; platelet refractoriness within the past 30 days, according to local criteria; a panel-reactive HLA antibody level of 20% or more; acute promyelocytic leukemia, idiopathic or thrombotic thrombocytopenic purpura, or the hemolytic–uremic syndrome; planned prophylactic transfusion of platelets at platelet counts of more than 10,000 per cubic millimeter10; major surgery within the previous 2 weeks; use of drugs intended to affect platelet number or function; pregnancy; and previous enrollment in this platelet-dose trial (PLADO trial).
STRATIFICATION AND RANDOMIZATION
Body-surface area was calculated from height and weight.11 Patients were randomly assigned in a 1:1:1 ratio, by means of computer-generated permuted blocks, to receive platelet transfusions of one of three doses — 1.1×1011, 2.2×1011, and 4.4×1011 platelets per square meter per transfusion (low-dose group, medium-dose group, and high-dose group, respectively) — according to four treatment strata12: allogeneic hematopoietic stem-cell transplantation, autologous or syngeneic hematopoietic stem-cell transplantation, chemotherapy for hematologic cancer, or chemotherapy for solid tumor. Treatment-group assignments were balanced within trial sites with the use of dynamic balancing.12 For typical adults, the medium dose is similar to a standard adult dose with regard to the number of platelets.
TRANSFUSIONS
The blood-transfusion service was given each patient’s assigned dose and the allowable range, ±25% of the assigned dose. Site staff were not told the patient’s assigned dose, but differences in transfusion volume prevented complete blinding. Selection of platelets for transfusion to achieve the correct “attempted dose” was specified as follows. For apheresis platelets, the platelet count at the time of collection was used to select a partial unit, a single unit, or multiple units. For pooled platelet concentrates, the mean platelet count per concentrate, based on quality-control data, was used to determine the number of platelet concentrates to combine. For each transfusion, an “at-issue” platelet count was obtained to determine the actual dose transfused. HLA-selected platelets were transfused completely to avoid product wastage, regardless of the patient’s assigned dose.
Platelets were transfused prophylactically if the morning count was 10,000 per cubic millimeter or lower (the “trigger” threshold). The patient’s physician could change the transfusion trigger threshold or dose if required by clinical indications, with a return to study guidelines as soon as possible. Local practice determined the indications for red-cell transfusion. Platelets and red cells were leukoreduced by means of filtration.
CLINICAL ASSESSMENTS
Research staff performed daily assessments of bleeding using physical examinations, interviews with patients, and chart reviews for bleeding events. They also collected data on all bleeding described in the WHO criteria,9 except they did not perform urine dipstick or stool guaiac tests. The bleeding data were used to calculate each patient’s daily bleeding grade. Daily platelet counts, hematocrit values, and hemoglobin levels were also measured.
The primary end point was bleeding of grade 2 or higher. Grade 2 bleeding was defined as oropharyngeal bleeding or epistaxis for more than 30 minutes during a 24-hour period, purpura of more than 1 in. (2.54 cm) in diameter, deep hematoma, joint bleeding, melena, hematochezia, hematemesis, gross hematuria, abnormal vaginal bleeding consisting of more than spotting, hemoptysis, blood in bronchopulmonary lavage specimens, visible blood in body-cavity fluid without symptoms, retinal bleeding without visual impairment, spinal-fluid specimens containing microscopic amounts of blood, or bleeding for more than 1 hour at invasive sites.9 (See the Supplementary Appendix for definitions of bleeding grades 0 through 4.) If an investigator indicated possible death from hemorrhage or if a deceased patient had had grade 3 or 4 bleeding, three nonstudy physicians adjudicated whether bleeding was the cause of death.
ADVERSE EVENTS
Information was collected on all serious adverse events and on events commonly associated with transfusion that occurred during transfusion or within 4 hours afterward.
STUDY COMPLETION
The study was considered to be completed at 30 days after the first platelet transfusion, after a 10-day period without a platelet transfusion, at hospital discharge, at death, or at withdrawal from the study — whichever occurred first.
STATISTICAL ANALYSIS
The primary end point was bleeding of grade 2 or higher; secondary end points were the highest grade of bleeding, total number of platelets transfused, and number of platelet transfusions. Other analyses were exploratory. To account for the three pairwise comparisons among the three treatment groups, two-sided P values of less than 0.017 were considered to indicate statistical significance. No other adjustment was made for analyzing multiple outcomes. The study was designed to have a statistical power of 85% to detect an absolute difference of 12.5% in the incidence of the primary end point for any pair among the three treatment groups, which required 450 patients per group.
Unless otherwise specified, all analyses were restricted to data for patients who received at least one platelet transfusion. Results were analyzed according to the patient’s treatment assignment, even if platelet transfusions were not received at the assigned dose or were not administered in accordance with the prophylactic transfusion trigger threshold of 10,000 platelets per cubic millimeter.
Dichotomous end points were compared among the groups with the use of Fisher’s exact test. The highest grade of bleeding was compared using an exact version of the Kruskall–Wallis test. The time to next transfusion and time to bleeding were compared among the groups by means of the log-rank test.13 Dose adherence for each transfusion, trigger-threshold adherence for each study day, pretransfusion and post-transfusion platelet-counts, platelet increments, and corrected count increments were compared among the groups with the use of generalized linear models to account for possible correlations between results within each patient.14,15 (The platelet increment is defined as the post-transfusion platelet count minus the pretransfusion count; the corrected count increment has a numerator of the platelet increment [in cubic millimeters] multiplied by the body-surface area [in square meters] and a denominator of the total number of platelets transfused divided by 1011.) Other continuous variables were analyzed with the use of the Wilcoxon rank-sum test. The post hoc subgroup analysis of data regarding the primary end point, according to randomization stratum, used a logistic-regression model with an interaction term.
RESULTS
STUDY POPULATION
Between 2004 and 2007, a total of 1351 patients were enrolled at 26 sites. Seventy-nine patients did not receive a platelet transfusion; however, including these patients in the analyses had a negligible effect on the results. The baseline characteristics of the study patients were well balanced among the three treatment groups (Table 1).
Table 1.
