Abstract
Dystonia is a neurological disorder characterized by involuntary movements. We examined striatal dopamine function in hyperactive transgenic mice generated as a model of dystonia. Evoked extracellular dopamine concentration was monitored with carbon-fiber microelectrodes and fast-scan cyclic voltammetry in striatal slices from non-transgenic mice, transgenic mice with a positive motor phenotype, and phenotype-negative transgenic littermates. Peak single-pulse evoked dopamine concentration was significantly lower in phenotype-positive mice than in non-transgenic or phenotype-negative mice, but indistinguishable between non-transgenic and phenotype-negative mice. Phenotype-positive mice also had higher functional D2 dopamine autoreceptor sensitivity than non-transgenic mice, which would be consistent with lower extracellular dopamine concentration in vivo. Multiple-pulse (phasic) stimulation (5 pulses, 10-100 Hz) revealed an enhanced frequency dependence of evoked dopamine release in phenotype-positive versus non-transgenic or phenotype-negative mice, which was exacerbated when extracellular Ca2+ concentration was lowered. Enhanced sensitivity to phasic stimulation in phenotype-positive mice was reminiscent of the pattern seen with antagonism of nicotinic acetylcholine receptors. Consistent with a role for altered cholinergic regulation, the difference in phasic responsiveness among groups was lost when nicotinic receptors were blocked by mecamylamine. Together, these data implicate compromised dopamine release regulation, possibly from cholinergic dysfunction, in the motor symptoms of this dystonia model.
Keywords: Acetylcholine, ACh, basal ganglia, brain slices, early-onset dystonia, voltammetry
Introduction
Dystonia is a neurological condition characterized by involuntary movements, sustained muscle contractions, and abnormal postures (Fahn et al., 1998; Bressman, 2004), and is commonly classified as a hyperkinetic movement disorder (Mink, 2003; Jankovic, 2009). The pathophysiology of dystonia is poorly understood; post mortem brain tissue from individuals with primary dystonia shows no obvious neuronal degeneration (Breakefield et al., 2008), in contrast to the marked loss, for example, of striatal projection neurons in Huntington’s disease (Vonsattel et al., 1985; Vonsattel and DiFiglia, 1998) or dopamine (DA) neurons of the substantia nigra pars compacta (SNc) in Parkinson’s disease (Albin et al., 1989). Hence, the development of primary dystonia appears to involve deficits in motor signaling and regulation at a synaptic or circuit level, rather than a gross change in brain structure.
Several lines of evidence implicate the involvement of DA dysfunction in the development of some forms of dystonia (Wichmann, 2008). First, dystonia can occur throughout the course of Parkinson’s disease (Nausieda et al., 1980; Katzenschlager et al., 2002; Bruno et al., 2004), after DA depletion by MPTP in non-human primates (Perlmutter et al., 1997a), and can be a complication of antiparkinsonian therapy (Hallett, 1981; Fabbrini et al., 2007). Second, PET imaging studies in individuals with dystonia suggest altered striatal DA receptor binding and DA uptake (Perlmutter et al., 1997a; Perlmutter et al., 1997b; Naumann et al., 1998; Playford et al., 1993). Third, dystonia is a consequence of DA deficiency in dopa-responsive dystonia that involves impaired DA synthesis (Ichinose et al., 1999; Sato et al., 2008).
Additional evidence implicating DA dysfunction has come from models of early-onset (DYT1) dystonia, which is an autosomal dominantly inherited movement disorder (Kramer et al., 1988, 1994; Risch et al., 1990; Ozelius et al., 1997; Bressman, 2004). The majority of cases are caused by a 3 bp (GAG) deletion in the DYT1 gene on chromosome 9q34 (Ozelius et al., 1989; 1997; Kramer et al., 1994; Risch et al., 1990). The protein encoded by the DYT1 gene, torsinA, is found throughout the brain (Ozelius et al., 1989; Augood et al., 1998, 1999, 2003; Walker et al., 2001). Overexpression of human mutant torsinA (ΔE-torsinA) in heterologous cells leads to formation of ΔE-torsinA-enriched inclusions that contain the vesicular monoamine transporter 2 (VMAT2), which is required for loading DA into vesicles (Misbahuddin et al., 2005). Such in vitro studies have been complemented by data from torsinA-overexpressing transgenic and knockdown mice showing altered striatal DA or metabolite content, albeit with inconsistent patterns of change (Shashidharan et al., 2005; Dang et al 2005, 2006; Grundmann et al., 2007; Zhao et al., 2008; Page et al., 2010). Additionally, decreased striatal DA release has been reported in a model with pan-cellular expression of ΔE-torsinA (Balcioglu et al., 2007) and one with selective ΔE-torsinA expression in DA neurons (Page et al., 2010).
We examined striatal DA release in a mouse model originally developed to express human ΔE-torsinA in neurons using the promoter for neuron-specific enolase (NSE) (Shashidharan et al., 2005). A proportion of these transgenic mice (30-40%) showed motor abnormalities including hyperactivity, bi-directional circling, and dystonic-like limb movements (Shashidharan et al. 2005). At the time our studies were conducted, symptomatic adult mice showed expression of ΔE-torsinA and a 40% decrease in striatal DA content (Shashidharan et al., 2005). Contemporaneous electrophysiological studies revealed aberrant firing patterns of basal ganglia output neurons and prolonged muscle contractions in mice with motor abnormalities (Chiken et al., 2008). We compared patterns of DA release and uptake in striatal slices from transgenic mice classified by their hyperactive motor phenotype [Phe(+)], transgenic littermates without overt changes in motor function [Phe(−)], and non-transgenic (non-Tg) mice, using single-pulse (1 p, tonic-like) stimulation or multiple-pulse (5 p, phasic-like) stimulation.
Materials and methods
Transgenic mice
All animal handling procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committees of the Mount Sinai School of Medicine and New York University School of Medicine. Transgenic mice were produced on a C57BL/6J × C3H background and bred as heterozygotes onto a C57BL/6J background (Shashidharan et al., 2005). Originally, four transgenic mouse lines were produced; although each had a different level of transgene expression, 30-40% of each line developed abnormal involuntary movements with dystonic-appearing self-clasping of limbs, hyperkinesia, and circling with no directional preference in a given mouse (Shashidharan et al., 2005). The mice examined in the present study were from line TG#13; Phe(+) mice from this line showed rotation rates of at least 1 cycle per minute (with the majority showing at least 5-10 cycles per minute) during a given monitoring period, whereas Phe(−) and non-Tg mice showed either no rotation behavior or less than one turn per 5 minute period (for methods, see Shashidharan et al., 2005). Genotype was confirmed in all mice used in this study, as previously (Shashidharan et al., 2005); ΔE-torsinA expression was not examined. Because no gender differences in motor dysfunction were seen in either transgenic group (Shashidharan et al., 2005), both male and female littermates were examined here; however, for each experimental group, mice were matched by age, sex, and weight.
Slice preparation
Coronal forebrain slices (350 μm thickness) were prepared from adult transgenic and non-Tg mice, and then maintained in HEPES-buffered artificial CSF (aCSF) at room temperature for at least 1 h before experimentation (Chen and Rice 2001; Avshalumov et al. 2003; Li et al., 2010). Recordings were made in a submersion chamber at 32 °C; the superfusing aCSF contained (in mM): 124 NaCl, 3.7 KCl, 26 NaHCO3, 1.5 or 2.4 CaCl2, 2.2 or 1.3 MgSO4, 1.3 KH2PO4, and 10 glucose (equilibrated with 95% O2-5% CO2). In experiments to examine the Ca2+-dependence of DA release, total divalent ion concentration was maintained by adjusting Mg2+ concentration.
