Abstract
The potential of pluripotent embryonic stem (ES) cells to develop into functional cells or tissue provides an opportunity in the development of new therapies for many diseases including neurodegenerative disorders. The survival of implanted cells usually requires systemic immunosuppression, however, which severely compromises the host immune system, leading to complications in clinical transplantation. An optimal therapy would therefore be the induction of specific tolerance to the donor cells, while otherwise preserving functional immune responses. Fas ligand (FasL) is expressed in activated lymphocytes as well as cells in “immune-privileged” sites including the central nervous system. Its receptor, Fas, is expressed on various immune-reactive cell types, such as activated natural killer and T cells, monocytes, and polymorphic mononucleocytes, which can undergo apoptosis upon interaction with FasL. To render transplanted cells tolerant to host cellular immune responses, we genetically engineered mouse ES cells to express rat FasL (rFasL). The rFasL-expressing ES cells were analyzed for survival during in vitro neurodifferentiation and after transplantation to the rat brain without further immunosuppression. Although control transfected HEK-293T cells expressed functional rFasL, immature and differentiated mouse ES cells did not express the recombinant rFasL surface protein. Furthermore, there was no evidence for functional endogenous Fas and FasL expression on either ES cells or on neural cells after in vitro differentiation. Moreover, implanted rFasL-engineered ES cells did not survive in the rat brains in the absence of the immunosuppressive agent cyclosporine A. Our results indicate that immature and differentiated mouse ES cells do not express a functional Fas/FasL system.
Keywords: Fas, Fas ligand, Fas/Fas ligand system, Embryonic stem cells, Neural differentiation, Immune response Immunosuppression, Brain, Transplantation
Introduction
Pluripotent embryonic stem (ES) cells can develop into functional mature cells or tissue, which has high implication in finding new treatments for diseases including neurodegenerative disorders. This includes the potential use of ES cells for cell replacement therapy in neurological diseases such as Parkinson disease (PD), Huntington disease, amyotrophic lateral sclerosis, and ischemia [1, 2]. However, survival of allogeneic or xenogeneic cell grafts usually requires nonspecific systemic immunosuppression. This can severely compromise the host immune system, leading to complications in clinical transplantation. A solution could be the induction of specific tolerance to the donor cells while otherwise preserving functional immune responses to avoid cell rejection without the use of immune-suppressive treatment [3, 4].
Fas ligand (FasL, CD178, or CD95L) is a type II membrane protein and is expressed in activated lymphocytes as well as cells in “immune-privileged” sites such as the testis, the eye, and the central nervous system (CNS) [5]. Its receptor, Fas (CD95 or Apo-1), a type I membrane protein and a member of the tumor necrosis factor receptor family, is expressed on various immune-reactive cell types including activated natural killer and T cells and nonlymphoid cells such as immature myeloid cells, monocytes, and polymorphic mononucleocytes [6]. Fas-expressing cells undergo apoptosis upon interaction with FasL. This process is involved in regulating cellular immunity, and it has been shown that FasL can be used as an immunomodulatory tool to protect allogeneic or xenogeneic cells against cellular immune responses [7].
The CNS is considered to be an immune-privileged site [7, 8], and expression of FasL has been shown to contribute to the protection of neural cells against Fas-expressing immune-reactive T-effector cells [9, 10]. In addition, the CNS provides a microenvironment that allows the survival of transplanted FasL-expressing Sertoli [11, 12] and astrocytoma cells [13] in allogeneic and xenogeneic brain transplantation models, indicating that FasL could be used as an immune-modulatory factor for grafted ES cells.
In a mouse-to-rat xenotransplantation situation, ∼90% of mouse ES (mES) cell grafts are rejected after implantation into the rat brain without immunosuppressive therapy (Table 1). In addition, mES cells do not express FasL (see below). We therefore hypothesized that ES cells, genetically engineered to express a recombinant FasL molecule, are protected against the host cellular immune response in the brain of rats, thus abolishing the need for systemic immunosuppressive drug treatment. We genetically engineered mouse ES cells to express rat (r)FasL and tested their survival ability in in vitro neurogenesis experiments and in vivo after transplantation into the rat brain. Surprisingly, we found that the ES cells did not express a functional recombinant rFasL protein. In addition, our data indicate that immature and differentiated mouse ES cells do not express a functional Fas/FasL system.
Table 1. Summary of graft development after transplantation of embryonic stem cells to the striatum of rats.
| Group | Transplant | CsA | 8-Week survival (n) | Teratoma (n)a | Neural graft (n)a | No graft (n) |
|---|---|---|---|---|---|---|
| 1 | ES.Xgfp | yes | 10b | 3 | 4 | 3 |
| 2 | ES.Xgfp | no | 12 | 0 | 0 | 12 |
| 3 | ES.XF1 | no | 12 | 0 | 0 | 12 |
Grafts were phenotypically analyzed as previously published [19].
Two animals in this group were sacrificed at 3 and 4 weeks post-transplantation due to reasons unrelated to the experimental conditions.
Abbreviation: CsA, cyclosporine A.
Materials and Methods
Cloning of Constructs
All cloning experiments were performed using standard molecular biological techniques. The pCE-3 plasmid was kindly provided by Drs. L. Adams and D.I. Gottlieb [14] and the rFasL-expressing plasmid pBL-KA15 by Dr. S. Nagata, Department of Genetics, Osaka University Medical School, Osaka, Japan. The plasmid pCX-EGFP was obtained from Clontech (Palo Alto, CA, http://www.clontech.com). The rFasL cDNA together with an internal ribosomal entry site (IRES) derived from pIRES-EGFP (Clontech) were inserted into either pCE-3 or pCX-EGFP downstream of the chicken-β-actin (CBA) promoter to generate pCE-rFasL-IRES-EGFP (EF) and pCX-rFasL-IRES-EGFP (XF). All constructs were confirmed by sequence analysis.
Stable Transfection and Isolation of rFasL-Expressing ES Cell Lines
All transfection experiments were performed using the Lipofectamine Plus reagent (Life Technologies, Rockville, MD, http://www.lifetech.com) in HEK-293T (HEK) cells (American Type Culture Collection [ATCC], Manassas, VA, http://www.atcc.org) and the mouse ES cell lines D3 (ATCC) and CE1 [14]. In the CE1 cells, pCE-rFasL-IRES-EGFP was cotransfected with the plasmid pOG231 expressing Cre recombinase (kindly provided by Stephen O. Gorman, Salk Institute, San Diego). Stably transfected cells were selected in D10 medium (HEK cells) or ES cell medium containing 2 μg/ml puromycin as described [15, 16]. In the case of the CE1 transfectants, puromycin-resistant clones were genotypically detected using a flanking polymerase chain reaction (PCR) according to Adams et al. [14]. In addition, cell clones were screened for rFasL transcription by reverse transcription-polymerase chain reaction (RT-PCR) (see below) and for green fluorescent protein (GFP) expression using fluorescent microscopy. The rFasL- and/or GFP-expressing cell clones were isolated and further expanded for use in the in vitro differentiation experiments.