Baseline Characteristics of the Study Patients, According to Treatment Group
| Characteristic | Platelet Dose* | |||||
|---|---|---|---|---|---|---|
| Low Dose (N = 417) |
P Value, Low vs. Medium Dose |
Medium Dose (N = 423) |
P Value, Medium vs. High Dose |
High Dose (N = 432) |
P Value, High vs. Low Dose |
|
| Age — yr | 0.18 | 0.20 | 0.02 | |||
| Median | 47 | 50 | 51 | |||
| Interquartile range | 30–57 | 34–58 | 32–62 | |||
| Sex — no. (%) | 0.44 | 0.83 | 0.33 | |||
| Male | 243 (58) | 258 (61) | 267 (62) | |||
| Female | 174 (42) | 165 (39) | 165 (38) | |||
| Weight — kg | 0.34 | 0.82 | 0.43 | |||
| Median | 80 | 78 | 78 | |||
| Interquartile range | 65–92 | 60–92 | 63–91 | |||
| Height — cm | 0.31 | 0.22 | 0.85 | |||
| Median | 170 | 170 | 170 | |||
| Interquartile range | 162–178 | 160–177 | 161–178 | |||
| Body-surface area — m2 | 0.36 | 0.74 | 0.54 | |||
| Median | 1.9 | 1.9 | 1.9 | |||
| Interquartile range | 1.7–2.1 | 1.6–2.1 | 1.7–2.1 | |||
| Previous pregnancy | ||||||
| No./total no. of women (%) | 111/174 (64) | 0.09 | 120/163 (74) | 0.11 | 110/164 (67) | 0.69 |
| No. of pregnancies | 0.33 | 0.97 | 0.32 | |||
| Median | 3 | 2 | 3 | |||
| Interquartile range | 2–4 | 2–4 | 2–3 | |||
| Previous transfusion — no. (%) | ||||||
| Platelets | 244 (59) | 0.67 | 240 (57) | 0.68 | 240 (56) | 0.40 |
| Red cells | 316 (76) | 0.62 | 326 (77) | 0.13 | 314 (73) | 0.32 |
| Spleen status — no. (%) | 0.41 | 0.43 | 0.75 | |||
| Nonpalpable | 393 (94) | 390 (92) | 403 (93) | |||
| Enlarged | 15 (4) | 18 (4) | 20 (5) | |||
| Splenectomized | 9 (2) | 15 (4) | 9 (2) | |||
| Primary diagnosis — no. (%) | 0.35 | 0.40 | 0.45 | |||
| Acute leukemia | 202 (48) | 186 (44) | 185 (43) | |||
| Lymphoma | 91 (22) | 89 (21) | 84 (19) | |||
| Myeloma | 39 (9) | 59 (14) | 56 (13) | |||
| Chronic leukemia | 24 (6) | 24 (6) | 33 (8) | |||
| Myelodysplasia | 16 (4) | 26 (6) | 14 (3) | |||
| Solid tumor | 5 (1) | 6 (1) | 7 (2) | |||
| Other | 40 (10) | 33 (8) | 53 (12) | |||
| Stratification category — no. (%) | 1.00 | 0.96 | 0.97 | |||
| Allogeneic stem-cell transplantation | 173 (41) | 173 (41) | 177 (41) | |||
| Autologous or syngeneic stem-cell transplantation |
138 (33) | 142 (34) | 149 (34) | |||
| Chemotherapy or radiation (i.e., non- transplantation) therapy for hematologic cancer |
104 (25) | 105 (25) | 104 (24) | |||
| Chemotherapy or radiation (i.e., non- transplantation) therapy for solid tumor |
2 (<1) | 3 (1) | 2 (<1) | |||
| Laboratory values | ||||||
| Hemoglobin | 0.29 | 0.41 | 0.78 | |||
| No. with data | 415 | 421 | 432 | |||
| Median — g/dl | 9.7 | 9.9 | 9.8 | |||
| Interquartile range — g/dl | 9.0–10.7 | 9.1–10.7 | 9.0–10.7 | |||
| Hematocrit | 0.64 | 0.87 | 0.76 | |||
| No. with data | 413 | 419 | 431 | |||
| Median — % | 28.0 | 28.2 | 28.0 | |||
| Interquartile range — % | 26.0–31.0 | 26.0–31.0 | 26.0–31.0 | |||
| Platelet count | 0.43 | 0.008 | 0.07 | |||
| No. with data | 415 | 421 | 432 | |||
| Median — ×10−3/mm3 | 39 | 40 | 35 | |||
| Interquartile range — ×10−3/mm3 | 25–62 | 26–66 | 24–54 | |||
| Prothrombin time† | 0.79 | 0.70 | 0.90 | |||
| No. with data | 356 | 369 | 377 | |||
| Median — ×ULN | 0.92 | 0.92 | 0.92 | |||
| Interquartile range — ×ULN | 0.85–0.99 | 0.85–0.99 | 0.86–0.99 | |||
| International normalized ratio | 0.83 | 0.82 | 0.99 | |||
| No. with data | 411 | 419 | 428 | |||
| Median | 1.0 | 1.0 | 1.1 | |||
| Interquartile range | 1.0–1.1 | 1.0–1.1 | 1.0–1.1 | |||
| Partial thromboplastin time | 0.64 | 0.78 | 0.52 | |||
| No. with data | 411 | 419 | 430 | |||
| Median — sec | 29.1 | 29.0 | 29.0 | |||
| Interquartile range — sec | 26.6–32.9 | 26.3–32.2 | 26.0–32.9 | |||
| Fibrinogen | 0.89 | 0.57 | 0.45 | |||
| No. with data | 409 | 417 | 430 | |||
| Median — mg/dl | 357 | 355 | 369 | |||
| Interquartile range — mg/dl | 279–459 | 276–465 | 283–468 | |||
| Positive for panel-reactive antibody | 0.97 | 0.98 | 0.95 | |||
| No. with data | 389 | 391 | 410 | |||
| Median — % of lymphocytes tested | 0.0 | 0.0 | 0.0 | |||
| Interquartile range — % of lympho- cytes tested |
0.0–0.0 | 0.0–0.0 | 0.0–0.0 | |||
Platelet doses were as follows: low dose, 1.1×1011 platelets per square meter of body-surface area; medium dose, 2.2×1011 platelets per square meter; and high dose, 4.4×1011 platelets per square meter.
ULN denotes upper limit of the normal range.
