Abstract
Alum-precipitated protein (alum protein) vaccines elicit long-lasting neutralizing antibody responses that prevent bacterial exotoxins and viruses from entering cells. Typically, these vaccines induce CD4 T cells to become T helper 2 (Th2) cells that induce Ig class switching to IgG1. We now report that CD8 T cells also respond to alum proteins, proliferating extensively and producing IFN-γ, a key Th1 cytokine. These findings led us to question whether adoptive transfer of antigen-specific CD8 T cells alters the characteristic CD4 Th2 response to alum proteins and the switching pattern in responding B cells. To this end, WT mice given transgenic ovalbumin (OVA)-specific CD4 (OTII) or CD8 (OTI) T cells, or both, were immunized with alum-precipitated OVA. Cotransfer of antigen-specific CD8 T cells skewed switching patterns in responding B cells from IgG1 to IgG2a and IgG2b. Blocking with anti–IFN-γ antibody largely inhibited this altered B-cell switching pattern. The transcription factor T-bet is required in B cells for IFN-γ–dependent switching to IgG2a. By contrast, we show that this transcription factor is dispensable in B cells both for IFN-γ–induced switching to IgG2b and for inhibition of switching to IgG1. Thus, T-bet dependence identifies distinct transcriptional pathways in B cells that regulate IFN-γ–induced switching to different IgG isotypes.
Keywords: B-cell Ig class switch, T helper 1 cells, T helper 2 cells, IgG2a, IgG2b
Ig class switch recombination (CSR) in B cells responding to T-dependent protein-based antigens is directed by intrinsic antigen components. These components govern the nature of CSR controlled by CD4 T helper cells (Th) and may evoke signals from accessory cells that affect the response. Thus proteins expressed by Salmonella Typhimurium promote T-helper 1 (Th1) responses that produce IFN-γ and B cell CSR to IgG2a (1–5), whereas alum-precipitated proteins (or alum proteins), used in vaccines (6, 7), induce T-helper 2 (Th2) responses that yield IL-4 and IL-13 (1, 8, 9) with CSR to IgG1 (9–11).
Although naive CD4 T-cell differentiation during Th1 or Th2 immune responses are well characterized, the effector functions acquired by naive CD8 T cells responding to Th1 or Th2-inducing antigens is less clear. Naive CD8 T cells stimulated in the presence of IL-4 in vitro differentiate into IL-4–producing CD8 T cells (12–15). In addition, IL-13– and IL-5–producing CD8 T cells have been reported in airway inflammation in mice (16, 17), and CD8 T cells producing Th2 cytokines have been derived from human blood (18, 19). Sometimes CD8 T cells respond to Th2 antigens by producing Th1 cytokines and influencing the differentiation of CD4 T cells and B cells. Thus, in low-level infection with Leishmania major, CD8 T-cell–derived IFN-γ alters CD4 T-cell differentiation from Th2 into Th1 (20). CD8 T cells can also suppress Th2-induction of IgE and inhibit airway inflammation (21, 22).
Alum-precipitated proteins can induce CD8 T cells to proliferate and produce IFN-γ (15, 23, 24), but are poor inducers of CD8 cytolytic T cells against syngenic target cells pulsed with the appropriate class I-restricted peptide (25). By contrast, CD4 T cells produce IL-4 and/or IL-13 in response to these antigens (15). These findings led us to investigate whether adoptively transferred antigen-specific CD8 T cells affect the normal Th2 bias of CD4 T-cell and B-cell responses to alum-precipitated ovalbumin (alumOVA). To test this, we studied popliteal lymph node responses to alumOVA in mice that had received transgenic ovalbumin (OVA)-specific CD4 T (OTII) cells, CD8 T (OTI) cells, or both of these. The results show that responding CD8 T cells profoundly modify CSR patterns in the specific B-cell response. Previous studies in mice deficient for the transcription factor T-bet indicate that switching to IgG2a requires this regulator (26, 27). Further studies in vitro have established that B-cell–intrinsic T-bet is needed for CSR to IgG2a, but only in B-cell responses to T-independent signals, including engagement of Toll-like receptor 4 (TLR4) by LPS (26, 28, 29). Here we show that, during T-cell–dependent responses to alum protein vaccines in vivo, CD8 T-cell–derived IFN-γ induces both T-bet–dependent and T-bet–independent pathways that modify CSR in B cells.