Carbon-fiber microelectrodes and DA recording in motor striatum
Evoked DA release was monitored using fast-scan cyclic voltammetry (FCV) with carbon-fiber microelectrodes purchased from WPI Inc (Sarasota, FL) or made in-house from 7-μm carbon fibers (Patel and Rice, 2006; Patel et al., 2009). Release of DA was elicited in the dorsolateral quadrant of striatum (motor striatum) of mouse brain slices using a bipolar stimulating electrode placed on the slice surface 100-150 μm ventromedially to the carbon-fiber microelectrode (Li et al., 2010). The stimulation paradigm was either a single pulse (1 p) or a five-pulse burst (5 p) at 10, 25 or 100 Hz (Patel et al., 2003; Rice and Cragg, 2004; Li et al., 2010). Pulse duration was 100 μs and pulse amplitude was 0.4-0.6 mA; evoked DA release under these conditions is action-potential (blocked by tetrodotoxin) and Ca2+ dependent (Chen and Rice, 2001: Patel et al., 2003). Inter-stimulus interval was 5 min with either paradigm.
Data were obtained using a Millar Voltammeter (available on request from Julian Millar, Queen Mary, University of London, UK), with data acquisition controlled by Clampex 7.0 software (Molecular Devices, Foster City, CA), which imported voltammograms to a PC via a DigiData 1200B A/D board (Molecular Devices). Scan rate for FCV was 800 V s−1 with a sampling interval of 100 ms. Voltammograms were obtained in two-electrode mode, with a Ag/AgCl wire in the recording chamber as the reference electrode. Identification of DA as the release signal was based on characteristic DA voltammograms, as previously (Bao et al., 2005; Patel and Rice, 2006). Electrodes were calibrated with DA in aCSF in the recording chamber at 32°C after each experiment in the presence of each reagent tested for calculation of evoked extracellular DA concentration ([DA]o).
Experimental design
Release of DA evoked by 1 p or 5 p at 10, 25 and 100 Hz applied at 5 min intervals was sampled at 2-4 sites in the dorsolateral striatum of each slice. In some slices from each mouse cohort examined, the falling phases of 1 p evoked [DA]o were compared as a qualitative index of DA uptake. At each site, peak 1 p evoked [DA]o was typically constant by the third stimulus; after stable release was obtained, 5 p stimulations at each frequency were applied in random order at the same site to examine the sensitivity of peak evoked [DA]o to stimulation frequencies that mimic characteristic DA neuron burst firing (see Rice and Cragg, 2004). After the last frequency, 1 p evoked [DA]o was again determined and averaged with the last pre-train 1 p evoked [DA]o to give control 1 p evoked [DA]o for that recording site; typically pre-train and post-train evoked [DA]o differed by <15%. To account for this slight difference, the ratio of 5 p to 1 p evoked [DA]o for each site was determined using this average 1 p evoked [DA]o.
To examine the sensitivity of D2 DA autoreceptor regulation of DA release, 1 p stimulation at 5 min intervals was used and peak [DA]o monitored during the application of the D2 agonist quinpirole. Under control conditions, peak single-pulse evoked [DA]o is stable for at least 2-3 hours at 5 min intervals (e.g., Bao et al., 2005). The use of 1 p stimulation enabled the efficacy of quinpirole on D2 release-regulating receptors to be assessed in the absence of competition from the endogenous ligand (Palij et al., 1990; Limberger et al., 1991; Patel et al., 1995; Patel et al., 2003) or from modulation by either glutamate or GABA via the local microcircuitry (Avshalumov et al., 2003; Chen et al., 2006). After four to five consistent 1 p evoked responses were obtained, quinpirole was applied at increasing concentrations (1 nM to 10 μM), with application of each concentration until peak evoked [DA]o was again stable (typically 20-30 min). Concentration-response data (expressed as % inhibition of [DA]o) for individual experiments were analyzed by non-linear regression to provide a one-component sigmoidal curve using Prism 3.0 software (GraphPad Software Inc. San Diego, CA). The pharmacological parameters for quinpirole, Imax (maximal response) and IC50 (drug concentration required to produce half maximal inhibition), were derived from these fitted curves, as previously (Patel et al., 1995; Patel et al., 2003).
To examine the Ca2+-dependence of the frequency response of DA release, 5 p to 1 p ratios were obtained in an extracellular Ca2+ concentration ([Ca2+]o) of 2.4 or 1.5 mM, then the experiment repeated at the same site after changing to the other [Ca2+]o. Although the order of frequency presentation was varied among recording sites, the order examined at a given site was the same for both [Ca2+]o tested. To examine the effect of nicotinic ACh receptor (nAChR) blockade on the frequency dependence of DA release, 5 p to 1 p ratios were obtained under control conditions, then in the presence of a nAChR antagonist, mecamylamine; the same order of 5 p frequencies was used before and after mecamylamine for a given site.
Statistical Analysis
Data are given as means ± SEM (where n indicates number of recording sites in 2-4 slices from 3-5 animals per group for each experimental series) and illustrated as either absolute [DA]o, the ratio of 5 p evoked [DA]o to 1 p, or percent inhibition of evoked [DA]o normalized with control 1 p evoked peak [DA]o taken as 100%. As previously (Chen and Rice, 2001; Rice and Cragg, 2004; Li et al., 2010), statistical comparisons were based on the number of sites rather than number of slices or animals, because site-to-site variability within striatum typically exceeds variability between slices or between animals in a given cohort. Significance of differences was assessed using one-way or two-way ANOVA, as appropriate. Two-way ANOVA was used to compare the falling phase of DA release profiles as an index of differences in DA uptake rate across the groups examined. Significance was considered to be p < 0.05.
Drugs and Chemicals
All experimental solutions were prepared immediately before use. Components of HEPES-buffered and superfusing aCSF solutions were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO), as were DA and mecamylamine. Quinpirole was from Tocris Cookson (Ellisville, MO). All drugs were water soluble and were prepared as aqueous stock solutions then dissolved directly in aCSF immediately before use.
Results
Decreased DA release, but unaltered DA uptake in Phe (+) mice
DA neurons display two modes of discharge: tonic background firing and phasic (burst) firing that is associated with motivation-related behaviors (Grace and Bunny, 1984; Romo and Schultz, 1990; Grace, 1991; Ljungberg et al., 1992). Here we considered 1 p evoked [DA]o to represent action-potential-dependent release from tonic activity, given the independence of peak 1 p evoked [DA]o from autoreceptor regulation and concurrently released transmitters, as already discussed. Local 5 p stimulation trains at 10, 25 or 100 Hz were chosen to mimic phasic firing (Schultz, 2002; Hyland et al., 2002). Normalized 5 p to 1 p [DA]o ratios were then used to compare phasic-to-tonic responsiveness (Trout and Kruk, 1992; Patel et al., 1992; Rice and Cragg, 2004) in non-Tg and transgenic Phe(−), and Phe(+) mice as an index of striatal DA signaling across groups.
