Abstract
Background
Many allergic conditions occur more frequently in African-American patients when compared with white patients; however it is not known whether this represents genetic predisposition or disparate environmental exposures.
Objective
To assess the relationship of self-reported race and genetic ancestry to allergic sensitization.
Methods
We included 601 women enrolled in a population-based cohort study whose self-reported race was African-American or white. Genetic ancestry was estimated using markers that differentiate West African and European ancestry. We assessed the relationship between allergic sensitization (defined as ≥1 allergen-specific IgE result) and both self-reported race and genetic ancestry. Regression models adjusted for socio-demographic variables, environmental exposures, and location of residence.
Results
The average proportion of West African ancestry in African-American participants was 0.69, whereas the mean proportion of European ancestry in white participants was 0.79. Self-reported African-American race was associated with allergic sensitization when compared with those who reported being white (adjusted odds ratio [aOR] 2.19; 95% confidence interval [CI] 1.22 – 3.93) even after adjusting for other variables. Genetic ancestry was not significantly associated with allergic sensitization after accounting for location of residence (aOR 2.09 for urban vs. suburban residence, 95% CI 1.32 −3.31).
Conclusion
Self-reported race and location of residence appeared to be more important predictors of allergic sensitization when compared with genetic ancestry, suggesting that the disparity in allergic sensitization by race may be primarily due to environmental factors rather than genetic differences.
Clinical Implications
These data suggest that efforts to eliminate disparities in allergic sensitization should focus on contributing environmental factors.
Keywords: self-reported race, race-ethnicity, continental population group, immunoglobulin E, allergic sensitization
INTRODUCTION
It is generally acknowledged that race is a complex construct, used to broadly categorize individuals into population groups.(1;2) Self-reported race may reflect biogeographic ancestry (e.g., West African, European, East Asian, or Native American);(3;4) however, racial categories are also correlated with socioeconomic status,(5) environmental exposures,(6) and location of residence.(7) While racial categories may correlate with ancestry and its attendant genetic variation, there may be considerable admixture within these groups. For example, African-Americans demonstrate a wide range of predominantly West African and European admixture, and on average comprise 80% West African ancestry.(8)
Differences in the prevalence of allergic conditions by race-ethnicity have now been well-described.(9–11) Total and allergen-specific levels of IgE, which are considered to be markers of allergic inflammation and sensitization, have been shown to be consistently higher among black or African-American individuals when compared with white individuals.(12;13) However, it is not known whether these differences in allergic status can be ascribed predominantly to genetic variation or extrinsic exposures.
Were differences in allergic sensitization by race due to genetic variation, we would expect allergic status to be more strongly correlated with genetic ancestry as opposed to self-reported race. Conversely, were environmental or social exposures of primary importance for sensitization we would expect self-reported race to be more strongly associated.
To examine either possibility we studied a well-characterized cohort of women enrolled in the Wayne County Health Environment Allergy and Asthma Longitudinal Study (WHEALS). Race was assessed by patient self-report and biogeographic ancestry was estimated using genetic, ancestral informative markers (AIMs). We studied the relationship between both self-reported race and geographic ancestry and allergic sensitization before and after adjusting for other potential explanatory variables, such as age, income, education, smoking status, pet and endotoxin exposure, and location of residence.
METHODS
Study population
This study was approved by the Institutional Review Board at Henry Ford Health System and was compliant with its Health Insurance Portability and Accountability Act policy. Recruitment for this study has been described previously.(14) Briefly, expectant mothers receiving care from a large, integrated health care delivery system in metropolitan Detroit, Michigan were invited to participate. To be eligible, pregnant women in their 2nd and 3rd trimester had to be at least 21 years of age; live in a geographically defined area of metropolitan Detroit; and attend one of five clinics in the area for their prenatal care. Mothers’ signed consent was required for participation. The intent of this study is to follow both the newborn and parents longitudinally in order to identify environmental and genetic factors associated with the development of atopy and associated conditions.
Data collection
The mothers and children participating in this study had multiple encounters following delivery (i.e., 1 month, 6 months, 12 months, and 24 months post-partum); however, for the purposes of this analysis we focus on data collected at the 1-month home visit. At this visit, field staff administered a survey which included socio-demographic questions, an assessment of smoking status, and items concerning environmental exposures, such as pets in the home. Mothers’ blood was collected for DNA, total serum IgE levels, and allergen-specific IgE levels. House dust samples were collected and analyzed for endotoxin levels.
Analysis of total serum IgE and allergic sensitization
From the collected venous blood, plasma was separated, frozen, and shipped in batches for analysis of total and allergen-specific IgE in the laboratory of one of the co-investigators (DRO). Total IgE was measured using the total IgE protocol of Pharmacia CAP (Pharmacia Diagnostics AB, Portage, MI) according to established protocols. Assays for allergen-specific immunoglobulin E (IgE) were performed using the same commercial assay procedure. The samples were analyzed for IgE specific to the following seven common aeroallergens: dog (Canis domesticus), cat (Felis domesticus), cockroach (Blatella germanica), ragweed (short, Ambrosia artemisilfolia), grass (timothy, Phleum pratense), Alternaria (Alternaria alternata), and dust mite (Dermatophagoides farinae). Individual allergen specific IgE results ≥0.35 international units per milliliter (IU/ml) were considered to be positive. Allergic sensitization was defined as having one or more positive allergen-specific IgE to one of the seven aeroallergens tested.
Collection of dust and measurement of endotoxin
Dust was collected from five locations in the house. However, for the purposes of this analysis we used dust collected from mothers’ bedroom floors as a proxy of endotoxin exposure. Our procedure for dust collection and endotoxin measurement has been described in detail previously.(14) In brief, a standardized sampling procedure was used to collect dust, whereby a measured string loop was laid out in the shape of a square covering an area of one square meter and the area vacuumed for 2 minutes. Dust samples were sieved through a 292 micrometer mesh filter (Spectra Mesh Polyethylene, Spectrum, Laguna Hills, California) on an orbital shaker for 2 hours. An aliquot of the dust passing through the sieve was extracted in PBS containing 0.05% Tween 20 at room temperature at a ratio of 50 micrograms of dust to 1 ml of PBS. Dust particles were removed from the extracting fluid using a serum filter system (Fisher Scientific, Pittsburgh, Pennsylvania) followed by centrifugation at 25,000 × G for 20 minutes.
