Abstract
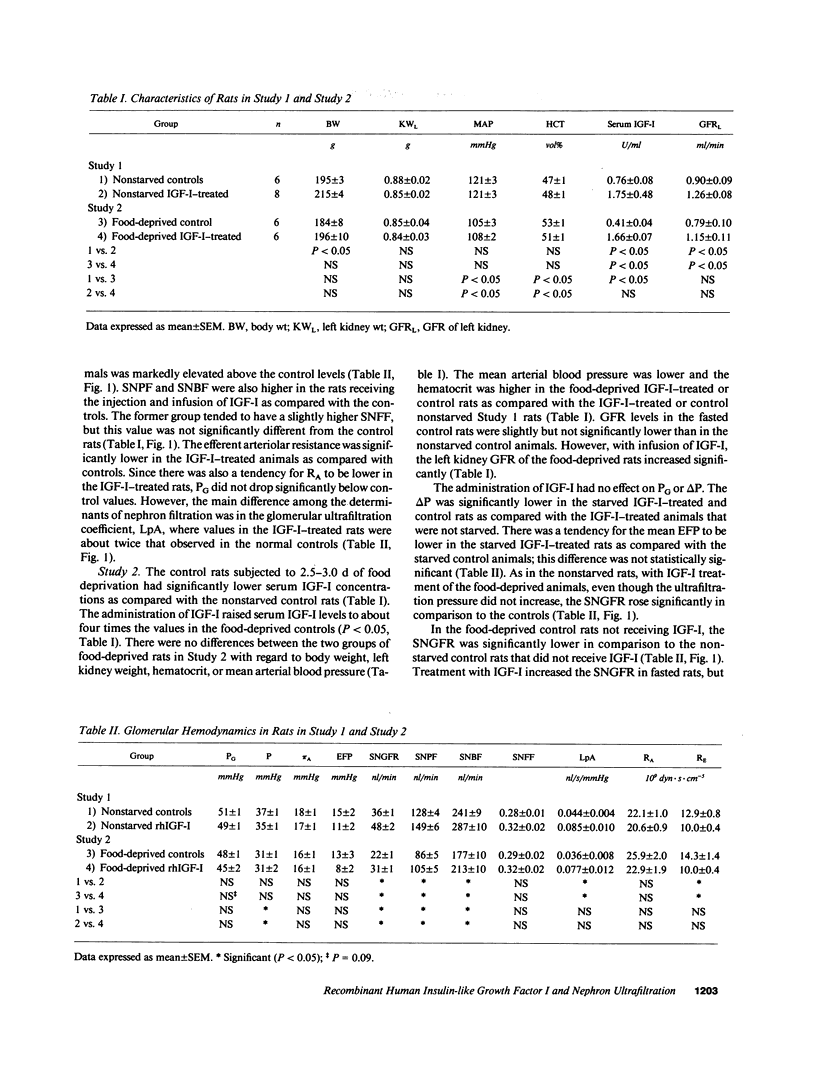
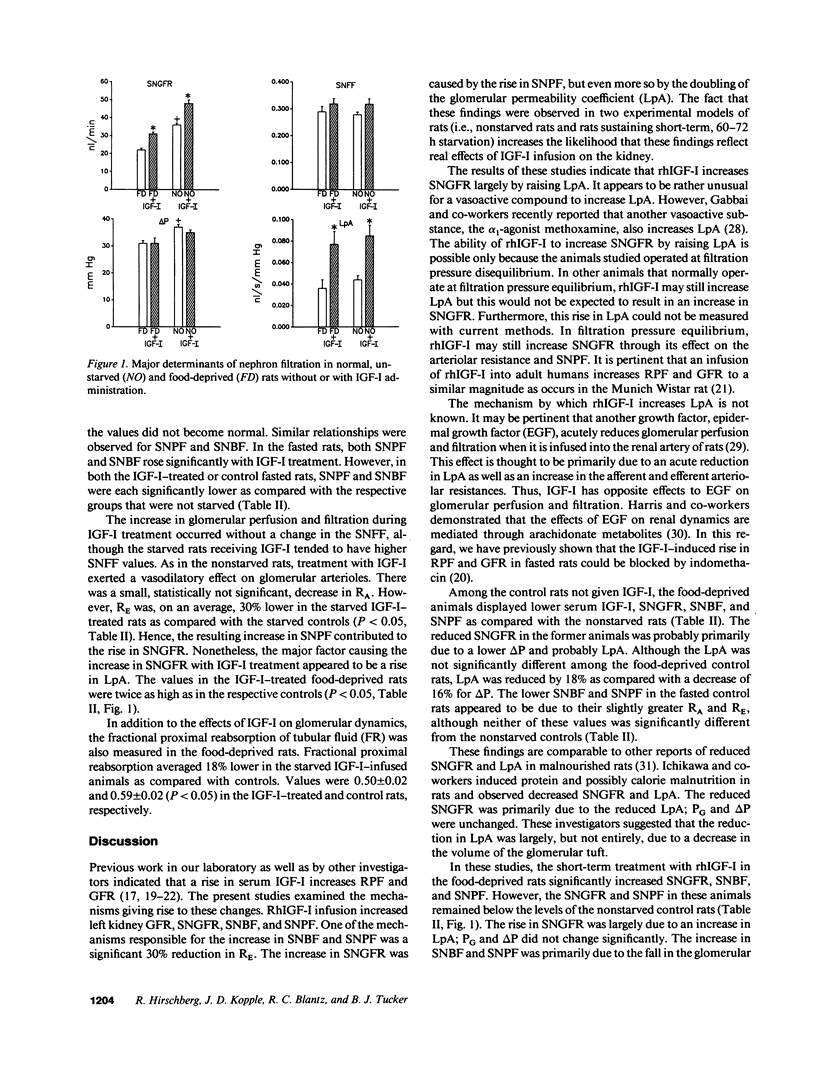
This study was undertaken to investigate the mechanisms by which an infusion of recombinant human insulin-like growth factor I (rhIGF-I) increases GFR and renal plasma flow (RPF) in rats. Glomerular micropuncture studies were carried out in 14 nonstarved Munich Wistar rats and in 12 rats deprived of food for 60-72 h. Animals were given an intravenous injection and infusion of either rhIGF-I or vehicle. In both nonstarved and starved animals, the IGF-I injection and infusion increased the serum IGF-I levels, left kidney GFR, single nephron glomerular filtration rate (SNGFR), single nephron blood flow rate (SNBF), and single nephron plasma flow rate (SNPF). The increase in SNPF and SNGFR was in part due to a fall in efferent arteriolar resistance (RE); there was a tendency, not significant, for afferent arteriolar resistance (RA) to fall in comparison to controls. The increase in SNGFR was partly caused by a rise in SNPF but was primarily due to an increase in glomerular ultrafiltration coefficient (LpA) to twice the control values. The increase in LpA resulted in an increase in SNGFR because the rats operated at ultrafiltration pressure disequilibrium. Control starved as compared with nonstarved rats had lower SNGFR, SNBF, and SNPF. This reduction was due to a tendency, not significant, for both RA and RE to be higher. Decreased SNGFR in food-deprived rats resulted from a reduced SNPF, a lower glomerular transcapillary hydrostatic pressure difference (delta P), and possibly a somewhat reduced LpA. These data indicate that IGF-I increases SNGFR, SNPF, and SNBF primarily by increasing LpA and also by decreasing RE without affecting delta P. Short-term starvation lowers SNGFR, SNPF, and SNBF primarily by decreasing delta P and possibly by lowering LpA and increasing RA and RE. IGF-I reverses some of the glomerular hemodynamic effects of short-term food deprivation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G. L., Skottner A., Jennische E. Immunocytochemical and biochemical localization of insulin-like growth factor I in the kidney of rats before and after uninephrectomy. Acta Endocrinol (Copenh) 1988 Dec;119(4):555–560. doi: 10.1530/acta.0.1190555. [DOI] [PubMed] [Google Scholar]