Cell Culture and In Vitro Differentiation of ES Cells
Cultures of human Fas-expressing T-cell lymphoma (Jurkat) cells were as described [15]. Rat lymphocytes were isolated, purified, and cultured for 7 days in the presence of 20 IU/ml interleukin-2 in adaptation of published protocols [15]. Propagation and differentiation of ES cells into neural progenitor cells and neurons were performed according to recent published protocols [16–19]. In some assays, the cultures were treated with soluble FasL (sFasL) (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) at concentrations of 10–100 ng/ml and with 100 ng/ml Fas/Fc chimera (Sigma).
RT-PCR
Total RNA was isolated using TRI Reagent (Sigma) followed by treatment with DNase I (Ambion, Austin, TX, http://www.ambion. com), cDNA preparation with the SuperScript Preamplification Kit (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and PCR analysis according to standard protocols and conditions as described elsewhere [16, 18, 19]. The oligonucleotide sequences for detecting mouse and rat FasL were 5′-ATGAGAGTCTTCTTAAGACC-3′ (forward) and 5′-TTGGCCATTTAACATCAGAC-3′ (reverse). This primer pair amplifies a 242-base pair (bp) fragment in the mouse and a 212-bp fragment in the rat sequence. The oligonucleotides for detecting mouse Fas were mFas497 (5′-CTTGAGCCATGCACAGCAACC-3′, forward) and mFas876 (5′-GATTGGTACCAGCACAGGAGC-3′, reverse).
Fluorescence-Activated Cell Sorting
Cell surface expression of Fas and FasL on HEK cells, ES cells, and cells during differentiation was detected according to standard protocols [15]. The following Fas- and FasL-specific antibodies were used: C13 (mouse anti-Fas; Transduction Laboratories, Lexington, KY, http://www.bdbiosciences.com/pharmingen; specificity: human, dog, chick, mouse, rat), 1:1,000; Jo2 (hamster anti-Fas; BD Pharmingen, San Diego, http://www.bdbiosciences.com/index_us. shtml; specificity: mouse), 0.2 μg/300 μl; N-20 (rabbit anti-FasL, sc-834; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com; specificity: mouse, rat, human), 1:200; MFL-3 (hamster anti-FasL; BD Pharmingen; specificity: mouse), 1 μg/300 μl; MFL-4 (hamster anti-FasL; BD Pharmingen; specificity: mouse, rat), 1 μg/300 μl; Kay-10 (mouse anti-FasL; BD Pharmingen; specificity: mouse), 1 μg/300 μl; AB1665 (rabbit anti-FasL; Chemicon, Temecula, CA, http://www.chemicon.com), 1:300; FLIM58 (hamster anti-FasL; MBL; specificity: rat, mouse), 1:300. Fluorescence-activated cell sorting (FACS) for detecting intracellular proteins was according to previously described protocols [20] using the following primary antibodies: rabbit anti-glial fibrillary acidic protein (GFAP; Dako, Glostrup, Denmark, http://www.dako. com), 1:500; mouse or rabbit anti-β-III-tubulin (Covance, Princeton, NJ, http://www.covance.com), 1:500 and 1:200, respectively; mouse anti-Nestin (Developmental Studies Hybridoma Bank, Iowa City, IA, http://www.uiowa.edu/∼dshbwww), 1 μg/ml. The secondary antibodies used were Alexa Fluor 488, 568, and 657 conjugated goat and donkey immunoglobulins (Molecular Probes, Eugene, OR, http://probes.invitrogen.com; 1:500). For FACS, cells were analyzed in a BD FACSAria Cell Sorter (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com).
Detection of Caspase-3 Activity
Caspase-3 was detected using an enzymatic fluorogenic substrate (PhiPhiLux-G1D2) kit by OncoImmunin, Inc. (Gaithersburg, MD, http://www.phiphilux.com). Cultured cells were mildly trypsinized, washed in FACS medium (phosphate-buffered saline supplemented with 5% fetal calf serum), incubated for 1 hour in 30 μl of PhiPhiLux-G1D2 solution, washed again, and analyzed in FACS using the GFP channel.
Immunocytochemistry and Western Blots
Immunocytochemistry assays were performed using standard procedures as described [16, 18, 19] using the rabbit anti-β-III-tubulin antibody Tuj1 (Covance). Western blot experiments were performed as described in [21] with certain modifications. Cells were disrupted in cell lysis buffer (pH of 8.0, 50 mM Tris-HCl, 150 mM NaCl, 0.2% sodium azide, 1% Triton X-100, and 1% Protease Inhibitor Cocktail [Sigma]), and equal amounts of protein were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and blotted onto Immuno-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, http://www.bio-rad.com). Washes were performed in Tris-buffered saline with 0.1% Tween 20 (TBS-T). Blocking solution contained 10% dried milk in TBS-T. The primary antibodies were diluted in 5% bovine serum albumin in TBS-T, and the enhanced chemiluminescence reaction was detected on X-Omat X-AR film (Sigma). The following antibodies were used (supplemental online Table 1): N-20 (goat and rabbit anti-FasL, sc-834; Santa Cruz Biotechnology; specificity: mouse, rat, human), 1 μg/ml; H11 (rat anti-FasL, ALX-804-010-C100; Alexis Laboratories, San Diego, http://www.axxora.com; specificity: mouse), 2 μg/ml; F2928 (rat anti-FasL; Sigma; specificity: mouse), 0.2 μg/ml; Ab-1 (rabbit anti-FasL, PC78; EMD Biosciences, San Diego, http://www.emdbiosciences.com; specificity: mouse), 5 μg/ml; and M-20 (rabbit anti-Fas, sc-716; Santa Cruz Biotechnology; specificity: mouse), 0.4 μg/ml.