Endotoxin activity in dust was measured using the fluorescent microplate assay based on recombinant Limulus factor C (PyroGene Recombinant Factor C, Cambrex Bio Science, Walkersville, Maryland) and an endotoxin standard (Cambrex Bio Science). Endotoxin-free microtiter plates and pipettes were used for all assays. Results were reported as endotoxin units (EU) per milligram of dust (EU/mg). Tests falling below the lower limit of the assay were assigned a value of 50% of the assay’s lower limit if there was satisfactory recovery of the internal control in the sample.
Genotyping
Participating mothers’ genomic DNA was isolated from whole blood using the FlexiGene DNA Kit (Qiagen, Valencia, California). Genotyping was performed on DNA isolated from 722 study participants by the genomics core at the University of California, San Francisco. Both the Mapping 500K Array and the Genome-Wide Human SNP Array 5.0 (Affymetrix, Inc., Santa Clara, California) were used for genotyping. The Mapping 500K Array uses two chips containing single nucleotide polymorphisms (SNPs) found within 200–1,100 base pair (bp), Nsp I and Sty I restriction fragments. The Genome-Wide Human SNP Array 5.0 differs from the Mapping 500K Array in that Nsp I and Sty I fractions are assayed on a single chip. For each individual, 500 nanograms of genomic DNA were digested with Nsp I and Sty I restriction enzymes. DNA was then ligated to adaptors which recognize the overhangs created by the restriction enzyme digest. These adaptors are recognized by PCR primers specific for these added sequences. Adaptor-ligated DNA fragments were amplified under conditions optimized to amplify fragments in the 200 to 1,100 bp size range. Amplified DNA was purified using polystyrene beads, fragmented, labeled, and hybridized to the GeneChip array. Affymetrix Power Tools software package was used to make genotype calls and assess the quality control (QC) call rates for both GeneChip arrays. We removed 42 individuals from the analysis because of missing data for one chip (i.e., among those genotyped with 2 chip arrays) or call rates which did not meet our QC standards. Average call rates exceeded 95% on the remaining individuals.
Admixture analysis
Genotype data from three ancestral populations (i.e., 42 Europeans, 37 West Africans, and 30 Native Americans from Mexico) was provided by two of the co-196 investigators (EGB, SC). The 37 West African samples were from individuals living in London, U.K. and South Carolina, U.S., who are either non-admixed or have very low levels of admixture. The 42 European samples were from Coriell's North American Caucasian panel (Coriell Institute for Medical Research, Camden, New Jersey). The Native American samples (Mayan, n = 15 and Nahua, n = 15) were recruited from villages in Tlapa in the state of Guerrero, Mexico by one of the co-investigators (MDS). DNA from these individuals had been genotyped using the GeneChip® Mapping 100K Set (Affymetrix Inc., Santa Clara, California). Delta (δ) values were calculated for the individual alleles, where δ is the absolute difference in allele frequency between two ancestral populations. A δ value of 1 implies that the allele is completely informative for ancestry, whereas a δ value of 0 implies that the marker is not informative for ancestry. Since a large majority of our study population self-reported either white or African-American race, we selected 493 SNPs that were informative (i.e., δ > 0.65) for estimating African and European ancestry. Each of these AIMs was also required to have <5% missing values in our study population and be in Hardy-Weinberg equilibrium within both ancestral groups.
We used the software package, PSMIX,(15) to estimate individual admixture among our study population. This software uses maximum likelihood estimation to estimate individual admixture, as described by Tang and colleagues.(16) The threshold for stopping was either a less than 10−6 change in the parameter estimate between consecutive iterations or a total of 10,000 iterations. To confirm our admixture estimates we repeated our estimates using another program, STRUCTURE,(17) which uses a Bayesian algorithm to compute individual admixture proportions. In addition, we separately restricted our analysis to AIMs greater than 2 centimorgans (cM) and 5 cM apart to reduce the influence of linkage disequilibrium. Correlation between ancestry estimates generated using STRUCTURE and PSMIX was greater than 0.99, even after stratifying by self-reported race.
Statistical Analysis
For the purposes of this study we restricted our analysis to the 601 (88.4%) of 680 study participants who reported being either white or African-American and who had genotype data. The primary outcome of this analysis was allergic sensitization. Analyses were also repeated assessing total serum IgE level as the outcome.
Chi-squared and Student’s t-test were used to compare differences between study participants who reported being of either white or African-American race. Regression models assessed the relationship of both total serum IgE (linear models) and seroatopy (logistic models) on the independent variables self-reported race-ethnicity (dichotomous) and ancestral admixture (continuous). Total serum IgE was log transformed to normalize the distribution and reduce the effect of extreme outliers. We separately adjusted regression models for other potential mediators, including socio-demographic variables (i.e., age, self-reported income, and level of education), environmental exposures (i.e., individual smoking status, endotoxin levels in home, and pet ownership), and location of residence (i.e., urban vs. suburban). Those living within the cities of Detroit, Hamtramck, or Highland Park (i.e., within the outer city limit of Detroit) were considered urban residents. Persons living outside the outer Detroit city limits were considered suburban residents. Pet ownership was the number of dogs and/or cats in the household and was categorized at 0, 1, and 2 or more pets, as we have done previously.(18) We performed separate analyses of the relationship of self-reported race, genetic ancestry, and location of residence to outcomes after stratifying by the other of these three variables.