- Andersson G., Jennische E. IGF-I immunoreactivity is expressed by regenerating renal tubular cells after ischaemic injury in the rat. Acta Physiol Scand. 1988 Apr;132(4):453–457. doi: 10.1111/j.1748-1716.1988.tb08352.x. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Boes M., Yorek M. Processing of insulin-like growth factors I and II by capillary and large vessel endothelial cells. Endocrinology. 1986 Mar;118(3):1072–1080. doi: 10.1210/endo-118-3-1072. [DOI] [PubMed] [Google Scholar]
- Bortz J. D., Rotwein P., DeVol D., Bechtel P. J., Hansen V. A., Hammerman M. R. Focal expression of insulin-like growth factor I in rat kidney collecting duct. J Cell Biol. 1988 Aug;107(2):811–819. doi: 10.1083/jcb.107.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F. G., Striker L. J., Elliot S. J., Andreani D., Striker G. E. Synthesis and release of insulinlike growth factor I by mesangial cells in culture. Am J Physiol. 1988 Dec;255(6 Pt 2):F1214–F1219. doi: 10.1152/ajprenal.1988.255.6.F1214. [DOI] [PubMed] [Google Scholar]
- Conti F. G., Striker L. J., Lesniak M. A., MacKay K., Roth J., Striker G. E. Studies on binding and mitogenic effect of insulin and insulin-like growth factor I in glomerular mesangial cells. Endocrinology. 1988 Jun;122(6):2788–2795. doi: 10.1210/endo-122-6-2788. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ercole A. J., Underwood L. E. Estimation of tissue concentrations of somatomedin C/insulin-like growth factor I. Methods Enzymol. 1987;146:227–233. doi: 10.1016/s0076-6879(87)46024-2. [DOI] [PubMed] [Google Scholar]
- Fagin J. A., Melmed S. Relative increase in insulin-like growth factor I messenger ribonucleic acid levels in compensatory renal hypertrophy. Endocrinology. 1987 Feb;120(2):718–724. doi: 10.1210/endo-120-2-718. [DOI] [PubMed] [Google Scholar]
- Furlanetto R. W., Underwood L. E., Van Wyk J. J., D'Ercole A. J. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J Clin Invest. 1977 Sep;60(3):648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler H. P., Eckardt K. U., Zapf J., Bauer C., Froesch E. R. Insulin-like growth factor I increase glomerular filtration rate and renal plasma flow in man. Acta Endocrinol (Copenh) 1989 Jul;121(1):101–106. doi: 10.1530/acta.0.1210101. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Schmid C., Zapf J., Froesch E. R. Effects of recombinant insulin-like growth factor I on insulin secretion and renal function in normal human subjects. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2868–2872. doi: 10.1073/pnas.86.8.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner D., Ritz E., Mehls O., Rosman J., Blum W., Heinrich U., Hübinger A. Growth hormone induced rise in glomerular filtration rate is not obliterated by angiotensin-converting enzyme inhibitors. Nephron. 1990;55(1):63–68. doi: 10.1159/000185920. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Rogers S. Distribution of IGF receptors in the plasma membrane of proximal tubular cells. Am J Physiol. 1987 Nov;253(5 Pt 2):F841–F847. doi: 10.1152/ajprenal.1987.253.5.F841. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hoover R. L., Jacobson H. R., Badr K. F. Evidence for glomerular actions of epidermal growth factor in the rat. J Clin Invest. 1988 Sep;82(3):1028–1039. doi: 10.1172/JCI113659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. C., Munger K. A., Badr K. F., Takahashi K. Mediation of renal vascular effects of epidermal growth factor by arachidonate metabolites. FASEB J. 1990 Apr 1;4(6):1654–1660. doi: 10.1096/fasebj.4.6.2138579. [DOI] [PubMed] [Google Scholar]
- Hirschberg R., Kopple J. D. Evidence that insulin-like growth factor I increases renal plasma flow and glomerular filtration rate in fasted rats. J Clin Invest. 1989 Jan;83(1):326–330. doi: 10.1172/JCI113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R., Rabb H., Bergamo R., Kopple J. D. The delayed effect of growth hormone on renal function in humans. Kidney Int. 1989 Mar;35(3):865–870. doi: 10.1038/ki.1989.65. [DOI] [PubMed] [Google Scholar]
- IKKOS D., LJUNGGREN H., LUFT R. Glomerular filtration rate and renal plasma flow in acromegaly. Acta Endocrinol (Copenh) 1956 Mar;21(3):226–236. doi: 10.1530/acta.0.0210226. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Purkerson M. L., Klahr S., Troy J. L., Martinez-Maldonado M., Brenner B. M. Mechanism of reduced glomerular filtration rate in chronic malnutrition. J Clin Invest. 1980 May;65(5):982–988. doi: 10.1172/JCI109784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isley W. L., Underwood L. E., Clemmons D. R. Dietary components that regulate serum somatomedin-C concentrations in humans. J Clin Invest. 