Jurkat Cell Assays
To determine functional FasL expression, 106 Fas-expressing Jurkat cells (human T-cell leukemia cell line; ATCC) were added into 24 wells containing either 2.5 × 105 control or rFasL-expressing HEK or immature ES cells or were added to ES cell cultures at late stages of differentiation (neural induction). After 36–48 hours in coculture, the cells were harvested by mild trypsinization (0.05%, 3 minutes at 37°C), transferred to FACS tubes, washed, and stained with 2 μg/ml propidium iodide (PI) prior to FACS analysis. As controls, Jurkat cells were treated with 50 ng/ml sFasL for 48 hours. Similar experiments were performed with conditioned media from the HEK and ES cell cultures. We cultured 106 Jurkat cells for 36 hours in 0.75 ml of conditioned medium supplemented with 0.75 ml of fresh medium, stained with 2 μg/ml PI, and analyzed by FACS.
Transplantation of ES Cells and Postmortem Analyses
Animal studies were approved by the Institutional Animal Care and Use Committee at McLean Hospital and Harvard Medical School. ES cells were harvested by mild trypsinization (0.05% for 5 minutes at 37°C), spun at 1,000 rpm for 5 minutes, rinsed in Ca2+- and Mg2+-free Dulbecco's phosphate-buffered saline (Gibco-BRL, Gaithersburg, MD, http://www.gibcobrl.com), and collected in ES cell culture medium. A total of 10,000 ES cells (5,000 cells per microliter) were stereotactically injected at two sites (1 μl each) in the striatum of female Sprague-Dawley rats (250–300 g) (Sprague-Dawley; Charles River Laboratories, Wilmington, MA, http://www.criver.com) according to published protocols [22]. Three groups of 12 animals each were implanted with either the GFP-expressing ES.Xgfp control (groups 1 and 2) or the rFasL-expressing ES.XF1 ES cells (group 3). Whereas animals in groups 2 and 3 did not receive immunosuppressive treatment, the rats in group 1 were daily subcutaneously injected with 15 mg/kg cyclosporine A (CsA) (Sandimmune; Sandoz, East Hanover, NJ, http://www.sandoz.com) diluted in extra virgin olive oil beginning 1 day prior to transplantation (2× dose of 30 mg/kg) and continuing through the course of the study. Eight weeks post-transplantation, animals were terminally anesthetized, and the brains were removed and prepared for immunohistochemistry as described [22].
Software and Statistical Analyses
All FACS data were analyzed using the FlowJo software program (Tree Star, Ashland, OR, http://www.treestar.com). Data were analyzed by unpaired Student's t test and two-way analysis of variance followed by Tukey-Kramer post hoc analysis. Data with p values <.05 were considered to be statistically significant.
Results
Functional rFasL Is Expressed in Recombinant HEK Cells
To overexpress rFasL, we used two different strategies. First, we cloned plasmid pCE-rFasL-IRES-EGFP, which was derived from the previously published CE-1 plasmid and can be used to insert a loxP-flanked expression cassette into the corresponding target locus in CE mES cells by Cre-recombinase expression [14]. Second, we cloned pCX-rFasL-IRES-EGFP, which was based on the pCX-EGFP (Xgfp) plasmid from Invitrogen. Both plasmids were tested in HEK cells demonstrating IRES-mediated enhanced green fluorescent protein expression in fluorescence microscopy (data not shown) and rFasL expression by RT-PCR (Fig. 1A). In addition, we tested several antibodies to detect the recombinant rFasL molecule by Western blot and by FACS (listed in Materials and Methods and supplemental online Table 1). In Western blot experiments, the antibodies F2928, H11, and N20 recognized a ∼32-kDa protein in rat thymus homogenates, but only the rabbit anti-FasL antibody N20 showed a similar band in rFasL-recombinant HEK cell clones (Fig. 1B, supplemental online Table 1). In addition, there were several additional bands indicating multiple forms of the FasL molecule. In the FACS analyses, we tested a total of six anti-FasL antibodies, but we were not able to detect a recombinant rFasL surface molecule (supplemental online Fig. S1).
Figure 1.
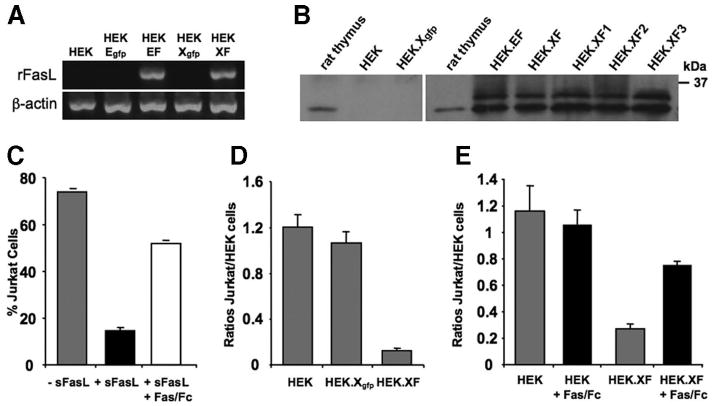
Expression of functional rFasL and enhanced green fluorescent protein in HEK-293T cells. HEK cells were transfected with the control plasmids CE3 [14] and pCX-eGFP or the rFasL-expressing plasmids pCE-rFasL-IRES-EGFP and pCX-rFasL-IRES-EGFP. (A): Reverse transcription-polymerase chain reaction experiments demonstrated transcription of the rFasL gene in EF- and XF- but not in the Egfp- and Xgfp control-transfected HEK cells. (B): Western blot assays using the rabbit anti-mouse FasL (N-20) antibody from Santa Cruz Biotechnology showed that naïve and Xgfp-transfected HEK cells had no expression of a FasL protein, whereas a FasL-specific protein was detected in rat thymus and rFasL-transfected HEK cells. The latter cells also showed several other forms of the rFasL protein. (C–E): The functionality of FasL expression was determined by coculture experiments with Jurkat cells. (C): As control for apoptosis induction, Jurkat cells were incubated with 50 ng/ml sFasL for 24–48 hours and analyzed by fluorescent activated cell sorting (FACS) using propidium iodide (PI) (supplemental online Fig. S2). There was a reduction of cells when sFasL was added to the cultures, and this effect could be blocked by Fas/Fc (100 ng/ml). (D): Control HEK, HEK.Xgfp, and HEK.XF cells were cocultured with Jurkat cells for 48 hours and analyzed by FACS in the presence of PI (see supplemental online Fig. S2 for details). The data were plotted as ratios of Jurkat cells over HEK cells. (E): When compared with cocultures with naïve or green fluorescent protein-expressing HEK cells, there was a reduction of cells in the Jurkat cell gate in the presence of rFasL-transfected HEK cells, and this effect could be blocked in the presence of Fas/Fc. Abbreviations: EF, pCE-rFasL-IRES-EGFP; Egfp, CE3; HEK, human embryonic kidney; rFasL, rat Fas ligand; sFasL, soluble Fas ligand; XF, pCX-rFasL-IRES-EGFP; Xgfp, pCX-eGFP.