As post-hoc analyses, we also examined factors associated with sensitization to individual aeroallergens. Also, as a check, we repeated our analyses incorporating Native American admixture in our regression models. The inclusion or exclusion of this variable had no substantive impact on our findings. We assessed collinearity between self- reported race, genetic ancestry, and location of residence by assessing the variance inflation factor (VIF) of each regression coefficient when regressing log transformed total serum IgE on these variables simultaneously. In this analysis, the VIF values for these variables were 2.31, 1.56, and 1.81, respectively. A high VIF suggests collinearity with at least one other variable in the regression model, and our VIF values were much less than the cut-off of 10 described elsewhere.(19) This supported our inclusion of these variables in the regression models simultaneously. All analyses were performed using SAS v9.1 (SAS Institute Inc., Cary, NC.)(20) A P-value <0.05 was considered statistically significant.
RESULTS
Of the 601 women included in this analysis, 385 (64.1%) reported being African-American and 216 (35.9%) reported being white. The comparison of characteristics among those reporting African-American and white race is shown in Table 1. Participants reporting being African-American were younger, had lower household income, differed in their degree of education, were more likely to reside in an urban location, and were less likely to have a dog or cat living in the home when compared with participants who reported being white. However, white participants were more likely to report Latino (6.0% vs. 0.8%, P-value <0.0001) or Arabic (8.3% vs. 0.0%, P-value <0.0001) ethnicity when compared with African-American participants. African-American participants were more likely to demonstrate allergic sensitization and had higher total serum IgE levels when compared with the white participants. There were also consistent differences in the individual allergen-specific IgE results between groups, and these reached statistical significance for cockroach, ragweed, timothy grass, and Alternaria sp.
Table 1.
Characteristics of women participants in the Wayne Health, Environment, Atopy and asthma Longitudinal Study (WHEALS) stratified by self-reported race.
| All women (n = 601) |
Self-reported race | P-value* | ||
|---|---|---|---|---|
| African American (n = 385) |
White (n = 216) |
|||
| Age in years – mean ± SD† |
29.8 ± 5.1 | 29.2 ± 5.2 | 31.0 ± 4.7 | <0.0001 |
| Self-reported Latino ethnicity – no. (%) |
16/601 (2.7) | 3/385 (0.8) | 13/216 (6.0) | <0.0001 |
| Self-reported Arabic ethnicity – no. (%) |
18/601 (3.0) | 0/385 (0.0) | 18/216 (8.3) | <0.0001 |
| Household income – mean ± SD‡ |
$59,231± $41,695 |
$50,782 ± $35,415 |
$74,455 ± $47,533 |
<0.0001 |
| Education – no. (%) |
<0.0001 | |||
| Less than high school |
29/600 (4.8) | 19/385 (4.9) | 10/215 (4.7) | |
| High school graduate or equivalent |
104/600 (17.3) | 77/385 (20.0) | 27/215 (12.6) | |
| Some college | 289/600 (48.1) | 211/385 (54.8) | 78/215 (36.3) | |
| College graduate or greater |
178/600 (29.7) | 78/385 (20.2) | 100/215 (46.5) | |
| Urban location of residence – no. (%) |
330/601 (54.9) | 304/385 (79.0) | 26/216 (12.0) | <0.0001 |
| Current smoker – no. (%) |
58/514 (11.3) | 37/319 (11.6) | 21/195 (10.8) | 0.773 |
| Dog living in home – no. (%) |
135/503 (26.8) | 61/312 (19.6) | 74/191 (38.7) | <0.0001 |
| Cat living in home – no. (%) |
89/503 (17.7) | 22/312 (7.1) | 67/191 (35.1) | <0.0001 |
| Home dust endotoxin level (EU/mg) – mean ± SD§ |
36.8 ± 79.6 | 40.7 ± 91.6 | 30.9 ± 55.9 | 0.199 |
| Allergic sensitization – no. (%) ∥ |
278/506 (54.9) | 203/320 (63.4) | 75/186 (40.3) | <0.0001 |
| Allergen-specific IgE results by allergen – no. (%) |
||||
| Dog | 97/506 (19.2) | 69/320 (21.6) | 28/186 (15.1) | 0.073 |
| Cat | 101/504 (20.0) | 67/319 (21.0) | 34/185 (18.4) | 0.478 |
| Cockroach | 65/505 (12.9) | 60/319 (18.8) | 5/186 (2.7) | <0.0001 |
| Ragweed | 147/506 (29.1) | 113/320 (35.3) | 34/186 (18.3) | <0.0001 |
| Timothy grass | 127/506 (25.1) | 100/320 (31.3) | 27/186 (14.5) | <0.0001 |
| Alternaria sp. | 101/503 (20.1) | 86/318 (27.0) | 15/185 (8.1) | <0.0001 |
| Dust mite | 112/506 (22.1) | 79/320 (24.7) | 33/186 (17.7) | 0.070 |
| Total serum IgE level (IU/ml) – geometric mean ± geometric SD¶ |
37.8 ± 4.4 | 52.6 ± 4.0 | 21.4 ± 4.4 | <0.0001 |
SD denotes standard deviation; IgE, immunoglobulin E; IU/mg, international units per milligram of dust; and IU/ml, international units per milliliter of serum.
P-value for the comparison of individuals reporting African-American race with those reporting white race.
Number with data 600, 385, and 215, respectively.
Number with data 566, 364, and 202, respectively.
Number with data 453, 275, and 178, respectively.
Allergic sensitization was defined as having one or more positive allergen-specific IgE to one of the seven aeroallergens tested.
Number with data 510, 323, and 187, respectively.