Under control conditions with 2.4 mM [Ca2+]o, average peak [DA]o evoked by 1 p in the striatum of non-Tg mice was 2.30 ± 0.16 μM (n = 49). Peak 1 p evoked [DA]o in the striatum of Phe(−) mice (2.29 ± 0.11 μM, n = 52) did not differ from that in non-Tgs (p > 0.05) (Fig. 1A,B). In striking contrast, hyperactive Phe(+) mice showed significantly lower 1 p evoked [DA]o than either non-Tg mice or Phe(−) littermates, with a mean 1 p evoked [DA]o of 1.49 ± 0.21 μM (n = 49) (p < 0.05 Phe(+) vs. non-Tg or Phe(−) mice) (Fig.1A,B).
Fig.1. Single-pulse (1 p) or five-pulse (5 p) evoked DA release in the striatum is lower in Phe(+) than Phe(−) or non-Tg mice.
A. Representative 1 p evoked [DA]o recorded in striatal slices from non-Tg mice, Phe(−) and Phe(+) mice in 2.4 mM [Ca2+]o. B. Mean 1 p evoked [DA]o for each group (*p < 0.05 non-Tg vs. Phe(+) mice; #p < 0.05 Phe(−) vs. Phe(+) mice; n = 49-52 sites per group). C. Time-course of 1 p evoked [DA]o in striatal slices from non-Tg, Phe(−) and Phe(+) mice, with peak evoked [DA]o taken as 100% and error bars omitted for clarity. The falling phase of averaged 1 p release records were indistinguishable indicating similar DA clearance (p > 0.05, n = 5 each). D. Mean 5 p evoked [DA]o at 10, 25 or 100 Hz in each group of mice (*p < 0.05 non-Tg vs. Phe(+) mice; #p < 0.05 Phe(−) vs. Phe(+) mice; n = 49-52 sites per group).
Monitored evoked [DA]o reflects both release and uptake. To investigate possible involvement of altered DA uptake by the DA transporter (DAT) in the transgenic mice examined, we compared the time-courses of DA clearance after release among the three groups. The falling phases of averaged representative 1 p evoked [DA]o records from these three groups were indistinguishable (p > 0.05 for any pairwise comparison, n = 5 per group) (Fig. 1C), indicating a lack of difference in DA uptake among the groups.
We next compared peak [DA]o evoked by 5-pulse trains at 10, 25 and 100 Hz among the three groups of mice. As with 1 p stimulation, 5 p evoked [DA]o at 10, 25 and 100 Hz in 2.4 mM [Ca2+]o, was significantly lower in Phe(+) mice (n = 49) than in non-Tg (n = 49) or Phe(−) mice (n = 52) (p < 0.05 Phe(+) vs. non-Tg or Phe(−) at each frequency) (Fig. 1D). The relatively limited frequency dependence of evoked DA release in all groups is consistent with previous observations that short-term depression of release occurs rapidly at striatal synapses and diminishes release probability for successive pulses (Trout and Kruk, 1992; Patel et al., 1992; Cragg, 2003). Nonetheless, 5 p evoked [DA]o at each frequency was significantly higher than 1 p evoked DA release (p < 0.05 vs. 1 p for each frequency in all groups, n = 49-52 sites).
Enhanced sensitivity of D2 autoreceptor regulation of DA release in Phe(+) mice
Release of DA from nigrostriatal terminals is regulated by inhibitory D2 DA autoreceptors on striatal DA axons (Sesack et al., 1994) both in vivo and in in vitro brain slices (Palij et al., 1990; Limberger et al., 1991; Benoit-Marand et al., 2001; Phillips et al., 2002; Patel et al., 2003). Moreover, decreased tonic DA release in vivo can lead to a compensatory up-regulation of D2 autoreceptor function that can be determined in vitro (Patel et al., 2003). We therefore compared D2 autoreceptor sensitivity among the three groups of mice. In all groups, activation of D2 receptors by quinpirole (1 nM to 10 μM) inhibited 1 p DA release in a concentration-dependent manner (Fig. 2A,B), as described previously (Palij et al., 1990; Patel et al., 2003). However, the quinpirole IC50, which is the concentration at which DA release inhibition was half-maximal, differed among the groups (Fig. 2B and Table 1). The IC50 in Phe(+) striatum was ~40% lower than non-Tg mice (p < 0.05 vs. non-Tg, n = 5 per group) (Table 1), indicating enhanced D2 receptor sensitivity. The maximum inhibition of DA release (Imax) did not differ among groups (Table 1).
Fig. 2. D2 receptor regulation of striatal DA release is enhanced in Phe(+) mice.
A.Representative records of 1 p evoked [DA]o in a non-Tg mouse under control conditions and in increasing concentrations of the D2 receptor agonist, quinpirole (1 nM to 10 μM). B. Mean concentration-response curves for the inhibition of DA release by quinpirole in non-Tg, Phe(−) and Phe(+) mice. Values are expressed as % inhibition of DA release (mean ± SEM, n = 5 for each group) against log concentrations of quinpirole; dashed lines indicate the quinpirole concentration at which peak 1 p evoked [DA]o was inhibited by 50%. Actual IC50 and Imax values for each group (see Table 1) were calculated from fitting one-component sigmoidal curves to concentration-response data from individual experiments.
Table 1. IC50 and Imax values for inhibition of 1 p evoked [DA]o by the selective D2 receptor agonist quinpirole in non-transgenic (non-Tg), phenotype positive (Phe(+)) and phenotype negative (Phe(−)) mice.
| Group | IC50 (nM) | Imax (%) |
|---|---|---|
| Non-Tg | 46.7 ± 5.3 | 96.8 ± 1.1 |
| Phe(−) | 35.6 ± 6.3 | 93.0 ± 1.4 |
| Phe(+) | 29.7 ± 5.2* | 91.0 ± 2.6 |
IC50 and Imax values were calculated by fitting a one-component sigmoidal curve to concentration-response data from individual experiments and are expressed as means ± SEM (n = 5 per group). The IC50 for DA release inhibition by quinpirole was significantly lower in Phe (+) mice (p < 0.05 vs. non-Tg) indicating enhanced sensitivity for D2 receptor regulation of DA release. See Methods for experimental details.
Enhanced sensitivity to phasic versus tonic stimulation in Phe(−) and Phe(+) mice
We next compared the 5 p to 1 p ratios for 10, 25 and 100 Hz among Phe(+), Phe(−), and non-Tg mice as an index of DA signaling in each group (Fig. 3). In the usual 2.4 mM [Ca2+]o, the ratio of 5 p to 1 p evoked [DA]o in non-Tg mice was slightly over unity for each of these frequencies (n = 49 sites). However, the ratios were significantly higher for Phe(+) (n = 49) than for non-Tg mice at 10, 25 and 100 Hz (p < 0.001 vs. non-Tg mice for each frequency) (Fig. 3A). The ratio of 5 p to 1 p release (n = 52) was also higher in Phe(−) than non-Tg mice at 10 and 25 Hz (p < 0.05 for each frequency) (Fig. 3A).
Fig. 3. Enhanced responsiveness of striatal DA release in Phe(+) mice to phasic versus tonic stimulation is Ca2+ dependent.