We then turned to a functional assay to demonstrate rFasL expression. Naïve, GFP-, and rFasL-transfected HEK cells were cocultured with Fas-expressing Jurkat cells, and cell survival was measured after 48 hours using FACS analysis (supplemental online Fig. S2). There was a >90% reduction of viable Jurkat cells when cultured together with the rFasL-transfected HEK cells (Fig. 1D) as also seen in soluble (s)FasL-treated control experiments (Fig. 1C), and this effect could be blocked by the Fas/Fc fragment (Fig. 1C, 1E). No effect was observed in cocultures with naïve or GFP-transfected HEK cells (Fig. 1D, 1E). These experiments indicated that a functional rFasL was expressed from either EF or XF plasmids.
mES Cells Do Not Express Functional FasL
To generate rFasL-expressing mES cell clones, we first used the CE ES cell lines (kindly provided by Drs. L. Adams and D. Gottlieb, Washington University [14]). CE1 ES cells were transfected with the EF plasmids and selected in puromycin drug resistance, and clones were genotypically screened by a flanking polymerase chain reaction as described [14]. To detect the recombinant rFasL transcript by RT-PCR, we designed an oligonucleotide primer pair that amplified a 212-bp fragment from the rFasL and a 242-bp fragment from the endogenous mouse FasL (mFasL) sequence (Fig. 2A). We found that EF ES cell clones expressed the rFasL by RT-PCR (Fig. 2A) but did not significantly increase the apoptosis of Jurkat cells in coculture experiments when compared with naïve ES cells (Fig. 2B). We then generated rFasL-expressing D3 ES cells using the XF plasmid, since the CBA promoter in this construct contains the CMV-IE enhancer, which increases its transcriptional activity. ES cell clones were screened by RT-PCR (Fig. 2A) and in Jurkat cell assays (Fig. 2B). From multiple clones, one clone (ES.XF1) showed the highest level of rFasL transcription (Fig. 2A) but had no statistically significant effect on Jurkat cell apoptosis (Fig. 2B). To demonstrate FasL protein expression, we performed FACS analysis and Western blot experiments. Although there was no evidence for surface FasL expression in FACS (supplemental online Fig. 1C), Western blots using the rabbit anti-FasL antibody N-20 showed multiple bands, including a band at 32 kDa that was also present in thymus lysates (Fig. 2C), indicating the expression of FasL protein.
Figure 2.
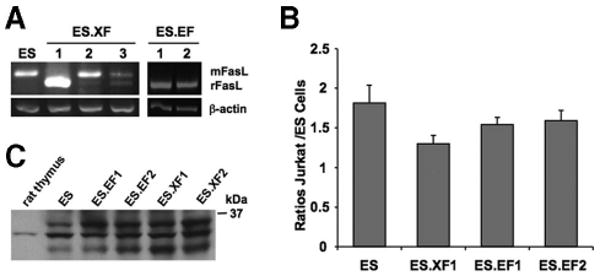
Generation of rFasL-expressing mouse ES cell clones. CE1 or D3 ES cells were transfected with the control plasmids CE3 [14] and Xgfp or the rFasL-expressing plasmids EF and XF, respectively. (A): Multiple ES cell clones were analyzed by reverse transcription-polymerase chain reaction for endogenous mFasL (242 base pairs) and the recombinant rFasL (212 base pairs) expression. From these ES cell lines, clone ES.XF1 had the highest rFasL transcription. (B): Functional FasL expression was analyzed in Jurkat cell assays. In at least three independent experiments, there was no significant effect of any of the rFasL-transfected ES cell lines on the induction of Jurkat cell apoptosis. (C): Western blot experiments using the rabbit anti-mouse FasL antibody N-20 demonstrated multiple bands including a band at 32 kDa, which was also present in rat thymus lysates. Abbreviations: EF, pCE-rFasL-IRES-EGFP; ES, embryonic stem; mFasL, mouse Fas ligand; rFasL, rat Fas ligand; XF, pCX-rFasL-IRES-EGFP.
A Functional FasL Is Not Expressed During mES Cell Differentiation
Although there was no indication that immature mES cells expressed a functional rFasL protein, this did not exclude the possibility that mature cells would do so. We therefore investigated a potential effect of rFasL during neural cell development. Naïve, GFP-, and rFasL-transfected ES cells were differentiated according to the five-stage differentiation protocol [16–19], and gene expression for endogenous mouse (m)Fas, mFasL, the recombinant rFasL, the neural precursor marker Nestin, and the neuronal marker Tuj1 were analyzed by RT-PCR (Fig. 3A) and FasL by FACS (supplemental online Fig. S1D) and Western blot experiments (Fig. 3B). In addition, we determined the fractions of neural precursors (Nestin), neurons (Tuj1), and astroglia (GFAP) by FACS at early (5:3) and late (5:8) stages of neural induction as previously described (Fig. 3C) [20]. All these experiments demonstrated that both the rFasL-expressing ES.EF and ES.XF cell clones developed similarly to naïve and GFP control ES cells. In addition, the Western blot experiments indicated the presence of FasL proteins during differentiation, but there was no evidence for surface FasL. To determine a possible effect of rFasL on apoptosis induction, we analyzed the fractions of PI-positive and PI-negative cell populations at late stages of differentiation (stages 5:3 and 5:8) (Fig. 3D) and in Jurkat cell assays (stage 5:8) (Fig. 3E). There was no increased uptake of PI or induced Jurkat cell death after differentiation of the rFasL-transfected ES cell clones.
Figure 3.