ADHERENCE WITH PLATELET DOSE AND TRANSFUSION TRIGGER THRESHOLD
Dose adherence was not required for 210 HLA-selected units of platelets and could not be reliably determined for an additional 331 volume-reduced units from which plasma was removed by centrifugation after collection. Among the 5466 prophylactic platelet transfusions with neither characteristic, the attempted dose was known for 5384 transfusions in 1162 patients — and there was dose adherence for 86% of these low-dose transfusions, 98% of these medium-dose transfusions, and 93% of these high-dose transfusions (P<0.001 for low dose vs. medium dose and medium dose vs. high dose) (Table 2). Among the 1162 patients, all known attempted doses were in adherence for 79%, 92%, and 86% of patients in the low-dose, medium-dose, and high-dose groups, respectively (P<0.001 for low dose vs. medium dose, and P = 0.004 for medium dose vs. high dose).
Table 2.
Platelet-Dose Adherence and Response to Prophylactic Platelet transfusions, According to Treatment Group
| Characteristic | Platelet Dose* | |||||
|---|---|---|---|---|---|---|
| Low Dose (N = 417) |
P Value, Low vs. Medium Dose |
Medium Dose (N = 423) |
P Value, Medium vs High Dose |
High Dose (N = 432) |
P Value, High vs. Low Dose |
|
| Dose adherence† | ||||||
| Attempted dose‡ | ||||||
| Transfusions that were neither HLA-selected nor volume-reduced and for which attempted dose was known | ||||||
| No. of transfusions | 2333 | 1708 | 1343 | |||
| Transfusions within assigned dose range — % | 86 | <0.001 | 98 | <0.001 | 93 | 0.06 |
| Patients who received ≥1 transfusion that was neither HLA-selected nor volume-reduced and had ≥1 transfusions with data on attempted dose | ||||||
| No. of patients | 395 | 386 | 381 | |||
| All transfusions within assigned dose range — % | 79 | <0.001 | 92 | 0.004 | 86 | 0.02 |
| At-issue dose§ | ||||||
| Transfusions that were neither HLA-selected nor volume-reduced and for which at–issue dose was known | ||||||
| No. of transfusions | 2278 | 1669 | 1320 | |||
| Transfusions within assigned dose range — % | 71 | 0.007 | 80 | <0.001 | 70 | 0.11 |
| Patients who received ≥1 transfusion that was neither HLA-selected nor volume-reduced and had ≥1 transfusions with data on at-issue dose | ||||||
| No. of patients | 392 | 380 | 380 | |||
| All transfusions within assigned dose range — % | 51 | <0.001 | 63 | 0.04 | 55 | 0.19 |
| Response to prophylactic platelet transfusions | ||||||
| No. of transfusions | 2547 | 1912 | 1572 | |||
| Days until next transfusion¶ | <0.001 | <0.001 | <0.001 | |||
| Median | 1.1 | 1.9 | 2.9 | |||
| Interquartile range | 0.7–2.1 | 0.9–3.1 | 1.2–4.7 | |||
| No. of transfusions with all data available to calculate 4-hr CCI‖ |
2193 | 1646 | 1386 | |||
| Pretransfusion platelet count — ×10−3/mm3 | 0.48 | 0.08 | 0.21 | |||
| Median | 9 | 9 | 9 | |||
| Interquartile range | 7–16 | 7–19 | 7–12 | |||
| Post-transfusion platelet count — ×10−3/mm3** | <0.001 | <0.001 | <0.001 | |||
| Median | 22 | 34 | 50 | |||
| Interquartile range | 16–30 | 24–48 | 33–68 | |||
| Platelet increment — ×10−3/mm3 ‖ | <0.001 | <0.001 | <0.001 | |||
| Median | 10 | 19 | 38 | |||
| Interquartile range | 5–17 | 11–30 | 22–54 | |||
| Post-transfusion CCI — ×10−3/mm3 | 0.08 | 0.03 | 0.98 | |||
| Median | 10 | 10 | 11 | |||
| Interquartile range | 5–15 | 6–16 | 6–15 | |||
Platelet doses were as follows: low dose, 1.1×1011 platelets per square meter of body-surface area; medium dose, 2.2×1011 platelets per square meter; and high dose, 4.4×1011 platelets per square meter.
Adherence to the prophylactic platelet dose was defined as receipt of a dose that was the assigned transfusion dose ±25%.
The “attempted dose” for apheresis platelets was based on the platelet count at the time of collection. For pooled platelet concentrates, the mean platelet count per concentrate, based on quality-control data, was used to determine how many concentrates to pool.
The “at-issue dose” was based on platelet counts obtained from all products just before distribution from the blood-transfusion service.
The analysis of days until next transfusion takes into account data censoring at the time of a granulocyte transfusion, a stem-cell transfusion, or study completion.
Calculations of the corrected count increment (CCI) require complete data on pretransfusion and post-transfusion counts and total platelet counts at issue. The unit of analysis is the transfusion. Here, actual medians and interquartile ranges are presented for pretransfusion and post-transfusion counts, the platelet increment, and the CCI, but the results of statistical comparisons are from a generalized linear model that takes into account that for each patient, the results for various transfusions may be correlated.
The post-transfusion platelet count was obtained within 4 hours after transfusion.
Platelet doses based on at-issue platelet counts were within the patient’s assigned range for 71%, 80%, and 70% of transfusions in the low-dose group, medium-dose group, and high-dose group, respectively (P = 0.007 for low dose vs. medium dose, and P<0.001 for medium dose vs. high dose). This result confirms that the platelet-selection procedure usually resulted in transfusions of at-issue doses in the assigned range. All known at-issue doses were within the assigned range for 51%, 63%, and 55% of patients in the low-dose, medium-dose, and high-dose groups, respectively (P<0.001 for low dose vs. medium dose).
Physicians ordered changes in the platelet dose for clinical reasons for 17% of patients in the low-dose group, 9% in the medium-dose group, and 7% in the high-dose group (P = 0.003 for low dose vs. medium dose and P<0.001 for low dose vs. high dose). Overall, only 3% of patients had a dose change before the onset of bleeding of grade 2 or higher.