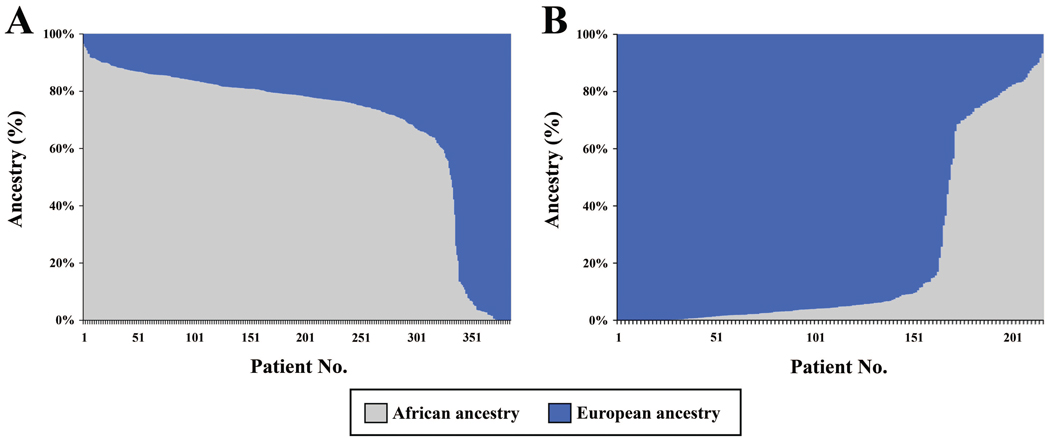
Figures 1A and 1B show the distribution of admixture among individuals who reported being African-American and white, respectively. As can be seen, there was considerable variation in West African and European ancestry in both groups. The average proportion of West African ancestry in self-reported African-American participants was 0.69 (±0.26 standard deviation [SD]), whereas the mean proportion of European ancestry in white participants was 0.79 (± 0.31 SD). As a check of our self-reported race data, we compared self-reported race to participants’ report of how they believed they were perceived by others. There were a total of 7 (1.2%) instances in which these were discordant, and in 5 of these cases participants felt others would categorize them as a race other than African-American or white. Including or excluding these 7 individuals did not substantively alter our findings; therefore, they were included in all analyses.
Figure 1.
Individual admixture estimates for the 385 participants who reported being African-American (A) and for the 216 participants who reported being white (B). Each patient number (column) comprises an individual and their admixture where gray represents African ancestry and blue, European ancestry.
Table 2 shows the unadjusted and adjusted relationship between self-reported race and allergic sensitization. Individuals who reported being African-American were significantly more likely to demonstrate allergic sensitization when compared with individuals who reported being white. This relationship persisted even after adjusting for age, income, educational level, smoking status, dog and cat ownership, house dust endotoxin levels, and location of residence.
Table 2.
Unadjusted and adjusted relationship between self-reported race and allergic sensitization.*
| Predictor variable(s) |
Odds of Allergic Sensitization | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1† | Model 2‡ | Model 3§ | Model 4∥ | |||||
| OR (95% CI) |
P-value | aOR (95% CI) |
P-value | aOR (95% CI) |
P-value | aOR (95% CI) |
P- Value |
|
| African- American self- reported race |
2.57 (1.77,3.72) |
<0.0001 | 2.88 (1.91,4.35) |
<0.0001 | 2.79 (1.74,4.49) |
<0.0001 | 2.19 (1.22,3.93) |
0.009 |
| Age | 0.85 (0.56,1.30) |
0.458 | 0.81 (0.51,1.29) |
0.364 | 0.81 (0.50,1.29) |
0.365 | ||
| Income | 1.01 (0.96,1.06) |
0.710 | 1.02 (0.96,1.07) |
0.592 | 1.02 (0.97,1.08) |
0.491 | ||
| Education | 1.08 (0.87,1.40) |
0.584 | 0.99 (0.73,1.35) |
0.964 | 1.00 (0.74,1.36) |
0.998 | ||
| Smoking status |
0.87 (0.44,1.72) |
0.682 | 0.86 (0.43,1.71) |
0.668 | ||||
| Pet ownership |
1.12 (0.84,1.49) |
0.455 | 1.11 (0.83,1.48) |
0.483 | ||||
| Dust endotoxin level |
0.95 (0.82,1.09) |
0.458 | 0.95 (0.82,1.09) |
0.448 | ||||
| Urban location of residence |
1.47 (0.85,2.52) |
0.167 | ||||||
OR denotes odds ratio; CI, confidence interval; and aOR, adjusted odds ratio
Allergic sensitization is defined as having one or more positive allergen-specific IgE to one of the seven aeroallergens tested. Logistic regression is used to model the relationship of allergic sensitization on the variables listed. Models adjust for all variables with results shown in the column.
Model 1 compares individuals who reported being of African-American race with those who reported being white.
Model 2 includes model 1 plus socio-demographic variables for age (per 10-year increase), income (per $10,000 increase), and education (categorized as less than high school, high school graduate, some college, and college graduate or greater).
Model 3 includes model 2 plus environmental exposure variables for smoking (current smoker = 1 and all others = 0), pet ownership (number of dogs and/or cats owned categorized as 0, 1, and ≥2), and dust endotoxin levels (loge transformed).
Model 4 includes model 3 plus location of residence (residing in the cities of Detroit, Hamtramck, or Highland Park = 1 and all others = 0)
West African ancestry was also predictive of allergic sensitization (Table 3). However, the relationship between West African ancestry and allergic sensitization was no longer statistically significantly after adjusting for location of residence (Table 3, model 4). In this model, urban residence was a significant predictor of allergic sensitization (OR 2.09, 95% CI 1.32 – 3.31).
Table 3.