1983 Feb;71(2):175–182. doi: 10.1172/JCI110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamberts S. W., Uitterlinden P., Verleun T. Relationship between growth hormone and somatomedin-C levels in untreated acromegaly, after surgery and radiotherapy and during medical therapy with sandostatin (SMS 201-995). Eur J Clin Invest. 1987 Aug;17(4):354–359. doi: 10.1111/j.1365-2362.1987.tb02200.x. [DOI] [PubMed] [Google Scholar]
- Lowe W. L., Jr, Adamo M., Werner H., Roberts C. T., Jr, LeRoith D. Regulation by fasting of rat insulin-like growth factor I and its receptor. Effects on gene expression and binding. J Clin Invest. 1989 Aug;84(2):619–626. doi: 10.1172/JCI114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski C. C., Chernausek S. D. Discordance of serum and tissue somatomedin levels in growth hormone-stimulated growth in the rat. Endocrinology. 1988 Jul;123(1):44–49. doi: 10.1210/endo-123-1-44. [DOI] [PubMed] [Google Scholar]
- Pillion D. J., Haskell J. F., Meezan E. Distinct receptors for insulin-like growth factor I in rat renal glomeruli and tubules. Am J Physiol. 1988 Oct;255(4 Pt 1):E504–E512. doi: 10.1152/ajpendo.1988.255.4.E504. [DOI] [PubMed] [Google Scholar]
- Roberts C. T., Jr, Lasky S. R., Lowe W. L., Jr, Seaman W. T., LeRoith D. Molecular cloning of rat insulin-like growth factor I complementary deoxyribonucleic acids: differential messenger ribonucleic acid processing and regulation by growth hormone in extrahepatic tissues. Mol Endocrinol. 1987 Mar;1(3):243–248. doi: 10.1210/mend-1-3-243. [DOI] [PubMed] [Google Scholar]
- Stiles A. D., Sosenko I. R., D'Ercole A. J., Smith B. T. Relation of kidney tissue somatomedin-C/insulin-like growth factor I to postnephrectomy renal growth in the rat. Endocrinology. 1985 Dec;117(6):2397–2401. doi: 10.1210/endo-117-6-2397. [DOI] [PubMed] [Google Scholar]
- Svoboda M. E., Van Wyk J. J. Purification of somatomedin-C/insulin-like growth factor I. Methods Enzymol. 1985;109:798–816. doi: 10.1016/0076-6879(85)09131-5. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Shiraga H., Yoshida Y., Fogo A., Glick A. D., Deen W. M., Hoyer J. R., Ichikawa I. "Intact nephrons" as the primary origin of proteinuria in chronic renal disease. Study in the rat model of subtotal nephrectomy. J Clin Invest. 1988 Nov;82(5):1614–1623. doi: 10.1172/JCI113773. [DOI] [PMC free article] [PubMed] [Google Scholar]