The trigger threshold of 10,000 platelets per cubic millimeter was adhered to on 90%, 92%, and 94% of patient-days in the low-dose group, medium-dose group, and high-dose group, respectively (P<0.001 for low dose vs. high dose). The trigger was adhered to on all study days for 53%, 62%, and 61% of patients, respectively (P = 0.01 for low dose vs. medium dose). Physicians ordered changes in the trigger threshold for clinical reasons for 32%, 26%, and 25% of patients, respectively, but a total of only 7% of patients had a change in the trigger threshold before the onset of bleeding of grade 2 or higher.
PRIMARY AND SECONDARY END POINTS
In the 1351 patients who underwent randomization, the percentage with at least one episode of bleeding of grade 2 or higher was 68% in the low-dose group, 67% in the medium-dose group, and 69% in the high-dose group, with no significant differences among the groups (P = 0.83 for low dose vs. medium dose and for low dose vs. high dose; P = 0.66 for medium dose vs. high dose). Among the 1272 patients who received at least one platelet transfusion, the percentage of patients in each group with at least one episode of bleeding of grade 2 or higher was not significantly different (71%, 69% and 70%, respectively) (Table 3).
Table 3.
Primary and Key Secondary End Points, According to Treatment Group.
| Characteristic | Platelet Dose* | |||||
|---|---|---|---|---|---|---|
| Low Dose (N = 417) |
P Value, Low vs. Medium Dose |
Medium Dose (N = 423) |
P Value, Medium vs. High Dose |
High Dose (N = 432) |
P Value, High vs. Low Dose |
|
| Primary end point | ||||||
| ≥1 Episode of bleeding of grade 2 or higher — % of patients |
71 | 0.60 | 69 | 0.71 | 70 | 0.94 |
| Secondary end points | ||||||
| Highest grade of bleeding during study — % of patients |
0.30 | 0.65 | 0.54 | |||
| No bleeding or grade 1 | 30 | 32 | 30 | |||
| Grade 2 | 58 | 59 | 60 | |||
| Grade 3 | 9 | 7 | 8 | |||
| Grade 4 | 3 | 2 | 2 | |||
| Death from hemorrhage — no. of patients | 0 | 0 | 1.00 | 1 | 1.00 | |
| No. of days with bleeding of grade 2 or higher |
0.90 | 0.91 | 0.99 | |||
| Median | 1 | 1 | 1 | |||
| Interquartile range | 0–4 | 0–4 | 0–4 | |||
| Days from randomization to onset of bleeding of grade 2 or higher |
0.85 | 0.66 | 0.55 | |||
| Median | 7 | 7 | 8 | |||
| Interquartile range | 3–18 | 3–19 | 3–19 | |||
| Red-cell transfusions | ||||||
| ≥1 Transfusion — % of patients | 95 | 0.12 | 92 | 1.00 | 92 | 0.09 |
| Total units per patient † | 0.62 | 0.70 | 0.90 | |||
| Median | 4 | 4 | 4 | |||
| Interquartile range | 2–8 | 2–8 | 2–8 | |||
| Platelet transfusions‡ | ||||||
| No. per patient | <0.001 | 0.09 | <0.001 | |||
| Median | 5 | 3 | 3 | |||
| Interquartile range | 3–9 | 2–6 | 2–6 | |||
| Total no. of platelets transfused, based on at-issue count — ×10−11 |
0.002 | <0.001 | <0.001 | |||
| Median | 9.25 | 11.25 | 19.63 | |||
| Interquartile range | 4.91–17.91 | 6.99–22.76 | 10.61–37.44 | |||
Platelet doses were as follows: low dose, 1.1×1011 platelets per square meter of body-surface area; medium dose, 2.2×1011 platelets per square meter; and high dose, 4.4×1011 platelets per square meter.
Analysis of the number of red-cell transfusions per patient was limited to data for patients who had at least one red-cell transfusion and who had no missing data on the number of units transfused: 394 patients in the low-dose group, 387 in the medium-dose group, and 397 in the high-dose group.
Analysis of the number of platelet transfusions per patient was limited to data for patients who had no missing data on the number of transfusion events and no missing data on the total number of platelets transfused: 295 patients in the low-dose group, 346 in the medium-dose group, and 359 in the high-dose group. In these patients, the tradeoffs between number of platelets and number of transfusion events can be assessed.
Overall, the highest bleeding grade observed was no bleeding or bleeding of grade 1 in 31% of patients, grade 2 in 59%, grade 3 in 8%, and grade 4 in 2%. In each group, the median number of days of bleeding of grade 2 or higher was 1. The median time from randomization to onset of bleeding ranged from 7 to 8 days. The median number of red-cell units transfused per patient was 4. A total of 93% of patients received at least one red-cell transfusion. There were no significant differences among the three groups for any of these end points.
Bleeding of grade 2 or higher occurred in 79% of recipients of allogeneic hematopoietic stem-cell transplants, 73% of patients who had hematologic cancers and were undergoing chemotherapy, and 57% of patients undergoing autologous or syngeneic hematopoietic stem-cell transplantation (P<0.001 for the comparison of the latter group with each of the first two groups). However, within each of these treatment categories, the platelet dose had no significant effect on bleeding.