A. Mean evoked [DA]o elicited by 5 pulse (5 p) stimulus trains at 10, 25 and 100 Hz in striatal slices from each group, normalized to 1 p evoked [DA]o in 2.4 mM [Ca2+]o (n = 49-52 sites). B. Mean evoked [DA]o elicited by 5 pulse (5 p) stimulus trains at 10, 25 and 100 Hz in striatal slices from each group, normalized to 1 p evoked [DA]o in 1.5 mM [Ca2+]o (n = 24-27). Significance of differences among the groups for each frequency in either A) 2.4 mM [Ca2+]o or B) 1.5 mM [Ca2+]o is indicated by *p < 0.05, **p < 0.01, ***p < 0.001 for non-Tg vs. Phe(+) or Phe(−) mice; #p < 0.05, ##p < 0.01, ###p < 0.001 for Phe(−) vs. Phe(+) mice (one- and two-way ANOVA for repeated measures with Bonferoni post hoc tests). C. Lowering [Ca2+]o from 2.4 to 1.5 mM selectively amplifies the phasic (5 p) to tonic (1 p) ratio of evoked [DA]o at 10, 25, and 100 Hz in Phe(+) mice (**p < 0.01 for 1.5 mM [Ca2+]o vs. same site response in 2.4 mM [Ca2+]o; n = 27-49 sites per point). No Ca2+ dependence of 5 p to 1 p ratio was seen in non-Tg or Phe(−) mice (n = 24-52 sites per group) (two-way ANOVA for all comparisons).
Axonal DA is released through classical vesicle exocytosis in a Ca2+-dependent manner (Bergquist et al., 1998; Phillips and Stamford, 2000; Chen and Rice, 2001). To test the possible involvement of altered exocytotic release in the enhanced phasic-to-tonic responsiveness seen in Phe(+) and Phe(−) mice, we assessed the Ca2+-dependence of the frequency dependence of DA release. As expected for Ca2+-dependent transmitter release, evoked [DA]o was significantly lower in 1.5 mM [Ca2+]o than in 2.4 mM [Ca2+]o at each frequency tested (p < 0.01 for 1.5 mM vs. 2.4 mM [Ca2+]o in all groups, n = 27-52 sites). Importantly, the difference in phasic-to-tonic responsiveness between Phe(+) and non-Tg and Phe(−) mice was amplified in 1.5 mM [Ca2+]o (p < 0.01 Phe(+) vs. Phe(−) or non-Tg mice; n = 24-27 sites) (Fig. 3B). By contrast, the difference in 5 p evoked [DA]o between Phe(−) and non-Tg mice was lost at 1.5 mM [Ca2+]o (p > 0.05 Phe(−) vs. non-Tg mice for each frequency; n = 24 sites each) (Fig. 3B).
The relative effects of [Ca2+]o on phasic-to-tonic responsiveness for each group can be seen when the data are plotted separately for each group of mice (Fig. 3C). This representation shows that the change in [Ca2+]o, and thus Ca2+ entry, had little influence on the 5 p to 1 p ratio in non-Tg and Phe(−) mice, reflecting a similar Ca2+ dependence for 1 p and burst-like stimulation. When [Ca2+]o was decreased in slices from Phe(+) mice, however, the phasic-to-tonic responsiveness of striatal DA release was significantly enhanced (Fig. 3C), implicating a role for impaired exocytosis.
Differences in phasic-to-tonic responsiveness are lost when nAChRs are blocked
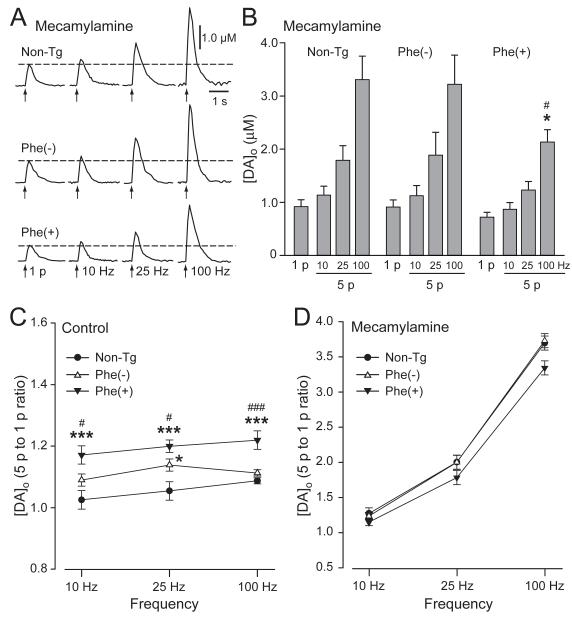
Striatal DA axon terminals express nAChRs (Dajas-Bailador and Wonnacott, 2004; Dani and Bertrand, 2007). Previous studies have shown that inhibition of striatal nAChRs suppresses DA release evoked by low tonic-like frequencies, but selectively enhances DA release evoked by higher, burst-like frequencies (Rice and Cragg, 2004; Zhang and Sulzer, 2004). This pattern of release is similar to the characteristics of evoked [DA]o in Phe(+) mice under control conditions described here (Fig. 3A,B). Moreover, there is evidence of cholinergic dysfunction in a previously described mouse model of DYT1 dystonia (Pisani et al, 2006; Martella et al., 2009). We therefore tested the hypothesis that enhanced phasic responsiveness of DA release in Phe(+) mice might involve altered cholinergic tone. Evoked [DA]o was monitored in Phe(+), Phe(−) and non-Tg mice in the absence, and then the presence of mecamylamine (10 μM; Rice and Cragg, 2004), a selective antagonist of nAChRs (Fig. 4). Consistent with facilitation of tonic DA release by endogenous ACh (Zhou et al., 2001), blockade of nAChRs by mecamylamine caused a similar ~50-60% decrease in 1 p evoked [DA]o, with control 1 p evoked [DA]o for each group taken as 100% for that group. In mecamylamine, evoked [DA]o was 0.92 ± 0.13 μM in non-Tg, 0.91 ± 0.13 μM in Phe(−), and 0.70 ± 0.09 μM in Phe(+) mice (n = 17-22). Mecamylamine also enhanced 5 p evoked [DA]o in a frequency-dependent manner in all three groups (Fig. 4A,B). However, the differences in the responsiveness of DA release to burst-like stimulation among Phe(+), Phe(−) and non-Tg mice seen under control conditions (Figs. 3A and 4C) were lost when nAChRs were blocked (Fig. 4D) (p > 0.05 for all comparisons; n = 17-22 sites). In fact, in mecamylamine there was a trend towards a lower 5 p to 1 p ratio in Phe(+) mice versus non-Tg or Phe(−) mice, especially at the higher frequencies tested (Fig. 4D).
Fig. 4. Differences in phasic versus tonic DA release between Phe(+) and non-Tg or Phe(−) mice are lost when nAChRs are blocked.
A. Representative 1 p and 5 p evoked [DA]o recorded in striatal slices from non-Tg, Phe(−) and Phe(+) mice in the presence of mecamylamine (10 μM), an antagonist of nAChRs. B. Mean 1 p or 5 p evoked [DA]o in mecamylamine in each group of mice (*p < 0.05 vs. non-Tg mice; #p < 0.05 Phe(−) vs. Phe(+); n = 17-22 per group). C. Ratio of 5 p to 1 p evoked [DA]o under control conditions showing enhanced sensitivity to phasic simulation in Phe(+) mice (*p < 0.05, ***p < 0.001 vs. non-Tg mice; #p < 0.05, ###p < 0.001 vs. Phe(−) mice (n = 17-22). D. Differences among the three groups were lost in mecamylamine (p > 0.05 for all comparisons; two-way ANOVA).