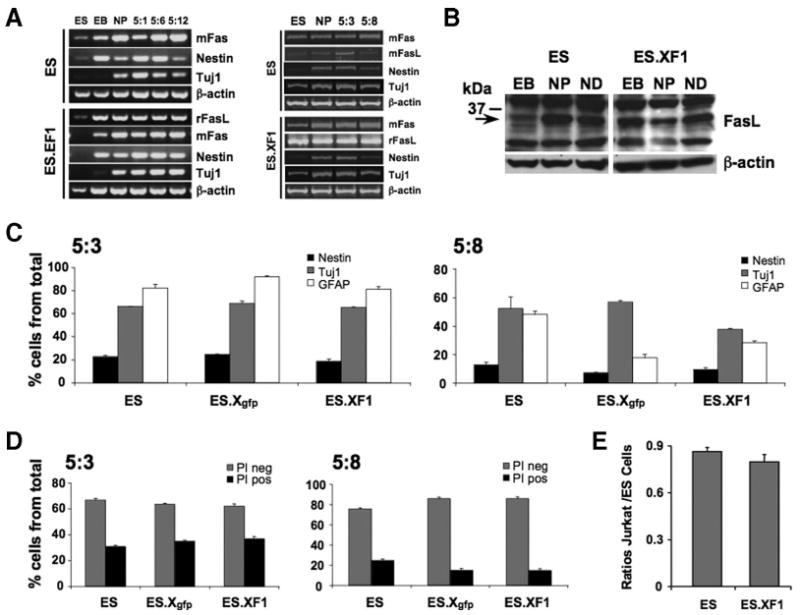
Differentiation of rFasL-expressing ES cells into neural lineages. Naïve and ES.EF or ES.XF mouse ES cell clones were differentiated according to the five-stage protocol to produce neural cell populations [16–20]. Cells were analyzed at different stages of differentiation to determine phenotype development and Fas/FasL expression. A series of duplicate or triplicate experiments was performed using multiple rFasL-recombinant ES cell clones. Shown are data from the representative clone ES.XF1. (A): Reverse transcription-polymerase chain reaction analyses for mFas, mFasL, rFasL, Nestin, and Tuj1 at ES, EB, NP, and ND (stage 5) day 5:1, 5:3, 5:6, 5:8, or 5:12. There were no differences in the gene expression profiles between the naïve and the rFasL-transfected ES cell lines. (B): Western blot experiments using the rabbit N-20 anti-FasL antibody showed multiple bands including a band at 32 kDa in naïve and rFasL-recombinant mouse ES cells. (C): Fluorescent activated cell sorting analyses according to previous published protocols [20] for Nestin (black), Tuj1 (gray), and GFAP (white) at ND stages 5:3 and 5:8 demonstrated no differences in cell phenotype development among naïve, control Xgfp-transfected (ES.Xgfp), and rFasL-recombinant ES cells (ES.XF1). (D): Analysis of cells at ND stages 5:3 and 5:8 using propidium iodide staining demonstrated no increase in apoptosis induction in rFasL-transfected ES cell clones when compared with naïve controls. (E): Jurkat cell assays as described in Figure 1 showed no increase in cell death at late stages of neural induction (5:8). Abbreviations: ES, embryonic stem; GFAP, glial fibrillary acidic protein; mFasL, mouse Fas ligand; ND, neural differentiation; NP, neural precursor; PI neg, propidium iodide negative; PI pos, propidium iodide positive; rFasL, rat Fas ligand.
Recombinant rFasL-Expressing mES Cells Are Rejected when Transplanted into the Midbrain of Rats
In addition to the in vitro experiments, we determined whether rFasL-transfected mES cells could survive in the brains of rats without immunosuppressive treatment. Two groups of 12 control animals received 10,000 of the GFP-expressing ES.Xgfp cells, and one of those groups was treated with the immune-suppressive agent CsA, whereas the other group of animals did not receive immunosuppression (Table 1). A third group of 12 rats was transplanted with similar amounts of the rFasL-transfected ES.XF1 cells and was also not treated with CsA. As expected, the majority of CsA-treated animals (7 out of 10 rats) developed grafts, which were either teratomas (three grafts) or of mainly neural cellular content (four grafts) [19], demonstrating initial survival of the ES cells. In contrast, animals that received ES.Xgfp cells and were not treated with CsA did not show any graft development, which was also true for rats that were transplanted with the ES.XF1 cells.
Immature and Differentiated mES Cells Do Not Express a Functional Fas/FasL System
The Western blot experiments indicated that naïve and the rFasL-recombinant immature and differentiated mES cells expressed FasL proteins (Figs. 2C, 3B), but we could not determine the presence of surface FasL expression. It is known that FasL can occur as a soluble molecule (sFasL), which is produced by cleavage through metalloproteinases at an extracellular metalloproteinase cleavage site [23, 24]. In addition, there is evidence that sFasL can be directly released by cells [25]. To investigate whether naïve and rFasL-recombinant mES cells express a sFasL molecule, we tested the media of cultured cells in the Jurkat cell apoptosis experiment. Using HEK cells, we found that conditioned medium from naïve cells did not induce Jurkat cell death, whereas medium from the rFasL-expressing HEK.XF cells induced apoptosis, and this effect could be blocked by Fas/Fc (Fig. 4A, 4B). In contrast, there was no difference in cell death observed when Jurkat cells were incubated in conditioned media derived from naive or the ES.XF1 ES cells (Fig. 4C). These results indicate that, in contrast to rFasL-transfected HEK cells, naïve and rFasL-recombinant mES cells do not express a sFasL molecule.
Figure 4.
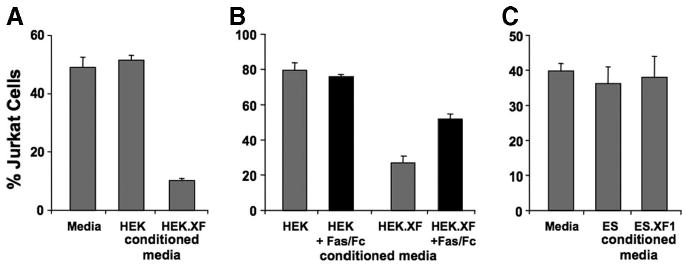
Testing the presence of soluble Fas ligand (sFasL) derived from naïve or rat Fas ligand-recombinant HEK and mouse ES cells. Jurkat cells, incubated in medium or conditioned medium, were analyzed for cell death in fluorescent activated cell sorting assays. Incubation of Jurkat cells in HEK.XF but not HEK cell conditioned medium induced apoptosis (A), and this effect could be blocked in the presence of Fas/Fc (B). In contrast, there was no death observed when Jurkat cells were cultured in conditioned media from naïve or ES.XF ES cells (C), demonstrating that the ES cells do not release sFasL. Abbreviation: ES, embryonic stem; HEK, human embryonic kidney.