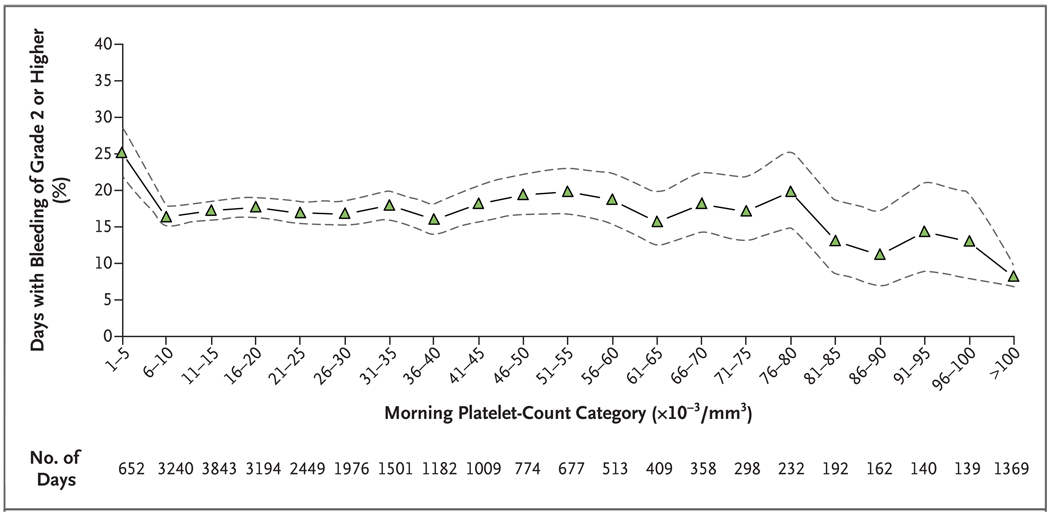
The 1272 patients who received at least one transfusion were observed for a total of 24,309 days. Figure 1 shows the percentage of days on which bleeding of grade 2 or higher occurred, according to the patient’s morning platelet-count category. Bleeding of grade 2 or higher occurred on 25% of days with morning platelet counts of 5000 per cubic millimeter or lower, 17% of days with morning platelet counts from 6000 to 80,000 per cubic millimeter, 13% of days with morning platelet counts from 81,000 to 100,000 per cubic millimeter, and 8% of days with morning platelet counts over 100,000 per cubic millimeter (P<0.001 for platelet counts of ≤5000 per cubic millimeter vs. counts of 6000 to 80,000; P = 0.001 for platelet counts of 81,000 to 100,000 per cubic millimeter vs. counts of 6000 to 80,000; and P<0.001 for platelet counts of >100,000 per cubic millimeter vs. counts of 6000 to 80,000) (Fig. 1).
Figure 1. Days with Bleeding of Grade 2 or Higher in All three Treatment Groups, According to Morning Platelet-Count Categories.
The percentage of days on which patients had bleeding of grade 2 or higher is shown, along with the associated 95% confidence intervals (dashed lines), according to the morning platelet-count category. Data are based on the 24,309 days during the study period on which patients had both a morning platelet count and information on bleeding of grade 2 or higher. Each patient-day was treated as a separate unit of analysis. Analyses were adjusted to take into account that for each patient, the results on various days may be correlated. The interaction between treatment group and morning platelet-count category was not significant, indicating that the effect of the morning platelet-count category did not differ significantly among the three treatment groups; therefore, the data from all three groups are combined.
We could calculate the total number of platelets transfused per patient for 1000 patients. The median number of platelets transfused was 9.25×1011, 11.25×1011, and 19.63×1011 in the low-dose, medium-dose, and high-dose groups, respectively (P = 0.002 for low dose vs. medium dose, P<0.001 for high dose vs. low dose and high dose vs. medium dose) (Table 3). The median number of platelet transfusions administered was five in the low-dose group as compared with three in the medium-dose group and three in the high-dose group (P<0.001 for low dose vs. medium dose and low dose vs. high dose).
RESPONSES TO PLATELET TRANSFUSIONS
The low-dose, medium-dose, and high- dose groups differed significantly in the median post –transfusion platelet count (22,000, 34,000, and 50,000 per cubic millimeter, respectively), the median increase in platelet count after transfusion (10,000, 19,000, and 38,000 per cubic millimeter, respectively), and the median number of days until the next transfusion (1.1, 1.9, and 2.9, respectively) (P<0.001 for all comparisons for all end points) (Table 2). The median 4-hour corrected count increment was 10,000 in the low-dose group, 10,000 in the medium-dose group, and 11,000 in the high-dose group, with no significant differences among groups.
ADVERSE EVENTS
There were no significant differences among the three groups in the occurrence of any specific category of serious adverse events or in the percentage of patients who had one or more serious adverse events (Table 4). Wheezing during or shortly after transfusion was significantly more common in the high-dose group than in the medium-dose group. Nine patients in the low-dose group died, as did four patients in the medium-dose group and seven in the high-dose group; the number of deaths did not differ significantly among the three groups.
Table 4.
Adverse Events, According to Treatment Group.*
| Event | Platelet Dose† | |||||
|---|---|---|---|---|---|---|
| Low Dose (N = 417) |
P Value, Low vs. Medium Dose |
Medium Dose (N = 423) |
P Value, Medium vs. High Dose |
High Dose (N = 432) |
P Value, High vs. Low Dose |
|
| Serious adverse event — no. of patients (%) | ||||||
| Anaphylaxis | 0 | 0 | 0.50 | 2 (<1) | 0.50 | |
| Bleeding | 5 (1) | 0.50 | 3 (1) | 0.73 | 5 (1) | 1.00 |
| Cardiac event | 3 (1) | 0.68 | 2 (<1) | 0.45 | 5 (1) | 0.73 |
| Central nervous system event | 4 (1) | 0.21 | 1 (<1) | 1.00 | 1 (<1) | 0.21 |
| Deep-vein thrombosis | 3 (1) | 0.12 | 0 | 0 | 0.12 | |
| Graft-versus-host disease | 0 | 0 | 0.25 | 3 (1) | 0.25 | |
| Infection | 10 (2) | 0.11 | 4 (1) | 0.12 | 11 (3) | 1.00 |
| Pulmonary event | 12 (3) | 0.84 | 14 (3) | 0.69 | 12 (3) | 1.00 |
| Renal failure | 3 (1) | 0.12 | 0 | 0.25 | 3 (1) | 1.00 |
| Veno-occlusive disease of liver | 6 (1) | 0.77 | 5 (1) | 0.28 | 2 (<1) | 0.17 |
| Other | 5 (1) | 0.75 | 4 (1) | 0.72 | 3 (1) | 0.50 |
| Any | 35 (8) | 0.29 | 27 (6) | 0.30 | 36 (8) | 1.00 |
| Event occurring during or ≤ 4 hr after a trans- fusion — no. of patients (%)‡ |
||||||
| Allergic reaction or hypersensitivity | 37 (9) | 0.42 | 45 (11) | 0.29 | 57 (13) | 0.05 |
| Sinus bradycardia | 4 (1) | 0.75 | 6 (1) | 1.00 | 7 (2) | 0.55 |
| Sinus tachycardia | 44 (11) | 0.73 | 41 (10) | 0.64 | 37 (9) | 0.35 |
| Hypertension | 39 (9) | 0.54 | 34 (8) | 0.20 | 46 (11) | 0.57 |
| Hypotension | 33 (8) | 0.28 | 25 (6) | 0.78 | 28 (6) | 0.43 |
| Dyspnea | 18 (4) | 0.74 | 21 (5) | 0.88 | 23 (5) | 0.53 |
| Hypoxia | 14 (3) | 0.85 | 16 (4) | 1.00 | 17 (4) | 0.72 |
| Wheezing | 7 (2) | 0.11 | 2 (<1) | 0.004 | 14 (3) | 0.19 |
| Cough | 3 (1) | 1.00 | 4 (1) | 0.38 | 8 (2) | 0.22 |
| Hemolysis | 2 (<1) | 0.62 | 1 (<1) | 1.00 | 2 (<1) | 1.00 |
| Rigors or chills | 37 (9) | 0.56 | 43 (10) | 0.51 | 50 (12) | 0.21 |
| Fever | 149 (36) | 0.17 | 132 (31) | 0.51 | 144 (33) | 0.47 |
| Infection | 5 (1) | 1.00 | 5 (1) | 0.77 | 7 (2) | 0.77 |
| Any event listed | 193 (46) | 0.33 | 181 (43) | 0.19 | 205 (47) | 0.78 |
Patients could have had more than one event.