Discussion
Here we examined striatal DA release in the dorsolateral striatum from transgenic mice originally developed to express human ΔE-torsinA in neurons (Shashidharan et al., 2005). We found that both tonically and phasically evoked DA release were significantly lower in striatal slices from Phe(+) mice than those from either asymptomatic Phe(−) littermates or non-Tg mice. Hyperactive Phe(+) mice also showed increased D2 DA autoreceptor sensitivity compared to non-Tg mice, which would be consistent with lower extracellular [DA]o in vivo. Additionally, evoked striatal DA release in Phe(+) mice showed an enhanced frequency dependence compared to Phe(−) or non-Tg mice; this difference was eliminated when nAChRs were blocked, implicating a role for cholinergic, as well as DAergic dysregulation in the motor phenotype of Phe(+) mice.
Correlations between any animal model of a movement disorder and the human phenotype must be made with caution, given the potential differences in expression of motor abnormalities in species-specific basal ganglia, in addition to potentially unknown effects of transgene manipulations. The mouse model we have studied, like many other genetic mouse models, does not exhibit overt dystonia. However, previous findings from these mice support the potential relevance of this mouse dystonia model. Basal ganglia output neurons in the internal and external segments of the globus pallidus (GPi and GPe) of Phe(+) mice show decreased firing rates and irregular bursts and pauses (Chiken et al. 2008), which are also seen in patients with dystonia (Vitek et al 1999; Zhuang et al. 2004; Starr et al. 2005). In addition, electromyography (EMG) of these mice demonstrate prolonged co-contractions of agonist and antagonist muscles (Chiken et al. 2008), which is also characteristic of human dystonia (Herz, 1944; Yanagisawa and Goto, 1971; Obeso et al., 1983; Marsden and Rothwell, 1987; Cohen and Hallett, 1988; Berardelli et al., 1998; Farmer et al., 1998; Liu et al., 2004). Although these similarities support the value of Phe(+) mice as a model of this movement disorder, another important limitation is that there are observations that suggest that the nature of the model may have changed over time, and raise questions about the relationship of the phenotypes to the expression of the torsinA transgene. Our studies were conducted in or before 2006, contemporaneous with other published studies of these animals. In later generations of this mouse line, some investigators observed that expression of the ΔE-torsinA transgene was no longer detectable, yet there was persistence, albeit in a diminished form, of the hyperactive phenotype (unpublished observations). These findings raise the possibility that some aspects of the phenotype may arise from insertional effects or other events not directly related to expression of the ΔE-torsinA protein.
Decreased DA release with unaltered DA uptake in Phe(+) mice
Evoked striatal [DA]o was markedly lower in Phe(+) mice than in either non-Tg or Phe(−) mice. Lower 1 p evoked [DA]o in Phe(+) mice is consistent with previous evaluation of striatal DA content in each group, which showed significantly lower striatal DA levels in Phe(+) than in non-Tg mice (Shashidharan et al., 2005). Decreased DA content and evoked [DA]o are unlikely to be consequences of increased activity per se in Phe(+) mice, because exercise has been shown to increase DA content and DA release in mouse striatum (Petzinger et al., 2007). Notably, Phe(−) mice have significantly higher striatal DA content than non-Tgs (Shashidharan et al., 2005). Coupled with our finding that 1 p evoked [DA]o in Phe(−) mice did not differ from that in non-Tg mice, this suggests that elevated tissue DA content might reflect a compensatory mechanism that helps maintain normal DA release levels in Phe(−) mice.
We found no evidence for increased [DA]o clearance as an explanation for lower evoked [DA]o in Phe(+) mice. Indeed, although wildtype torsinA has been shown to influence DAT trafficking to the plasma membrane, ΔE-torsinA does not (Torres et al., 2003). These data are consistent with other studies indicating either no change or a possible decrease in DAT binding sites or DA uptake in mice overexpressing ΔE-torsinA (Shashidharan et al., 2005; Balcioglu et al., 2007; Hewett et al., 2010; Page et al., 2010). Thus, lower evoked [DA]o in Phe(+) mice compared to non-Tg or Phe(−) mice reflects lower DA release, not enhanced DA uptake.
Altered DA content and/or DA release has been found in several transgenic mouse models of DYT1 dystonia (Dang et al., 2005, 2006; Grundmann et al., 2007; Balcioglu et al., 2007; Zhao et al 2008; Page et al., 2010). Moreover, Misbahuddin et al. (2005) showed that ΔE-torsinA-enriched inclusions contain VMAT2. This suggests that ΔE-torsinA might interfere with filling DA vesicles by VMAT2, which could contribute to the lower DA content of Phe(+) mice (Shashidharan et al., 2005), despite similar numbers of striatal VMAT2 binding sites in ΔE-torsinA transgenic mice and their non-Tg littermates (Balcioglu et al., 2007). Data from our evaluation of striatal D2 DA autoreceptor function would also be consistent with lower [DA]o in vivo in Phe(+) versus non-Tg mice, with a lower IC50 for quinpirole-induced inhibition of DA release in Phe(+) indicating enhanced D2 receptor sensitivity. Previous studies have shown that increased DA receptor sensitivity is a compensatory response to low [DA]o (Kim et al., 2000), including that seen in VMAT2 mutant mice (Patel et al., 2003). Although there is evidence for a decrease in total D2 receptor number in human dystonia (Perlmutter et al., 1997b), such measurements do not distinguish between pre- and postsynaptic D2 receptors or provide an indication of the functional efficacy of D2 autoreceptor activation, as examined here.
Enhanced sensitivity of DA release to phasic stimulation in Phe(+) dorsolateral striatum
In contrast to the generally lower evoked [DA]o in Phe(+) mice, the responsiveness of evoked [DA]o to phasic versus tonic stimulation (5 p to 1 p ratio) was significantly enhanced compared to that in either Phe(−) or non-Tg mice. The phasic-to-tonic responsiveness of DA release was also slightly enhanced in Phe(−) mice compared to that in non-Tg mice, indicating some level of altered DA release patterns, whether or not the mice exhibited the positive motor phenotype. The difference in the sensitivity of DA release to phasic stimulation between Phe(+) and Phe(−) or non-Tg mice was Ca2+ dependent, with amplification of differences when Ca2+ availability was lowered. Such Ca2+ dependence would be consistent with an impairment in Ca2+-dependent vesicle exocytosis in Phe(+) mice. Enhanced phasic-to-tonic DA signaling, especially when paired with increased DA receptor sensitivity, could be a potential contributing factor to the motor characteristics of Phe(+) mice.