Unadjusted and adjusted relationship between genetic ancestry and allergic sensitization.*
| Predictor variable(s) |
Odds of Allergic Sensitization | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1† | Model 2‡ | Model 3§ | Model 4∥ | |||||
| OR (95% CI) |
P- value |
aOR (95% CI) |
P- value |
aOR (95% CI) |
P- value |
aOR (95% CI) |
P- value |
|
| African ancestry |
1.75 (1.08,2.82) |
0.022 | 1.73 (1.04,2.86) |
0.034 | 1.84(1.05,3.22) | 0.032 | 1.34 (0.73,2.43) |
0.343 |
| Age | 0.80 (0.53,1.20) |
0.285 | 0.76 (0.48,1.20) |
0.241 | 0.78 (0.49,1.24) |
0.289 | ||
| Income | 0.99 (0.94,1.04) |
0.630 | 1.00 (0.95,1.05) |
0.950 | 1.01 (0.96,1.07) |
0.612 | ||
| Education | 1.02 (0.79,1.32) |
0.875 | 0.94 (0.70,1.26) |
0.672 | 0.97 (0.72,1.32) |
0.866 | ||
| Smoking status |
0.87 (0.44,1.70) |
0.676 | 0.85 (0.43,1.69) |
0.644 | ||||
| Pet ownership |
0.96 (0.73,1.25) |
0.747 | 1.01 (0.77,1.33) |
0.947 | ||||
| Dust endotoxin level |
0.95 (0.82,1.09) |
0.452 | 0.94 (0.82,1.09) |
0.435 | ||||
| Urban location of residence |
2.09 (1.32,3.31) |
0.002 | ||||||
OR denotes odds ratio; CI, confidence interval; and aOR, adjusted odds ratio
Allergic sensitization is defined as having one or more positive allergen-specific IgE to one of the seven aeroallergens tested. Logistic regression is used to model the relationship of allergic sensitization on the variables listed. Models adjust for all variables with results shown in the column. Proportion of African ancestry (i.e. admixture) was assessed with 493 ancestral informative markers which differentiate African and European ancestry.
Model 1 includes proportion of African ancestry (range 0–1).
Model 2 includes model 1 plus socio-demographic variables for age (per 10-year increase), income (per $10,000 increase), and education (categorized as less than high school, high school graduate, some college, and college graduate or greater).
Model 3 includes model 2 plus environmental exposure variables for smoking (current smoker = 1 and all others = 0), pet ownership (number of dogs and/or cats owned categorized as 0, 1, and ≥2), and dust endotoxin levels (loge transformed).
Model 4 includes model 3 plus location of residence (residing in the cities of Detroit, Hamtramck, or Highland Park = 1 and all others = 0)
The same pattern was seen for total IgE levels in that self-reported African-American race was a consistent positive predictor of IgE levels even after adjusting for age, income, educational level, smoking status, dog and cat ownership, house dust endotoxin levels, and location of residence (Table E1 in the online supplemental material). In contrast, West African ancestry was not associated with IgE levels after accounting for location of residence (Table E2, model 4 in the online supplemental material). In this model, urban residence and not genetic ancestry was a significant positive predictor of total serum IgE levels. The consistent relationship between location of residence and both allergic sensitization and total serum IgE levels after stratification by ancestral proportion is also shown in the online supplement (Tables E3 and E4).
Including both self-reported race and admixture simultaneously in regression models showed that only the former remained significantly associated with allergic sensitization and total serum IgE levels (data not shown). Similarly, West African ancestry was no longer significantly associated with allergic sensitization or total serum IgE levels after stratifying by self-reported race (Table 4 and Table E5 in the online supplemental material, respectively). However, after stratifying by ancestral proportion, there were still significant differences in the prevalence of allergic sensitization and total serum IgE levels by self-reported race (Table 5 and Table E6 in the online supplemental material, respectively).
Table 4.
Adjusted relationship between genetic ancestry and allergic sensitization after stratifying by self-reported race*
| Predictor variable(s) |
Odds of allergic sensitization | |||
|---|---|---|---|---|
| Individuals reporting African- American race |
Individuals reporting white race | |||
| aOR (95% CI) | P-value | aOR (95% CI) | P-value | |
| African ancestry | 1.29 (0.50,3.32) | 0.593 | 0.51 (0.18,1.47) | 0.214 |
| Age | 0.96 (0.53,1.73) | 0.878 | 0.58 (0.25,1.31) | 0.187 |
| Income | 1.00 (0.92,1.10) | 0.938 | 1.02 (0.95,1.10) | 0.574 |
| Education | 0.86 (0.58,1.28) | 0.452 | 1.40 (0.81,2.43) | 0.229 |
| Smoking status | 1.09 (0.45,2.63) | 0.848 | 0.62 (0.17,2.29) | 0.473 |
| Pet ownership | 1.18 (0.76,1.83) | 0.465 | 1.02 (0.69,1.52) | 0.915 |
| Dust endotoxin level |
0.91 (0.76,1.09) | 0.315 | 1.02 (0.79,1.33) | 0.866 |
| Urban location of residence |
1.77 (0.92,3.37) | 0.085 | 0.82 (0.28,2.41) | 0.722 |
aOR denotes adjusted odds ratio and CI, confidence interval.
Allergic sensitization is defined as having one or more positive allergen-specific IgE to one of the seven aeroallergens tested. Logistic regression is used to model the relationship of allergic sensitization on the variables listed. Proportion of African ancestry (i.e. admixture) was assessed with 493 ancestral informative markers which differentiate African and European ancestry. Models adjust for all variables shown in the column, including age (per 10-year increase), income (per $10,000 increase), education (categorized as less than high school, high school graduate, some college, and college graduate or greater), smoking status (current smoker = 1 and all others = 0), pet ownership (number of dogs and/or cats owned categorized as 0, 1, and ≥2), dust endotoxin levels (loge transformed), and location of residence (residing in the cities of Detroit, Hamtramck, or Highland Park = 1 and all others = 0).
Table 5.
Difference in allergic sensitization among those who reported African-608 American and white race stratified by the proportion of African Ancestry.*
| African Ancestry (%) | Allergic Sensitization – no. (%) | P-value† | |
|---|---|---|---|
| Self-reported African-American race |
Self-reported white race |
||
| 0–33% | 30/47 (63.8%) | 61/139 (43.9%) | 0.019 |
| 34–66% | 16/29 (55.2%) | 4/6 (66.7%) | 0.680 |
| 67–100% | 157/244 (64.3%) | 10/41 (24.4%) | <.0001 |
Allergic sensitization is defined as having one or more positive allergen-specific IgE to one of the seven aeroallergens tested. Proportion of African ancestry (i.e. admixture) was assessed with 493 ancestral informative markers which differentiate African and European ancestry.