To gain further insight into the function of the Fas/FasL system in mouse ES cells and during differentiation, we additionally analyzed the functionality of the mFas molecule. Naïve as well as rFasL-transfected mES cells had low transcriptional Fas activity, which was increased during differentiation (Figs. 3A, 5A). Although we could not detect a Fas surface molecule using the anti-Fas antibody Jo2 in FACS, Western blot experiments with the M20 antibody detected a strong band in ES cells, which was larger than the expected 48-kDa product (Fig. 5A). In contrast, there was a ∼48-kDa band visible in neural precursor cells and during neural differentiation (stages 5:3 and 5:10). To test whether a functional Fas molecule was expressed, we supplemented the cultures with soluble FasL. ES cells (data not shown) and differentiated mES cells were treated with sFasL starting at the neural differentiation stage 5:1 for 4 hours to 12 days (Fig. 5B). There was no morphological difference between untreated and sFasL-treated cells. In addition, caspase-3 activity assays and PI-staining to detect the induction of cell death did not reveal any evidence of Fas-induced apoptosis (Fig. 5C, 5D). These data suggest that a functional Fas molecule was not expressed in immature ES and differentiated mES cells.
Figure 5.
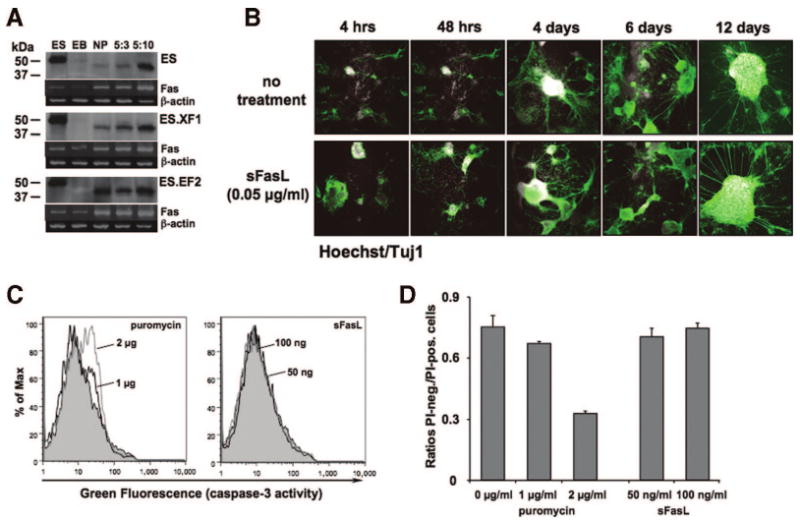
Testing the expression of functional mFas expression during ES cell differentiation. (A): Western blot experiments using the anti-Fas antibody M20 revealed the presence of two bands: a band higher than the expected 48 kDa in ES cells and a ∼48-kDa band in NPs and at the neural differentiation (ND) stages 5:3 and 5:10. The intensities of the latter band correlated with those in corresponding reverse transcription-polymerase chain reaction experiments (lower rows). Although a Fas-specific transcript was visible in ES cells and EBs, no corresponding product was detected in the Western blots, indicating the expression of a Fas protein at later stages during cell differentiation. (B–D): Testing the functionality of the Fas molecule in differentiated ES cells. (B): Naïve ES cells were differentiated and treated with three different concentrations (10, 50, and 100 ng/ml) of sFasL at ND stage 5, and cells were stained with the anti-β-III-tubulin antibody Tuj1 (green) and the nuclear dye Hoechst (white). There was no morphologic difference between untreated and sFasL-treated cells; magnification, ×5. (C): To test whether sFasL induced apoptosis, cells at ND stage 5 were analyzed by fluorescent activated cell sorting (FACS) using a caspase-3 assay (see Materials and Methods for details). Although treatment of cells in control conditions with 1 (black) or 2 (gray) μg/ml puromycin for 18 hours induced caspase-3 activity (green fluorescence), cells grown for 6 days with 50 (black) or 100 (gray) ng/ml sFasL did not shift in the FACS. (D): Puromycin- and sFasL-treated cells were analyzed by FACS after propidium iodide (PI) staining and plotted as ratios of PI-negative over PI-positive cells as described [20]. Consistent with the caspase-3 results, puromycin treatment revealed an increase in apoptotic PI-positive cells, whereas sFasL had no effect. Abbreviations: ES, embryonic stem; hrs, hours; NP, neural precursor; PI-neg., propidium iodide negative; PI-pos., propidium iodide positive; sFasL, soluble Fas ligand.
Discussion
The purpose of this study was to determine whether overexpression of rFasL in mouse ES cells would abrogate some of the cellular immune responses after transplantation to the brain of recipient rats. To our surprise, we found that there was no in vitro or in vivo evidence for the expression of a functional rat or endogenous mouse FasL protein in immature ES cells or during differentiation toward neural cell populations. In addition, we could not demonstrate the expression of a functional Fas molecule regarding induction of apoptosis or cell proliferation and neurite sprouting [26–28]. These results suggest that mES cells do not express a functional Fas/FasL system in the immature state as well as during in vitro neurogenesis.
The expression of recombinant rFasL plasmids in HEK-293T cells revealed that functional rFasL molecules could be expressed. Although surface FasL could not be detected by FACS, Western blot experiments using the rabbit anti-mouse FasL antibody N-20 showed multiple forms of the FasL protein and, in coculture and conditioned medium assays, Jurkat cell apoptosis was induced. HEK-293T and other cell lines have widely been used to express full-length FasL, demonstrating that FasL is glycosylated [29] and expressed as soluble and the surface molecule [5, 29–31]. The production of sFasL has been attributed to metalloproteinase cleavage sites (matrix metalloproteinase) within the transmembrane domain (TM) of the FasL molecule [23, 24] or as a differentially spliced form of the membrane-bound protein [25]. It is therefore possible that the inability to detect membrane-bound rFasL was due to the proteolytic cleavage of sFasL at the TM, resulting in an incomplete surface molecule that could not be recognized by the antibodies.