The role of cholinergic tone in enhanced phasic sensitivity of DA release in Phe(+) mice
Decreased 1 p evoked striatal DA release in Phe(+) mice and enhanced sensitivity to phasic versus tonic stimulation mirror the pattern of changes in DA release seen when nAChRs are blocked (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004). The loss of enhanced phasic responsiveness of DA release in Phe(+) compared to non-Tg or Phe(−) mice with mecamylamine suggests that altered striatal cholinergic transmission might also contribute to the neurochemical phenotype of symptomatic Phe(+) versus non-Tg and asymptomatic Phe(−) mice. Previous electrophysiological studies support a role for altered cholinergic transmission in a mouse dystonia model with pan-cellular ΔE-torsinA overexpression (Pisani et al, 2006; Martella et al., 2009). Because nAChR regulation of striatal DA release is lost when nAChRs are desensitized by excessive agonist, as well as by nAChR blockade (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004), either excessive or impaired ACh release could contribute to the enhancement of phasic-to-tonic DA release in Phe(+) mice. However, the Ca2+ dependence of this neurochemical phenotype argues for too little, rather than too much ACh. Lowering [Ca2+]o would be expected to decrease Ca2+-dependent release of ACh, as well as DA. If elevated ACh and consequent nAChR desensitization were responsible for the patterns of evoked [DA]o in Phe(+) mice, then this should have been ameliorated when ACh release was decreased in lower [Ca2+]o. Instead, however, the phasic-to-tonic responsiveness of DA release in Phe(+) mice was exacerbated, suggesting that Ca2+-dependent, exocytotic release of ACh, as well as DA, may be compromised in Phe(+) mice. At first glance, this indirect evidence for decreased cholinergic tone in Phe(+) mice seems inconsistent with the fact that anticholinergic therapy is one of few effective pharmacologic approaches for some forms of human dystonia (Fahn, 1983). However, the anticholinergic agents used are antagonists of muscarinic ACh receptors (mAChRs). Recent studies indicate that in the striatum, mAChR agonists act at cholinergic autoreceptors to suppress ACh release, resulting in enhanced phasic-to-tonic sensitivity of striatal DA release that was nAChR dependent (Threlfell et al., 2010). Thus, properly controlled levels of mAChR antagonists should boost striatal cholinergic tone, thereby restoring cholinergic-DAergic balance for proper basal ganglia function (e.g., Morris et al., 2004).
Conclusions
We show here that in a mouse model of dystonia with motor hyperactivity, aberrant muscle contractions, and abnormal basal ganglia output, tonic striatal DA release is low, but the responsiveness to phasic stimulation is enhanced. Assuming that DA motor signals are encoded by DA neuron burst firing, our data suggest that falsely amplified DA signals on a low tonic [DA]o background in symptomatic Phe(+) mice might contribute to overactive DA motor signaling and consequent uncontrolled movement. Consistent with this hypothesis, pharmacological agents that deplete DA stores can improve the symptoms of dystonia (Jankovic, 2009). We also provide evidence for a role of impaired cholinergic regulation of DA release in Phe(+), but not Phe(−) mice, which with other recent findings (Threlfell et al., 2010) may contribute mechanistic insight into the efficacy of anticholinergic (mAChR antagonist) therapy in DYT1 dystonia (Fahn, 1983).
Several other mouse models of DYT1 dystonia have been developed and characterized, with most showing subtle neurological phenotypes (Goodchild et al., 2005; Sharma et al., 2005; Dang et al., 2006; Grundmann et al., 2007; Zhao et al., 2008), rather than the pronounced motor symptoms of the Phe(+) mice examined here (Shashidharan et al., 2005). Differences could include the target of ΔE-torsinA expression, e.g., pan-cellular vs. pan-neuronal, levels of expression, and/or developmental differences in the timing of expression. Additionally, as noted above, ΔE-torsinA expression was lost in much later generations of genotype-positive mice than those examined here (unpublished observations), despite persistent hyperkinesia, possibly pointing to an insertional mutation or other effect independent of the mutant torsinA protein as a contributor to the motor phenotypes. At the time our studies were conducted, there was reliable transgene expression in the mouse line (Shashidharan et al., 2005), although this was not determined in the specific mice examined. It is therefore likely, but not certain, that the mice we examined expressed ΔE-torsinA. Thus, the relationship of the motor and neurochemical phenotypes of these mice to ΔE-torsinA expression is in some respects uncertain. Regardless, our characterization of the neurochemical phenotype of these animals, like the previous physiological assessment of prolonged muscle contractions and altered neuronal activity in the GPi and GPe of Phe(+) mice (Chiken et al., 2008), provides new information about the roles of striatal DA and ACh release regulation in coordinated motor behavior. Our findings may also contribute to a better understanding of the pathophysiology of dystonia and other hyperkinetic disorders and suggest more effective therapeutic approaches.
Acknowledgments
This work was supported by the Bachmann-Strauss Dystonia and Parkinson Foundation (M.E.R. and PS), and by NIH/NINDS grants NS036362 (M.E.R) and NS043038 (P.S.).
Abbreviations
- ACh
acetylcholine
- aCSF
artificial CSF
- DA
dopamine
- [DA]o
extracellular dopamine concentration
- ΔE-torsinA
human mutant torsinA
- FCV
fast-scan cyclic voltammetry
- GPe
globus pallidus external segment
- GPi
globus pallidus internal segment
- h-torsinA
human wildtype torsinA
- mAChR
muscarinic ACh receptor
- nAChR
nicotinic ACh receptor
- non-Tg
non-transgenic
- NSE
neuron-specific enolase
- Phe(−)
transgenic with no motor phenotype
- Phe(+)
transgenic with a positive motor phenotype
- SNc
substantia nigra pars compacta
- VMAT2
vesicular monoamine transporter 2
Footnotes
The authors declare no conflicts of interest.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Keller-McGandy CE, Siriani A, Hewett J, Ramesh V, Sapp E, DiFiglia M, Breakefield XO, Standaert DG. Distribution and ultrastructural localization of torsinA immunoreactivity in the human brain. Brain Res. 2003;986:12–21. doi: 10.1016/s0006-8993(03)03164-0. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Martin DM, Ozelius LJ, Breakefield XO, Penney JB, Jr., Standaert DG. Distribution of the mRNAs encoding torsinA and torsinB in the normal adult human brain. Ann Neurol. 1999;46:761–769. [PubMed] [Google Scholar]
- Augood SJ, Penney JB, Jr, Friberg IK, Breakefield XO, Young AB, Ozelius LJ, Standaert DG. Expression of the early-onset torsion dystonia gene (DYT1) in human brain. Ann. Neurol. 1998;43:669–673. doi: 10.1002/ana.410430518. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Marshall SP, Peña DM, Rice ME. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J. Neurosci. 2003;23:2744–2750. doi: 10.1523/JNEUROSCI.23-07-02744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG. Dopamine release is impaired in a mouse model of DYT1 dystonia. J. Neurochem. 2007;102:783–788. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Rice ME. Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. J. Neurosci. 2005;25:10029–10040. doi: 10.1523/JNEUROSCI.2652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Jonason J, Pileblad E, Nissbrandt H. Effects of local administration of L-, N-, and P/Q-type calcium channel blockers on spontaneous dopamine release in the striatum and the substantia nigra: a microdialysis study in rat. J. Neurochem. 1998;70:1532–1540. doi: 10.1046/j.1471-4159.1998.70041532.x. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallet M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat. Rev. Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv. Neurol. 2004;94:101–107. [PubMed] [Google Scholar]