P-value is for testing the difference in the proportion with allergic sensitization between those who reported African-American race and those who reported white race.
Likewise, there were still significant differences in the prevalence of allergic sensitization and total serum IgE levels by self-reported race after stratifying by location of residence. Among individuals with an urban location of residence, 60.5% of African-American participants demonstrated allergic sensitization when compared with 30.0% of white participants (P-value = 0.002), and geometric mean total serum IgE levels were 52.8 (± 4.01 geometric standard deviation [gSD]) and 21.0 (± 4.8 gSD) among African-American and white participants, respectively (P-value <0.0001). Among individuals living in suburban locations, 56.5% of African-American participants demonstrated allergic sensitization when compared with 41.6% of white participants (P-value = 0.045), and geometric mean total serum IgE levels were 51.9 (± 4.06 gSD) and 21.4 (± 4.4 gSD) among African-American and white participants, respectively (P-value = 0.006).
Post-hoc regression analysis demonstrated that self-reported African-American race was significantly associated with allergic sensitization to dog, cockroach, timothy grass, and Alternaria sp. (and was of borderline statistical significance with ragweed sensitization) even after adjusting for age, income, education, smoking status, pet ownership, home endotoxin levels, and location of residence (Table E7 in the online supplemental material). Urban residence was also significantly associated with cockroach sensitization in these models (OR 2.86, 95% CI 1.05 – 7.82). On the other hand, West African ancestry was not significantly associated with sensitization with any specific allergen; however urban residence was significantly associated with sensitization to cockroach (OR 4.60, 95% CI 1.83 – 11.54) and timothy grass (OR 1.94, 95% 1.13 – 3.31) in these models (Table E8 in the online supplemental material).
Removing individuals who reported Latino or Arabic ethnicity (either each group alone or both groups together) from the analysis had no bearing on the results presented; the same relationships remained statistically significant (data not shown). The estimated average proportion of African ancestry among those reporting both Latino ethnicity and African-American race (n = 3) was 0.66 (± 0.07 SD); those reporting Latino ethnicity and white race (n = 13), 0.06 (± 0.04 SD); and those reporting Arabic ethnicity (n = 18), 0.16 (± 0.26 SD).
DISCUSSION
Differences between continental groups (e.g., West African, East Asia, and Europe) account for approximately 10–15% of total genetic variation.(21) This genetic variation could contribute to differences in disease prevalence between populations as a result of differences in either the frequency or effect of alleles implicated in disease pathogenesis.(22;23) Since between-group genetic variation tends to follow historic geographic boundaries,(24) we would expect that the difference in allergic sensitization among African-Americans and whites in the United States would be closely associated with continental ancestry (i.e., West African and European, respectively) if this disparity had a primarily genetic explanation. However, we found that current location of residence (i.e., urban versus suburban) to be more strongly predictive of allergic sensitization when compared with geographic ancestry, suggesting the primacy of current environmental exposures for this outcome.
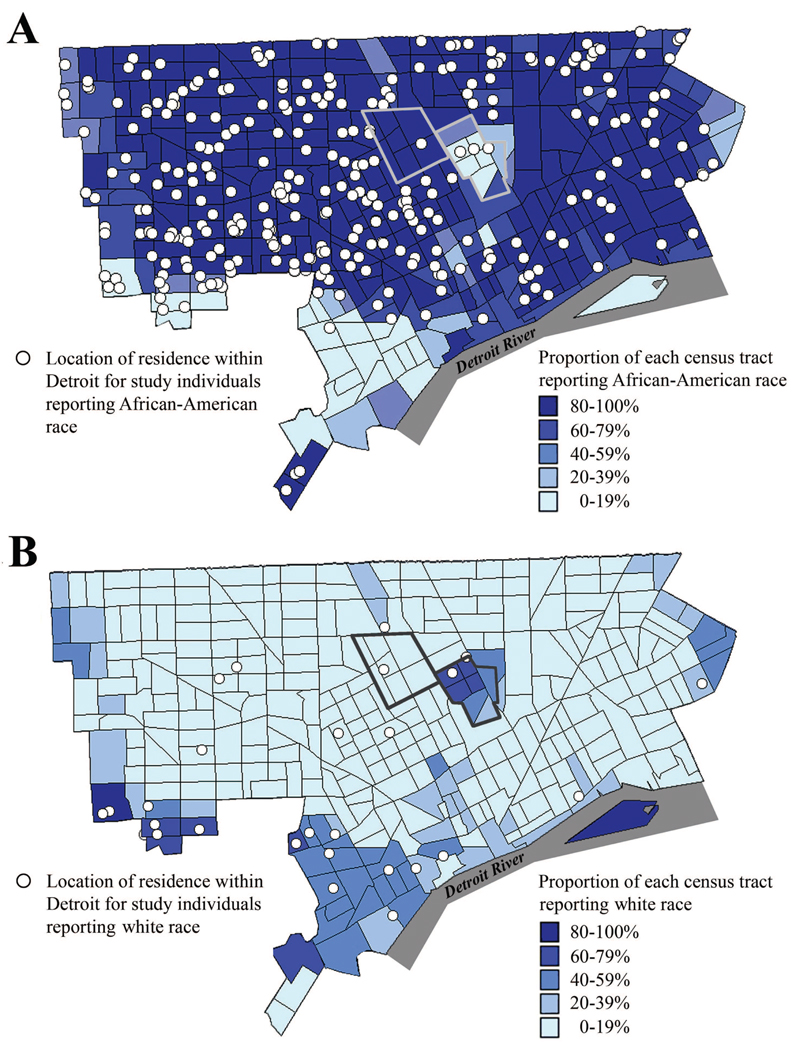
In contrast to ancestry, we also found that self-reported race was a robust predictor of allergic sensitization even after adjusting for socio-demographic variables, environmental exposures, and location of residence. For example, even among persons living within the city of Detroit, 60.5% of African-American participants demonstrated allergic sensitization when compared with 30.0% of white participants (P-value = 0.002). This suggests that additional, unmeasured social or environmental factors may underlie the relationship between self-reported race and allergic sensitization, even among those residing in seemingly similar locations. As Detroit is a highly segregated metropolitan area,(25;26) it is quite plausible that African-American and white participants had different exposures regardless of urban or suburban location of residence. For example, African-American individuals comprise 77% of the city’s population but only 7% of the surrounding population,(27) and figures 2A and 2B show the relative geographic separation of African-American and white study participants even within the city.