Platelet doses were as follows: low dose, 1.1×1011 platelets per square meter of body-surface area; medium dose, 2.2×1011 platelets per square meter; and high dose, 4.4×1011 platelets per square meter.
For events occurring during or within 4 hours after a transfusion, data were missing for one patient in each of the three treatment groups.
STUDY COMPLETION
Completion of the study occurred at the time of hospital discharge for most patients (71%). Other causes of study completion were an absence of platelet transfusion for 10 days (in 14% of patients), the elapsing of 30 days from first platelet transfusion (10%), withdrawal from the study (4%), and death (2%). The three groups did not differ significantly with regard to reasons for study completion.
DISCUSSION
This study evaluated the effects of platelet dose on hemostasis and transfusion end points. For a typical adult, the medium dose was equivalent to the standard dose currently used in clinical practice.16 The low dose was half the medium dose, and the high dose was twice the medium dose. There were high rates of adherence for platelet doses and transfusion trigger thresholds. Physicians changed the dose to a nonstudy dose for patients in the low-dose group more often than for those in the medium-dose and high-dose groups, but the changes were made primarily after the onset of bleeding of grade 2 or higher.
The percentages of patients who received at least one platelet transfusion and had bleeding of grade 2 or higher — 71%, 69%, and 70% in the low-, medium-, and high-dose groups, respectively — were not significantly different. The only death from hemorrhagic causes occurred in the high-dose group (from pulmonary hemorrhage). The highest grade of bleeding, the number of days of bleeding of grade 2 or higher, the number of days before the occurrence of bleeding of grade 2 or higher, and the occurrence and number of red-cell transfusions also did not differ significantly among the three groups. These findings confirm that the dose per prophylactic platelet transfusion, within the range of doses studied, did not significantly affect bleeding.
The percentage of patients with bleeding of grade 2 or higher was significantly less among those undergoing autologous or syngeneic hematopoietic stem-cell transplantation (57%) than among those with hematologic cancers who were undergoing chemotherapy (73%) or those undergoing allogeneic hematopoietic stem-cell transplantation (79%), as has been described previously.17 However, platelet dose had no significant effect on bleeding in any of these treatment categories.
Patients had a 25% risk of having bleeding of grade 2 or higher on days on which the morning platelet count was 5000 per cubic millimeter or lower, as compared with a 17% risk on days with counts from 6000 to 80,000 per cubic millimeter. Previous reports suggest that endothelial integrity can be maintained with platelet counts of 5000 per cubic millimeter or higher.18,19 Further reductions in the risk of bleeding at platelet counts of 80,000 per cubic millimeter or higher are postulated to be due to an improved clinical status.
The rates of bleeding seen in our trial are higher than those in several other platelet-transfusion trials.20–22 The reported incidence of bleeding depends on factors that often differ among studies, such as assessment method, frequency of assessment, criteria used for bleeding grade, and population of patients.20 In our study, consistent data collection was achieved with the use of daily hemostatic assessments performed by research staff who were unaware of the treatment assignments and who followed detailed instructions. Bleeding grades were ascertained on the basis of objective criteria, thus reducing bias.
To our knowledge, only two previously reported randomized trials, with limited enrollment, have evaluated the use of low-dose platelet transfusions as compared with standard-dose transfusions.5,6 Neither trial showed any significant differences regarding bleeding of any WHO grades — findings similar to those in our study.
The Strategies for Transfusion of Platelets (SToP) study (NCT00420914)6 was halted because of grade 4 bleeding in three patients in the low-dose group (1.5×1011 to 3.0×1011 platelets per transfusion), as compared with none in the standard-dose group (3.0×1011 to 6.0×1011 platelets per transfusion). A stopping rule of a 5% absolute difference between the two groups in the incidence of grade 4 bleeding was reached after only 58 and 61 patients were enrolled in the low-dose group and the standard-dose group, respectively. We did not find a significant difference in the incidence of grade 4 bleeding in our similar, but much larger, cohort. An important difference between our study and the StoP study was that we adjusted the dose of platelets for body-surface area, whereas in the StoP study, the same dose range was used for all patients in each of the two groups.
In our trial, the total number of platelets transfused — 9.25×1011 for the low dose, 11.25×1011 for the medium dose, and 19.63×1011 for the high dose — was significantly different among the three groups. However, the median number of transfusions per patient was significantly greater in the low-dose group (five transfusions) than in the medium-or high-dose group (three transfusions in each group) (P<0.001 for both comparisons). In the SToP study,6 the low-dose group also required significantly more transfusions than the standard-dose group.
As expected, the median platelet increment after transfusion in the low-dose group (10,000 per cubic millimeter) was approximately half the median increment in the medium-dose group (19,000 per cubic millimeter), and the median increment in the high-dose group (38,000 per cubic millimeter) was approximately twice the increment in the medium-dose group. The number of days until the next transfusion also differed significantly among the three groups: 1.1 days in the low-dose group, 1.9 in the medium-dose group, and 2.9 in the high-dose group. These data, data from other, smaller studies, and mathematical models all suggest that larger doses give higher increments and prolonged intervals until the next transfusion.3,23–27 However, the corrected count increment did not differ significantly among the three groups (i.e., differences in the increment were explained by differences in the number of platelets transfused).