- Bruno MK, Ravina B, Garraux G, Hallett M, Ptacek L, Singleton A, Johnson J, Singleton A, Hanson M, Considine E, Gwinn-Hardy K. Exercise-induced dystonia as a preceding symptom of familial Parkinson’s disease. Mov. Disord. 2004;19:228–230. doi: 10.1002/mds.10626. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J. Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Moran KA, Avshalumov MV, Rice ME. Limited regulation of somatodendritic dopamine release by voltage-sensitive Ca2+ channels contrasted with strong regulation of axonal dopamine release. J. Neurochem. 2006;96:645–655. doi: 10.1111/j.1471-4159.2005.03519.x. [DOI] [PubMed] [Google Scholar]
- Chiken S, Shashidharan P, Nambu A. Cortically evoked long-lasting inhibition of pallidal neurons in a transgenic mouse model of dystonia. J. Neurosci. 2008;28:13967–13977. doi: 10.1523/JNEUROSCI.3834-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Hallett M. Hand cramps: clinical features and electromyographic patterns in a focal dystonia. Neurology. 1988;38:1005–1012. doi: 10.1212/wnl.38.7.1005. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J. Neurosci. 2003;23:4378–4385. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004;25:317–24. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, McNaught KS, Jengelley TA, Jackson T, Li J, Li Y. Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Exp. Neurol. 2005;196:452–463. doi: 10.1016/j.expneurol.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, Pence MA, Li Y. Motor deficits and hyperactivity in Dyt1 knockdown mice. Neurosci. Res. 2006;56:470–474. doi: 10.1016/j.neures.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov. Disord. 2007;22:1379–1389. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- Fahn S. High dosage anticholinergic therapy in dystonia. Neurology. 1983;33:1255–1261. doi: 10.1212/wnl.33.10.1255. [DOI] [PubMed] [Google Scholar]
- Fahn S, Bressman S,B, Marsden CD. Classification of dystonia. Adv. Neurol. 1998;78:1–10. [PubMed] [Google Scholar]
- Farmer SF, Sheean GL, Mayston MJ, Rothwell JC, Marsden CD, Conway BA, Halliday DM, Rosenberg JR, Stephens JA. Abnormal motor unit synchronization of antagonist muscles underlies pathological co-contraction in upper limb dystonia. Brain. 1998;121:801–814. doi: 10.1093/brain/121.5.801. [DOI] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann K, Reischmann B, Vanhoutte G, Hübener J, Teismann P, Hauser TK, Bonin M, Wilbertz J, Horn S, Nguyen HP, Kuhn M, Chanarat S, Wolburg H, Van der Linden A, Riess O. Overexpression of human wildtype torsinA and human DeltaGAG torsinA in a transgenic mouse model causes phenotypic abnormalities. Neurobiol. Dis. 2007;27:190–206. doi: 10.1016/j.nbd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hallett M. Bromocriptine for levodopa-induced end-of-dose dystonia. Lancet. 1981;317:616. doi: 10.1016/s0140-6736(81)92066-3. [DOI] [PubMed] [Google Scholar]
- Hewett J, Johanson P, Sharma N, Standaert D, Balcioglu A. Function of dopamine transporter is compromised in DYT1 transgenic animal model in vivo. J. Neurochem. 2010;113:228–253. doi: 10.1111/j.1471-4159.2010.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz E. Dystonia. 1. Historical review: analysis of dystonic symptoms and physiologic mechanisms involved. Arch Neurol. Psychiatry. 1944;51:305–318. [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ichinose H, Suzuki T, Inagaki H, Ohye T, Nagatsu T. Molecular genetics of dopa-responsive dystonia. Biol. Chem. 1999;380:1355–1364. doi: 10.1515/BC.1999.175. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844–856. doi: 10.1016/S1474-4422(09)70183-8. [DOI] [PubMed] [Google Scholar]
- Katzenschlager R, Costa D, Gacinovic S, Lees AJ. 123I]-FP-CIT-SPECT in the early diagnosis of PD presenting as exercise-induced dystonia. Neurology. 2002;59:1974–1976. doi: 10.1212/01.wnl.0000037484.28297.66. [DOI] [PubMed] [Google Scholar]
- Kim DS, Szczypka MS, Palmiter RD. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J. Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PL, Heiman GA, Gasser T, Ozelius LJ, de Leon D, Brin MF, Burke RE, Hewett J, Hunt AL, Moskowitz C, et al. The DYT1 gene on 9q34 is responsible for most cases of early limb-onset idiopathic torsion dystonia in non-Jews. Am. J. Hum. Genet. 1994;55:468–475. [PMC free article] [PubMed] [Google Scholar]
- Kramer PL, Ozelius L, Brin MF, Fahn S, Kidd KK, Gusella J, Breakefield XO. Molecular genetics of an autosomal dominant form of torsion dystonia. Adv. Neurol. 1988;50:57–66. [PubMed] [Google Scholar]
- Li X, Patel JC, Wang J, Avshalumov MV, Nicholson C, Buxbaum JD, Elder GA, Rice ME, Yue Z. Enhanced motor performance and striatal dopamine transmission with LRRK2 overexpression in mice is eliminated by familial Parkinson’s disease mutation G2019S. J. Neurosci. 2010;30:1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger N, Trout SJ, Kruk ZL, Starke K. “Real time” measurement of endogenous dopamine release during short trains of pulses in slices of rat neostriatum and nucleus accumbens: role of autoinhibition. Naunyn Schmiedebergs Arch. Pharmacol. 1991;344:623–629. doi: 10.1007/BF00174745. [DOI] [PubMed] [Google Scholar]
- Liu X, Tailor J, Wang S, Yianni J, Gregory R, Stein J, Aziz T. Reversal of hypertonic co-contraction after bilateral pallidal stimulation in generalised dystonia: a clinical and electromyogram case study. Mov. Disord. 2004;19:336–340. doi: 10.1002/mds.10655. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J. Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC. The physiology of idiopathic dystonia. Can. J. Neurol. Sci. 1987;14:521–527. doi: 10.1017/s031716710003804x. [DOI] [PubMed] [Google Scholar]
- Martella G, Tassone A, Sciamanna G, Platania P, Cuomo D, Viscomi MT, Bonsi P, Cacci E, Biagioni S, Usiello A, Bernardi G, Sharma N, Standaert DG, Pisani A. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain. 2009;132:2336–2349. doi: 10.1093/brain/awp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch. Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Misbahuddin A, Placzek MR, Taanman JW, Gschmeissner S, Schiavo G, Cooper JM, Warner TT. Mutant torsinA, which causes early-onset primary torsion dystonia, is redistributed to membranous structures enriched in vesicular monoamine transporter in cultured human SH-SY5Y cells. Mov. Disord. 2005;20:432–440. doi: 10.1002/mds.20351. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Naumann M, Pirker W, Reiners K, Lange KW, Becker G, Brücke T. Imaging the pre- and postsynaptic side of striatal dopaminergic synapses in idiopathic cervical dystonia: a SPECT study using [123I] epidepride and [123I] beta-CIT. Mov. Disord. 1998;13:319–323. doi: 10.1002/mds.870130219. [DOI] [PubMed] [Google Scholar]