Figure 2.
Distribution of study participants within the city limits of Detroit (also includes Hamtramck and Highland Park shown with bolded outlines). Participants who reported being African-American are shown against the proportion of individuals within each census tract who reported being African-American (A), and participants who reported being white are shown against the proportion of individuals within each census tract who reported being white (B). Census tract proportions are taken from year 2000 U.S. Census data.
Sensitization to cockroach antigen appeared to be the one exception where another predictor, urban location of residence, was statistically significant when adjusting for self-reported race. Perhaps this is because cockroach exposure is highly skewed to urban settings. For example, in a study of the homes of children with asthma in the Baltimore area, 64% of homes in the city had evidence of cockroach infestation compared with 0% of suburban homes.(28) In addition, the housing characteristics associated with cockroach infestation, such as living in an apartment (as compared with a house)(29) and increasing physical deterioration of the dwelling,(30) may also be associated with urban living.
Increased likelihood of dog sensitization despite lower pet ownership suggests that factors other than higher allergen exposure may contribute to increased allergic sensitization in African-American patients when compared with white patients. Historically expressways were built through African-American neighborhoods in the city of Detroit and African-Americans increasingly occupied the surrounding areas,(26) this may contribute to greater exposure to air pollution and particulate matter. Studies in both humans and animal models suggest that exposure to traffic particulate matter enhances allergic sensitization and IgE production to aeroallergens.(31–33) Perhaps this and other local environmental exposures may explain in part the general increased allergic sensitization seen in African-American participants.
It is important to note that although biogeographic ancestry did not appear to explain differences in allergic sensitization, genetics may still play an important role in determining allergic sensitization. However, these genetic determinants may occur at a similar frequency and have a similar effect among persons with West African and European ancestry. There is also the possibility of gene-environment interactions which differ by race-ethnicity but were not explored.(34) In addition, because this is a cross-sectional study in adults, we may have passed the window in which the exposures assessed, such as pet exposure and endotoxin, may influence IgE levels. However, this would not influence our conclusions regarding the effect of ancestry, which did not vary.
Persons who reported being African-American and white in our study also had on average less West African (i.e., 69%) and European ancestry (i.e., 79%), respectively, than has been reported elsewhere.(4;8) Perhaps this reflects instances in which racial identity was based in part upon socio-cultural factors related to one’s surroundings rather than genetic ancestry. However, we did not observe differences in West African ancestry between African-American participants with either an urban or suburban residence, and similarly we did not observe differences in European ancestry among white participants residing in those broad categories (Table E9 in the online supplemental material). It is also possible that these estimates are unique to Wayne County, Michigan where 571 (95%) of the 601 participants resided at the time of the study. Native American ancestry was also present to a much lesser extent within our cohort. However, post-hoc analysis did not find that including Native American ancestry would have affected our results. To ensure that the self-reported race categories and ancestry estimates were entered correctly, we compared self-reported race to the race that participants felt others saw them as and we calculated ancestry using multiple methods. The consistency of these checks lends further support to our findings. Despite a relatively large study size, our numbers for analysis became much smaller after stratifying (e.g., by self-reported race, genetic ancestry, or location of residence). Although we have no reason to believe that this negatively influenced our results, our findings should be replicated in other large and diverse, population-based cohorts.
In conclusion we found that location of residence was a stronger predictor of allergic sensitization when compared with ancestry. These findings suggest that the disparity in atopy between African-American and white patients may be primarily due to environmental rather than genetic factors. However, self-reported race continued to be a strong predictor of allergic sensitization even after adjusting for socio-demographic variables, environmental exposures, and location of residence, suggesting that other important social and environmental confounders exist and have yet to be fully characterized. Until this time, self-reported race will likely continue to be an important predictor of allergic status and related conditions.(35)
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the entire WHEALS research staff, especially the members of the Henry Ford Hospital Molecular Epidemiology Research Laboratory. Without your hard work and devotion this paper would not have been possible.