In conclusion, when prophylactic transfusions are given after a trigger threshold of 10,000 platelets per cubic millimeter or lower is reached, the platelet dose has no significant effect on the incidence of bleeding in patients with hypoproliferative thrombocytopenia, probably because few platelets are needed to maintain hemostasis.18,19 A strategy of low-dose transfusion significantly reduces the quantity of platelets transfused, which could preserve these scarce blood components but could also increase the number of platelet transfusions.
Supplementary Material
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health to the Data Coordinating Center at New England Research Institutes (HL072268), Case Western Reserve University (HL072033), Children’s Hospital Boston (HL072291), Cornell University (HL072196), Duke University (HL072289), Emory University (HL072248), Johns Hopkins University (HL072191), Massachusetts General Hospital (HL072299), Puget Sound Blood Center (HL072305), Tulane University (HL072274), University of Iowa (HL072028), University of Maryland (HL072359), University of Minnesota (HL072072), University of North Carolina (HL072355), University of Oklahoma (HL072283), University of Pennsylvania (HL072346), University of Pittsburgh (HL072331), and the Blood Center of Wisconsin (HL072290).
Dr. Slichter reports receiving grant support from U.S. Army Medical Research Acquisition Activity (Department of Defense), Navigant Biotechnologies, and Pall Medical; Dr. Assmann, receiving grant support from Z-Medica; Dr. Triulzi, receiving consulting fees from Fenwal Laboratories and Cerus and lecture fees from Pall; Dr. Strauss, receiving consulting fees from CaridianBCT; Dr. Ness, receiving consulting fees from Fenwal Laboratories and CaridianBCT; Dr. Brecher, receiving consulting fees from Fenwal Laboratories; Dr. Josephson, receiving lecture fees from Mediware; Dr. George, receiving consulting fees and grant support from Amgen; and Dr. Manno, receiving consulting fees and grant support from Baxter Healthcare, consulting fees from Bayer Healthcare, and lecture fees from EMD Healthcare Communications Scientific Communication Group. No other potential conflicts of interest relevant to this article were reported.
We thank all the patients who volunteered to take part in this study, all the study investigators and committee members, and Ginny Knight at the Puget Sound Blood Center for administrative assistance.
APPENDIX
The following investigators and staff participated in the study: Case Western Reserve University, Cleveland: K.R. McCrae (principal investigator), V. Exum, D. Hendrix; BloodCenter of Wisconsin, Milwaukee, and University of Wisconsin–Madison, Madison: J.G. McFarland (principal investigator), R.D. Woodson, N. Ruys, J. Werndli, K. Koenig, N. Turman, J. Stublaski; Froedert Memorial Lutheran Hospital, Milwaukee: M. Lankiewicz, D. Miller-Metcalfe, S. Graminske, R. Dora, S. Heldke; St. Luke’s Medical Center, Milwaukee: A.B. Divgi, J. Boos, S. Beekman; Children’s Hospital of Wisconsin, Milwaukee: R. Punzalan; Children’s Hospital Boston, Boston: E.J. Neufeld (principal investigator), S.R. Sloan, T. Kang, J. Hedstrom, K. Harney; Beth Israel Deaconess Medical Center, Boston: L. Uhl, B. Malynn; Brigham and Women’s Hospital, Boston: R.M. Kaufman, M. Kelly, B. Rowe, K. Wallace, S. Slate; Weill College of Cornell University, New York: J. Bussel (principal investigator), D.L. Skerrett, E. Feldman, C. Nguyen, J. Cruz, M. Wissert; Duke University, Durham, NC: T.L. Ortel (principal investigator), S. Adams, T. Anderson, C. Higgins, C. Mette, E. Petzold, C. Pinaroc, C. Thornburg, M.J. Telen; Emory University, Atlanta: C.D. Hillyer (principal investigator), C.D. Josephson, M.-I. Castillejo, J. Newman, J. Ieong, J. Johnson; Johns Hopkins University, Baltimore: P.M. Ness (principal investigator), R. Case, A. Fuller, A.K. Fuller; Puget Sound Blood Center and University of Washington Medical Center, Seattle: S.J. Slichter (principal investigator), T.B. Gernsheimer, A. Deyle, J. Corson, A. Hirata; Virginia Mason Medical Center, Seattle: D. Aboulafia; Children’s Hospital Medical Center, Seattle: D. Matthews; Tulane University Hospital and Clinics, New Orleans: C. Leissinger (principal investigator), R. Kruse-Jarres, H. Safah, C. Schmidt; University of Iowa, Iowa City: R.G. Strauss (principal investigator), B. Link, T. Raife, J. Swift, D. Schrock; University of Maryland, Baltimore: J.R. Hess (principal investigator), M. Mitrou, J. Spanfelner; Fairview–University Medical Center, Minneapolis: J. McCullough (principal investigator), T. Carr, T.Chlebeck, S. Malcolm, S. Pulkrabek; University of North Carolina, Chapel Hill: M.E. Brecher (principal investigator), A. Tsui, M. Miller, S. Hay; University of Oklahoma, Oklahoma City: J.N. George (principal investigator), D. Terrell, X. Li; University of Texas Southwestern Medical Center, Dallas: G. Buchanan (principal investigator), V.M. Aquino, J. Cox, T. Hoffman, G. Paranjape; University of Pennsylvania, Philadelphia: B.A. Konkle (principal investigator), E.A. Stadtmauer, I. Tarng, M. Kelty, M. Einarson, K. Hinkle; Children’s Hospital of Philadelphia, Philadelphia: C.S. Manno (principal investigator), A. Parker, A. Wade, J. Nathanson, M. Braun; University of Pittsburgh, Pittsburgh: D.J. Triulzi (principal investigator), P. D’Andrea, L. Blanker; Dartmouth–Hitchcock Medical Center, Lebanon, NH: J. AuBuchon (medical monitor); New England Research Institutes (Data Coordinating Center), Watertown, MA: S.F. Assmann (principal investigator), D. Brambilla, A.S. Corbett, E. Devlin, J. Erickson, E. Gerstenberger, S. Granger, K. Hayes, J. Miller, J. Scott; National Heart, Lung, and Blood Institute, Bethesda, MD: G. Nemo (project officer), S. Glynn, L. Harvath, E. Leifer, T. Mondoro, E. Wagner; Data and Safety Monitoring Board: S. Geyer, T. Lane, J. Lusher, B. McLeod, A. Reitsma, P. Roberson, A. Shapiro, C. Whitsett.