- Nausieda PA, Weiner WJ, Klawans HL. Dystonic foot response of Parkinsonism. Arch. Neurol. 1980;37:132–6. doi: 10.1001/archneur.1980.00500520030003. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rothwell JC, Lang AE, Marsden CD. Myoclonic dystonia. Neurology. 1983;33:825–830. doi: 10.1212/wnl.33.7.825. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett J, Kramer P, Bressman SB, Shalish C, de Leon D, Rutter M, Risch N, Brin MF, Markova ED, Limborska SA, Ivanova-Smolenskaya IA, McCormick MK, Fahn S, Buckler AJ, Gusella JF, Breakefield XO. Fine localization of the torsion dystonia gene (DYT1) on human chromosome 9q34: YAC map and linkage disequilibrium. Genome Res. 1997;7:483–494. doi: 10.1101/gr.7.5.483. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Kramer PL, Moskowitz CB, Kwiatkowski DJ, Brin MF, Bressman SB, Schuback DE, Falk CT, Risch N, de Leon D. Human gene for torsion dystonia located on chromosome 9q32-q34. Neuron. 1989;2:1427–1434. doi: 10.1016/0896-6273(89)90188-8. [DOI] [PubMed] [Google Scholar]
- Page ME, Bao L, Andre P, Pelta-Heller J, Sluzas E, Gonzalez-Alegre P, Iacovitti L, Rice ME, Ehrlich ME. Cell-autonomous alteration of dopaminergic transmission by wild type and mutant (ΔE) TorsinA in transgenic mice. Neurobiol. Dis. 2010;39:318–326. doi: 10.1016/j.nbd.2010.04.016. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palij P, Bull DR, Sheehan MJ, Millar J, Stamford J, Kruk ZL, Humphrey PPA. Presynaptic regulation of in corpus striatum monitored in vitro in real time by fast cyclic voltammetry. Brain Res. 1990;509:172–174. doi: 10.1016/0006-8993(90)90329-a. [DOI] [PubMed] [Google Scholar]
- Patel J, Rice ME. In: Dopamine release in brain slices, in Encyclopedia of Sensors. Grimes CA, Dickey EC, Pishko MV, editors. Vol. 6. American Scientific Publishers; Stevenson Ranch, CA: 2006. pp. 313–334. [Google Scholar]
- Patel J, Mooslehner KA, Chan PM, Emson PC, Stamford JA. Presynaptic control of striatal dopamine neurotransmission in adult vesicular monoamine transporter 2 (VMAT2) mutant mice. J. Neurochem. 2003;85:898–910. doi: 10.1046/j.1471-4159.2003.01732.x. [DOI] [PubMed] [Google Scholar]
- Patel J, Trout SJ, Kruk ZL. Regional differences in evoked dopamine efflux in brain slices of rat anterior and posterior caudate putamen. Naunyn Schmiedebergs Arch. Pharmacol. 1992;346:267–276. doi: 10.1007/BF00173539. [DOI] [PubMed] [Google Scholar]
- Patel J, Trout SJ, Palij P, Whelpton R, Kruk ZL. Biphasic inhibition of stimulated endogenous dopamine release by 7-OH-DPAT in slices of rat nucleus accumbens. Br. J. Pharmacol. 1995;115:421–426. doi: 10.1111/j.1476-5381.1995.tb16350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JC, Witkovsky P, Avshalumov MV, Rice ME. Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J. Neurosci. 2009;29:6568–6579. doi: 10.1523/JNEUROSCI.0181-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Tempel LW, Black KJ, Parkinson D, Todd RD. MPTP induces dystonia and parkinsonism. Clues to the pathophysiology of dystonia. Neurology. 1997a;49:1432–1438. doi: 10.1212/wnl.49.5.1432. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee-Minnich L, Jankovic J, Moerlein SM. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J. Neurosci. 1997b;17:843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vucković M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PEM, Stamford JA. Differential recruitment of N-, P- and Q-type voltage-operated calcium channels in striatal dopamine release evoked by ‘regular’ and ‘burst’ firing. Brain Res. 2000;884:139–146. doi: 10.1016/s0006-8993(00)02958-9. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Pisani A, Martella G, Tscherter A, Bonsi P, Sharma N, Bernardi G, Standaert DG. Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiol. Dis. 2006;24:318–325. doi: 10.1016/j.nbd.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Playford ED, Fletcher NA, Sawle GV, Marsden CD, Brooks DJ. Striatal [18F]dopa uptake in familial idiopathic dystonia. Brain. 1993;116:1191–1199. doi: 10.1093/brain/116.5.1191. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Risch NJ, Bressman SB, deLeon D, Brin MF, Burke RE, Greene PE, Shale H, Claus EB, Cupples LA, Fahn S. Segregation analysis of idiopathic torsion dystonia in Ashkenazi Jews suggests autosomal dominant inheritance. Am. J. Hum. Genet. 1990;46:533–538. [PMC free article] [PubMed] [Google Scholar]
- Romo R, Schultz W. Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J. Neurophysiol. 1990;63:592–606. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]
- Sato K, Sumi-Ichinose C, Kaji R, Ikemoto K, Nomura T, Nagatsu I, Ichinose H, Ito M, Sako W, Nagahiro S, Graybiel AM, Goto S. Differential involvement of striosome and matrix dopamine systems in a transgenic model of dopa-responsive dystonia. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12551–12556. doi: 10.1073/pnas.0806065105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J. Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Baxter MG, Petravicz J, Bragg DC, Schienda A, Standaert DG, Breakefield XO. Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. J. Neurosci. 2005;25:5351–5355. doi: 10.1523/JNEUROSCI.0855-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharan P, Sandu D, Potla U, Armata IA, Walker RH, McNaught KS, Weisz D, Sreenath T, Brin MF, Olanow CW. Transgenic mouse model of early-onset DYT1 dystonia. Hum. Mol. Genet. 2005;14:125–133. doi: 10.1093/hmg/ddi012. [DOI] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr., Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J. Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J. Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Sweeney AL, Beaulieu JM, Shashidharan P, Caron MG. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated ΔE-torsinA mutant. Proc. Natl. Acad. Sci., U.S.A. 2004;101:15650–15655. doi: 10.1073/pnas.0308088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout SJ, Kruk ZL. Differences in evoked dopamine efflux in rat caudate putamen, nucleus accumbens and tuberculum olfactorium in the absence of uptake inhibition: influence of autoreceptors. Br. J. Pharmacol. 1992;106:452–458. doi: 10.1111/j.1476-5381.1992.tb14355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, Triche S, Mewes K, Hashimoto T, Bakay RA. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann. Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J. Neuropathol. Exp. Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr. Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Walker RH, Brin MF, Sandu D, Gujjari P, Hof PR, Olanow C, Shashidharan P. Distribution and immunohistochemical characterization of TorsinA immunoreactivity in rat brain. Brain Res. 2001;900:348–354. doi: 10.1016/s0006-8993(01)02302-2. [DOI] [PubMed] [Google Scholar]
- Wichmann T. Commentary: dopaminergic dysfunction in DYT1 dystonia. Exp. Neurol. 2008;212:242–246. doi: 10.1016/j.expneurol.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N, Goto A. Dystonia musculorum deformans. Analysis with electromyography. J. Neurol. Sci. 1971;13:39–65. doi: 10.1016/0022-510x(71)90206-1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhao Y, DeCuypere M, LeDoux MS. Abnormal motor function and dopamine neurotransmission in DYT1 ΔGAG transgenic mice. Exp. Neurol. 2008;210:719–730. doi: 10.1016/j.expneurol.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zhuang P, Li Y, Hallett M. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin. Neurophysiol. 2004;115:2542–2557. doi: 10.1016/j.clinph.2004.06.006. [DOI] [PubMed] [Google Scholar]