In the ES cells, we used two different strategies to express rFasL by targeted gene expression from a loxP locus [14] (EF clones) and using a strong CBA promoter with the CMV-IE enhancer (XF clones). Although the rat and endogenous mouse FasL gene was transcribed and Western blot experiments using the N-20 antibody indicated that FasL protein was expressed, we could not detect soluble or membrane-bound FasL in naïve or rFasL-transfected ES cells. Published data about the expression of FasL on ES cells are contradictory. Drukker et al. demonstrated that human ES cells do not express FasL mRNA and surface protein [32], whereas Fabricius et al. reported surface FasL expression on mES cells [33] and Kim et al. on human ES cells [34]. It should be noted that in the study performed by Fabricius et al., a not further defined anti-FasL antibody was used, which revealed a shift in the FACS assay. However, there was no control experiment to rule out possible cross-reactivity with nonspecific antigens. Our results show that, although endogenous or recombinant FasL are transcribed and probably translated, the mES cells lack the intrinsic ability to synthesize a functional FasL protein. The reasons for this are unclear, and further studies are required (e.g., using a tagged version of the FasL protein to track the intracellular processes involved in gene translation and protein expression). It is interesting to note that similar experiments as described for the mouse ES cells using the rat neuroblastoma cell line B104 revealed the same inability to express a functional soluble or membrane-bound recombinant or endogenous rat FasL protein (supplemental online Fig. S3). This indicates that the inability to produce functional FasL proteins does not seem to be restricted to ES cells and their neural derivatives.
Fas and FasL can be detected in the brains of embryos and postnatal mice [35, 36], and a functional role of the Fas/FasL system during brain development has been studied in Fas- (lpr/lpr) or FasL-defective (gld/gld) mice [37]. These mice develop a lymphoproliferative autoimmune disease and also have some degree of hippocampal malfunction, which was attributed to an involvement of Fas in controlling the branching of dendrites in the developing hippocampus [36]. In the adult neural system, the expression of Fas and FasL has been shown on astrocytes, neurons, and glia [38]. Both molecules are involved in maintenance of the immune suppressed status of the adult CNS, induction of neuronal cell death and inflammation in a variety of neurologic diseases (reviewed in [38]), and neurite growth as a possible mechanism in neuroregeneration [26–28]. Altogether, the Fas/FasL system in the CNS underlies complex regulatory mechanisms and Fas signaling seems to be associated with stress, such as injury or disease, whereas naïve and unencumbered cells are mostly resistant to Fas activation. In the differentiating ES cells, we could detect endogenous Fas at late stages of in vitro differentiation by Western blot experiments, but we were not able to demonstrate a functional surface Fas molecule. To this end, we have not determined whether the immature and differentiated ES cells express molecules involved in Fas-mediated signaling (e.g., FADD, c-FliP, and caspase-8) [39]. It would be interesting to determine whether cell stress can lead to the expression of a functional Fas protein including the activation of intracellular signaling cascades.
In clinical cell transplantation, long-term survival and function of embryo-derived neural cells have been observed in patients with PD, indicating that the CNS immunity does not necessarily reject allogeneic cells in the brain [40]. However, it is a common observation that the initial number of transplanted cells in the CNS dramatically decreases over time, and in animal models there is evidence that immune responses could play a role in grafting to the CNS. The mechanisms of these responses are complex, strongly depend on the cell type implanted, and involve active regulation of immune reactivity by the CNS microenvironment [41–43]. Without immunosuppression, allogeneic ES cells can be tolerated in the brains of recipient animals, whereas xenogeneic ES cells are rejected, and the latter has been confirmed in our study. It has been shown that ES cells can express a variety of factors potentially involved in immunogenicity (reviewed in [43]), but a functional role of the Fas/FasL system has not been described. Recently, it was demonstrated that human ES cells do not express FasL and are rejected when transplanted under the kidney capsula of immune-competent (xenogeneic situation), but are tolerated in “allogeneic,” human peripheral blood mononuclear cell-reconstituted mice (allogeneic situation), suggesting that the Fas/FasL system is not involved in ES cell immunity [32]. In the CNS, FasL can contribute to allogeneic or xenogeneic immune-tolerance toward Sertoli or astrocytoma cell grafts [11–13]. In addition to these data, we now have demonstrated that immature and neurally differentiated ES cells lack the intrinsic ability to express endogenous or recombinant FasL, implying that FasL might not be a useful tool to achieve immune tolerance in transplantation paradigms using ES cells and their cellular derivatives.
Supplementary Material
Acknowledgments
We thank Dr. Angel Vinuela, Casper Reske-Nielsen, Michaela Patterson, and Yalda Sadeghi for excellent technical help and Dr. Rosario Sanchez-Pernaute for helpful discussion. This study was supported by National Institute of Neurological Disorders and Stroke Grant NS048551 (to K.-C.S.) and Udall Ctr. P50 NS-39793, Orchard, Stern and Consolidated Anti-Aging Foundation (OI).
Footnotes
Disclosure of Potential Conflicts of Interest: The authors indicate no potential conflicts of interest.
See www.StemCells.com for supplemental material available online.
References
- 1.Isacson O. The production and use of cells as therapeutic agents in neurodegenerative diseases. Lancet Neurol. 2003;2:417–424. doi: 10.1016/s1474-4422(03)00437-x. [DOI] [PubMed] [Google Scholar]
- 2.Sonntag KC, Sanchez-Pernaute R. Tailoring human embryonic stem cells for neurodegenerative disease therapy. Curr Opin Investig Drugs. 2006;7:614–618. [PubMed] [Google Scholar]
- 3.LeGuern C. Regulation of T-cell functions by MHC class II self-presentation. Trends Immunol. 2003;24:633–638. doi: 10.1016/j.it.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Sonntag KC, Emery DW, Yasumoto A, et al. Tolerance to solid organ transplants through transfer of MHC class II genes. J Clin Invest. 2001;107:65–71. doi: 10.1172/JCI11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suda T, Takahashi T, Golstein P, et al. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 6.Leithauser F, Dhein J, Mechtersheimer G, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 7.Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int Rev Immunol. 2002;21:153–172. doi: 10.1080/08830180212058. [DOI] [PubMed] [Google Scholar]