REFERENCES
- 1.Tinmouth AT, Freedman J. Prophylactic platelet transfusions: which dose is the best dose? A review of the literature. Transfus Med Rev. 2003;17:181–193. doi: 10.1016/s0887-7963(03)00018-x. [DOI] [PubMed] [Google Scholar]
- 2.Heddle NM. Controversy concerning platelet dose. ISBT Sci Ser. 2007;2:220–225. [Google Scholar]
- 3.Stanworth SJ, Hyde C, Brunskill S, Murphy MF. Platelet transfusion prophylaxis for patients with haematological malignancies: where to now? Br J Haematol. 2005;131:588–595. doi: 10.1111/j.1365-2141.2005.05769.x. [DOI] [PubMed] [Google Scholar]
- 4.Norol F, Bieling P, Roudot-Thoraval F, et al. Platelet transfusion: a dose-response study. Blood. 1998;92:1448–1453. [PubMed] [Google Scholar]
- 5.Tinmouth A, Tannock IF, Crump M, et al. Low-dose prophylactic platelet transfusions in recipients of an autologous peripheral blood progenitor cell transplant and patients with acute leukemia: a randomized controlled trial with a sequential Bayesian design. Transfusion. 2004;44:1711–1719. doi: 10.1111/j.0041-1132.2004.04118.x. [DOI] [PubMed] [Google Scholar]
- 6.Heddle NM, Cook RJ, Tinmouth A, et al. A randomized controlled trial comparing standard-and low-dose strategies for transfusion of platelets (SToP) to patients with thrombocytopenia. Blood. 2009;113:1564–1573. doi: 10.1182/blood-2008-09-178236. [DOI] [PubMed] [Google Scholar]
- 7.Slichter SJ. Background, rationale, and design of a clinical trial to assess the effects of platelet dose on bleeding risk in thrombocytopenic patients. J Clin Apher. 2006;21:78–84. doi: 10.1002/jca.20090. [DOI] [PubMed] [Google Scholar]
- 8.SAS/STAT software, version 9.2. Cary, NC: SAS Institute; 2000–2008. [Google Scholar]
- 9.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Rebulla P, Finazzi G, Marangoni F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. N Engl J Med. 1997;337:1870–1875. doi: 10.1056/NEJM199712253372602. [DOI] [PubMed] [Google Scholar]
- 11.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 12.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 13.Cox DR, Oakes D. Analysis of survival data. London: Chapman & Hall. 1984 [Google Scholar]
- 14.Hardin JW, Hilbe JM. Generalized estimating equations. Boca Raton, FL: Chapman & Hall/CRC; 2003. [Google Scholar]
- 15.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 16.Whitaker BI, Green J, King MR, et al. The 2007 National Blood Collection and Utilization Survey report. (Accessed January 25, 2010, at. http://www.hhs.gov/ophs/bloodsafety/2007nbcus_survey.pdf.)
- 17.Nevo S, Swan V, Enger C, et al. Acute bleeding after bone marrow transplantation (BMT ) — incidence and effect on survival: a quantitative analysis in 1,402 patients. Blood. 1998;91:1469–1477. [PubMed] [Google Scholar]
- 18.Slichter SJ. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfus Med Rev. 2004;18:153–167. doi: 10.1016/j.tmrv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Heddle NM, Cook RJ, Sigouin C, Slichter SJ, Murphy M, Rebulla P. A descriptive analysis of international transfusion practice and bleeding outcomes in patients with acute leukemia. Transfusion. 2006;46:903–911. doi: 10.1111/j.1537-2995.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 20.Heddle NM, Cook RJ, Webert KE, Sigouin C, Rebulla P. Methodologic issues in the use of bleeding as an outcome in transfusion medicine studies. Transfusion. 2003;43:742–752. doi: 10.1046/j.1537-2995.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 21.Wandt H, Frank M, Schaefer-Eckart K, Wilhelm M. Routine prophylactic platelet transfusions are not necessary in patients with acute myeloid leukemia — a therapeutic transfusion strategy is safe and cost effective. Blood. 2005;106:129a. abstract. [Google Scholar]
- 22.McCullough J, Vesole DH, Benjamin RJ, et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT trial. Blood. 2004;104:1534–1541. doi: 10.1182/blood-2003-12-4443. [DOI] [PubMed] [Google Scholar]
- 23.Hersh JK, Hom EG, Brecher ME. Mathematical modeling of platelet survival with implications for optimal transfusion practice in the chronically platelet transfusion-dependent patient. Transfusion. 1998;38:637–644. doi: 10.1046/j.1537-2995.1998.38798346631.x. [DOI] [PubMed] [Google Scholar]
- 24.Brecher ME, Hom EG, Hersh JK. Optimal platelet dosing. Transfusion. 1999;39:431–434. doi: 10.1046/j.1537-2995.1999.39499235680.x. [DOI] [PubMed] [Google Scholar]
- 25.Klumpp TR, Herman JH, Gaughan JP, et al. Clinical consequences of alterations in platelet transfusion dose: a prospective, randomized, double-blind trial. Transfusion. 1999;39:674–681. doi: 10.1046/j.1537-2995.1999.39070674.x. [DOI] [PubMed] [Google Scholar]
- 26.Sensebé L, Giraudeau B, Bardiaux L, et al. The efficiency of transfusing high doses of platelets in hematologic patients with thrombocytopenia: results of a prospective, randomized, open, blinded end point (PROBE) study. Blood. 2005;105:862–864. doi: 10.1182/blood-2004-05-1841. [DOI] [PubMed] [Google Scholar]
- 27.Goodnough LT, Kuter DJ, McCullough J, et al. Prophylactic platelet transfusions from healthy apheresis platelet donors undergoing treatment with thrombopoietin. Blood. 2001;98:1346–1351. doi: 10.1182/blood.v98.5.1346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.