This work was supported by grants from the Fund for Henry Ford Hospital, the Sandler Program for Asthma Research, and the National Institute of Allergy and Infectious Diseases (AI61774, AI50681, AI59415) and the National Heart, Lung, and Blood Institute (HL79055), National Institutes of Health. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- 1.Winker MA. Measuring race and ethnicity: why and how? JAMA. 2004;292(13):1612–1614. doi: 10.1001/jama.292.13.1612. [DOI] [PubMed] [Google Scholar]
- 2.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348(12):1170–1175. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 3.Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: genes, race and disease. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-comment2007. comment2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha M, Larkin EK, Elston RC, Redline S. Self-reported race and genetic admixture. N Engl J Med. 2006;354(4):421–422. doi: 10.1056/NEJMc052515. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs SL, Schroeder SA. Class - the ignored determinant of the nation's health. N Engl J Med. 2004;351(11):1137–1142. doi: 10.1056/NEJMsb040329. [DOI] [PubMed] [Google Scholar]
- 6.Perlin SA, Sexton K, Wong DW. An examination of race and poverty for populations living near industrial sources of air pollution. J Expo Anal Environ Epidemiol. 1999;9(1):29–48. doi: 10.1038/sj.jea.7500024. [DOI] [PubMed] [Google Scholar]
- 7.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parra EJ, Marcini A, Akey J, Martinson J, Batzer MA, Cooper R, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63(6):1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph CL, Williams LK, Ownby DR, Saltzgaber J, Johnson CC. Applying epidemiologic concepts of primary, secondary, and tertiary prevention to the elimination of racial disparities in asthma. J Allergy Clin Immunol. 2006;117(2):233–240. doi: 10.1016/j.jaci.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asthma prevalence and control characteristics by race/ethnicity--United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;53(7):145–148. [PubMed] [Google Scholar]
- 11.Fagan JK, Scheff PA, Hryhorczuk D, Ramakrishnan V, Ross M, Persky V. Prevalence of asthma and other allergic diseases in an adolescent population: association with gender and race. Ann Allergy Asthma Immunol. 2001;86(2):177–184. doi: 10.1016/S1081-1206(10)62688-9. [DOI] [PubMed] [Google Scholar]
- 12.Grundbacher FJ, Massie FS. Levels of immunoglobulin G, M, A, and E at various ages in allergic and nonallergic black and white individuals. J Allergy Clin Immunol. 1985;75(6):651–658. doi: 10.1016/0091-6749(85)90089-2. [DOI] [PubMed] [Google Scholar]
- 13.Litonjua AA, Celedon JC, Hausmann J, Nikolov M, Sredl D, Ryan L, et al. Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol. 2005;115(4):751–757. doi: 10.1016/j.jaci.2004.12.1138. [DOI] [PubMed] [Google Scholar]
- 14.Williams LK, McPhee RA, Ownby DR, Peterson EL, James M, Zoratti EM, et al. Gene-environment interactions with CD14 C-260T and their relationship to total serum IgE levels in adults. J Allergy Clin Immunol. 2006;118(4):851–857. doi: 10.1016/j.jaci.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Wu B, Liu N, Zhao H. PSMIX: an R package for population structure inference via maximum likelihood method. BMC Bioinformatics. 2006;7:317. doi: 10.1186/1471-2105-7-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang H, Peng J, Wang P, Risch NJ. Estimation of individual admixture: analytical and study design considerations. Genet Epidemiol. 2005;28(4):289–301. doi: 10.1002/gepi.20064. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288(8):963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 19.Myers RH. Classical and modern regression with applications. 2nd ed ed. Boston: PWS-KENT; 1990. [Google Scholar]
- 20.SAS Institute Inc. SAS/STAT Users Guide. Version 9.1 ed. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- 21.Jorde LB, Wooding SP. Genetic variation, classification and 'race'. Nat Genet. 2004;36(11 Suppl):S28–S33. doi: 10.1038/ng1435. [DOI] [PubMed] [Google Scholar]
- 22.Guthery SL, Salisbury BA, Pungliya MS, Stephens JC, Bamshad M. The structure of common genetic variation in United States populations. Am J Hum Genet. 2007;81(6):1221–1231. doi: 10.1086/522239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamshad M, Guthery SL. Race, genetics and medicine: does the color of a leopard's spots matter? Curr Opin Pediatr. 2007;19(6):613–618. doi: 10.1097/MOP.0b013e3282f163ca. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, et al. Genetic structure of human populations. Science. 2002;298(5602):2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 25.Schulz AJ, Williams DR, Israel BA, Lempert LB. Racial and spatial relations as fundamental determinants of health in Detroit. Milbank Q. 2002;80(4):677–707. iv. doi: 10.1111/1468-0009.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugrue TJ. The origins of the urban crisis race and inequality in postwar Detroit : with a new preface by the author. 1st Princeton Classic ed ed. Princeton: Princeton University Press; 2005. [Google Scholar]
- 27.Schulz A, Israel B, Williams D, Parker E, Becker A, James S. Social inequalities, stressors and self reported health status among African American and white women in the Detroit metropolitan area. Soc Sci Med. 2000;51(11):1639–1653. doi: 10.1016/s0277-9536(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 28.Simons E, Curtin-Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor environmental differences between inner city and suburban homes of children with asthma. J Urban Health. 2007;84(4):577–590. doi: 10.1007/s11524-007-9205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chew GL, Higgins KM, Gold DR, Muilenberg ML, Burge HA. Monthly measurements of indoor allergens and the influence of housing type in a northeastern US city. Allergy. 1999;54(10):1058–1066. doi: 10.1034/j.1398-9995.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 30.Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspect. 2002;110 Suppl 2:323–327. doi: 10.1289/ehp.02110s2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastain TM, Gilliland FD, Li YF, Saxon A, az-Sanchez D. Intraindividual reproducibility of nasal allergic responses to diesel exhaust particles indicates a susceptible phenotype. Clin Immunol. 2003;109(2):130–136. doi: 10.1016/s1521-6616(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 32.Fernvik E, Scharnweber T, Knopp D, Niessner R, Vargaftig BB, Peltre G. Effects of fractions of traffic particulate matter on TH2-cytokines, IgE levels, and bronchial hyperresponsiveness in mice. J Toxicol Environ Health A. 2002;65(15):1025–1045. doi: 10.1080/152873902760125200. [DOI] [PubMed] [Google Scholar]
- 33.Fernvik E, Peltre G, Senechal H, Vargaftig BB. Effects of birch pollen and traffic particulate matter on Th2 cytokines, immunoglobulin E levels and bronchial hyper-responsiveness in mice. Clin Exp Allergy. 2002;32(4):602–611. doi: 10.1046/j.0954-7894.2002.01347.x. [DOI] [PubMed] [Google Scholar]
- 34.Williams LK, Oliver J, Peterson EL, Bobbitt KR, McCabe MJ, Smolarek D, et al. Gene-environment interactions between CD14 C-260T and endotoxin exposure on Foxp3+ and Foxp3- CD4+ lymphocyte numbers and total serum IgE levels in early childhood. Ann Allergy Asthma Immunol. 2008;100(2):128–136. doi: 10.1016/S1081-1206(10)60421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mountain JL, Risch N. Assessing genetic contributions to phenotypic differences among 'racial' and 'ethnic' groups. Nat Genet. 2004;36(11 Suppl):S48–S53. doi: 10.1038/ng1456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.