- 8.Ksander BR, Streilein JW. Regulation of the immune response within privileged sites. Chem Immunol. 1994;58:117–145. [PubMed] [Google Scholar]
- 9.Medana I, Li Z, Flugel A, et al. Fas ligand (CD95L) protects neurons against perforin-mediated T lymphocyte cytotoxicity. J Immunol. 2001;167:674–681. doi: 10.4049/jimmunol.167.2.674. [DOI] [PubMed] [Google Scholar]
- 10.Moalem G, Monsonego A, Shani Y, et al. Differential T cell response in central and peripheral nerve injury: Connection with immune privilege. FASEB J. 1999;13:1207–1217. doi: 10.1096/fasebj.13.10.1207. [DOI] [PubMed] [Google Scholar]
- 11.Sanberg P, Borlongan C, Othberg A, et al. Testis-derived Sertoli cells have a trophic effect on dopamine neurons and alleviate hemiparkinsonism in rats. Nat Med. 1997;3:1129–1132. doi: 10.1038/nm1097-1129. [DOI] [PubMed] [Google Scholar]
- 12.Saporta S, Cameron D, Borlongan C, et al. Survival of rat and porcine sertoli cell transplants in the rat striatum without cyclosporin-A immunosuppression. Exp Neurol. 1997;146:299–304. doi: 10.1006/exnr.1997.6493. [DOI] [PubMed] [Google Scholar]
- 13.Saas P, Walker PR, Hahne M, et al. Fas ligand expression by astrocytoma in vivo: Maintaining immune privilege in the brain? J Clin Invest. 1997;99:1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams LD, Choi L, Xian HQ, et al. Double lox targeting for neural cell transgenesis. Brain Res Mol Brain Res. 2003;110:220–233. doi: 10.1016/s0169-328x(02)00651-4. [DOI] [PubMed] [Google Scholar]
- 15.Matter-Reissmann UB, Sonntag KC, Gilli UO, et al. Human Fas-ligand expression on porcine endothelial cells does not protect against xenogeneic natural killer cytotoxicity. Xenotransplantation. 2004;11:43–52. doi: 10.1111/j.1399-3089.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 16.Sonntag KC, Simantov R, Kim KS, et al. Temporally induced Nurr1 can induce a non-neuronal dopaminergic cell type in embryonic stem cell differentiation. Eur J Neurosci. 2004;19:1141–1152. doi: 10.1111/j.1460-9568.2004.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SH, Lumelsky N, Studer L, et al. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 18.Chung S, Sonntag KC, Andersson T, et al. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci. 2002;16:1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonntag KC, Simantov R, Bjorklund L, et al. Context-dependent neuronal differentiation and germ layer induction of Smad4–/– and Cripto–/– embryonic stem cells. Mol Cell Neurosci. 2005;28:417–429. doi: 10.1016/j.mcn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Krichevsky AM, Sonntag KC, Isacson O, et al. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. STEM CELLS. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eberle J, Fecker LF, Hossini AM, et al. CD95/Fas signaling in human melanoma cells: Conditional expression of CD95L/FasL overcomes the intrinsic apoptosis resistance of malignant melanoma and inhibits growth and progression of human melanoma xenotransplants. Oncogene. 2003;22:9131–9141. doi: 10.1038/sj.onc.1207228. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Pernaute R, Studer L, Ferrari D, et al. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. STEM CELLS. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 24.Vargo-Gogola T, Crawford HC, Fingleton B, et al. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys. 2002;408:155–161. doi: 10.1016/s0003-9861(02)00525-8. [DOI] [PubMed] [Google Scholar]
- 25.Ayroldi E, D'Adamio F, Zollo O, et al. Cloning and expression of a short Fas ligand: A new alternatively spliced product of the mouse Fas ligand gene. Blood. 1999;94:3456–3467. [PubMed] [Google Scholar]
- 26.Desbarats J, Birge RB, Mimouni-Rongy M, et al. Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol. 2003;5:118–125. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- 27.Lambert C, Landau AM, Desbarats J. Fas-beyond death: A regenerative role for Fas in the nervous system. Apoptosis. 2003;8:551–562. doi: 10.1023/A:1026113222478. [DOI] [PubMed] [Google Scholar]
- 28.Pettmann B, Henderson CE. Killer wiles: Growing interest in Fas. Nat Cell Biol. 2003;5:91–92. doi: 10.1038/ncb0203-91. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Suda T, Takahashi T, et al. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nachbur U, Kassahn D, Yousefi S, et al. Posttranscriptional regulation of Fas (CD95) ligand killing activity by lipid rafts. Blood. 2006;107:2790–2796. doi: 10.1182/blood-2005-07-2744. [DOI] [PubMed] [Google Scholar]
- 31.Schneider P, Bodmer JL, Holler N, et al. Characterization of Fas (Apo-1, CD95)-Fas ligand interaction. J Biol Chem. 1997;272:18827–18833. doi: 10.1074/jbc.272.30.18827. [DOI] [PubMed] [Google Scholar]
- 32.Drukker M, Katchman H, Katz G, et al. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. STEM CELLS. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 33.Fabricius D, Bonde S, Zavazava N. Induction of stable mixed chimerism by embryonic stem cells requires functional Fas/FasL engagement. Transplantation. 2005;79:1040–1044. doi: 10.1097/01.tp.0000159142.62535.37. [DOI] [PubMed] [Google Scholar]
- 34.Kim SK, Kim BK, Shim JH, et al. Nonylphenol and octylphenol-induced apoptosis in human embryonic stem cells is related to fas-fas ligand pathway. Toxicol Sci. 2006;94:310–321. doi: 10.1093/toxsci/kfl114. [DOI] [PubMed] [Google Scholar]
- 35.Park C, Sakamaki K, Tachibana O, et al. Expression of fas antigen in the normal mouse brain. Biochem Biophys Res Commun. 1998;252:623–628. doi: 10.1006/bbrc.1998.9572. [DOI] [PubMed] [Google Scholar]
- 36.Zuliani C, Kleber S, Klussmann S, et al. Control of neuronal branching by the death receptor CD95 (Fas/Apo-1) Cell Death Differ. 2006;13:31–40. doi: 10.1038/sj.cdd.4401720. [DOI] [PubMed] [Google Scholar]
- 37.Cohen PL, Eisenberg RA. The lpr and gld genes in systemic autoimmunity: Life and death in the Fas lane. Immunol Today. 1992;13:427–428. doi: 10.1016/0167-5699(92)90066-G. [DOI] [PubMed] [Google Scholar]
- 38.Choi C, Benveniste EN. Fas ligand/Fas system in the brain: Regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004;44:65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Grivennikov SI, Kuprash DV, Liu ZG, et al. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: From simple paradigms to complex mechanisms. Int Rev Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 40.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brevig T, Pedersen EB, Finsen B. Molecular and cellular mechanisms in immune rejection of intracerebral neural transplants. Novartis Found Symp. 2000;231:166–177. doi: 10.1002/0470870834.ch11. [DOI] [PubMed] [Google Scholar]
- 42.Tambur AR. Transplantation immunology and the central nervous system. Neurol Res. 2004;26:243–255. doi: 10.1179/016164104225013932. [DOI] [PubMed] [Google Scholar]
- 43.Sonntag KC. Immunological considerations in CNS transplants. In: Emerich DF, Halberstadt CR, editors. Cellular Transplants: From Lab to Clinic. San Diego, CA: Academic Press Elsevier Inc.; 2006. pp. 305–326. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