Results
CD8 T Cells Responding to alumOVA Proliferate, Produce IFN-γ and Induce Responding CD4 T Cells to Produce IFN-γ.
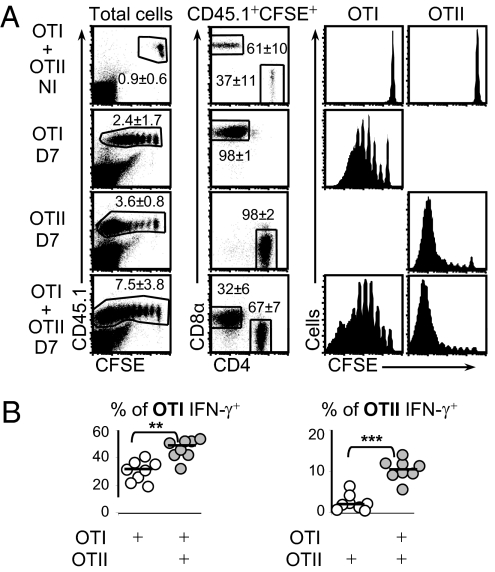
We tested whether and how CD8 T cells influence B cell responses to alumOVA in chimeric mice that had received OVA-specific CD4 and/or CD8 T cells. The CD4 (OTII) and CD8 (OTI) T cells were CD45.1+, distinguishing them from the CD45.2+ cells of the congenic WT recipient mice. The transferred cells were prelabeled with the fluorescent dye CFSE to assess proliferation in the recipient by CFSE dilution (30). The chimeric mice were immunized in the footpads with endotoxin-free alumOVA, and the response of the transferred cells in the draining popliteal lymph node (LN) was assessed 7 d later. Both OTII cells and OTI cells in the popliteal LN proliferate in response to alumOVA (15) (Fig. 1A). Although OTI cells produced IFN-γ protein (Fig. 1B, Left), as expected (1, 11, 31), in those chimeras with OTII cells alone, very few of the OTII cells produced IFN-γ (Fig. 1B, Right). When OTI and OTII cells were cotransferred, the proportion of OTI cells producing IFN-γ increased significantly, and a proportion of the OTII cells produced IFN-γ (Fig. 1B). At the same time, fewer OTII cells produced IL-4 protein (Fig. S1A). These changes in IFN-γ and IL-4 protein production were reflected in the levels of mRNA encoding these cytokines in the responding LN (Fig. S1B).
Fig. 1.
Comparison of proliferative response and IFN-γ production by CD4 OTII and CD8 OTI cells in response to alumOVA. Chimeras were constructed by transfer of CFSE-labeled CD45.1+OTI and/or CD45.1+OTII cells into congenic CD45.2+ C57BL6 mice. Most chimeras were immunized with alumOVA in both footpads, whereas some were not immunized (NI). (A) On day 7 (D7) postimmunization, draining popliteal LN were taken and proliferative response of OTI and OTII cells was assessed by CFSE dilution. (B) On day 7, popliteal LN suspensions were cultured for 5 h with the OVA peptides recognized respectively by OTI and OTII cells. OTI cells and OTII cells identified by the gating strategy used in A were analyzed for the production of IFN-γ. Data are derived from two independent experiments with eight mice in total. Each symbol represents data from the two pooled popliteal LN of one mouse. Statistics assessed by a two-tailed Mann–Whitney test are shown as follows: NS, not significant, **P < 0.01, ***P < 0.001.
CSR induced by cognate CD4 T-cell interaction with B cells can occur in the outer T zone as well as in germinal centers. Therefore, we tested whether the change of cytokine production from IL-4 to IFN-γ occurs in the responding CD4 T cells in both these tissue compartments. To this end, the OTII T cells from chimeras responding to alumOVA were sorted by flow cytometry into PD-1+CXCR5+ T follicular helper cells (TFh OTII) and other effector T cells (Eff OTII) (Fig. S1C). In the mixed chimeras, both fractions of OTII cells produced more IFN-γ and less IL-4 mRNA than the OTII cell fractions isolated from chimeras constructed with OTII cells alone (Fig. S1C). Thus, the OTI cell-induced alteration of OTII cell-cytokine production has the potential to alter CSR patterns in both the outer T zone and germinal centers.
CD8 OTI Cells Diversify B Cell-Switching Pattern in Response to alumOVA.
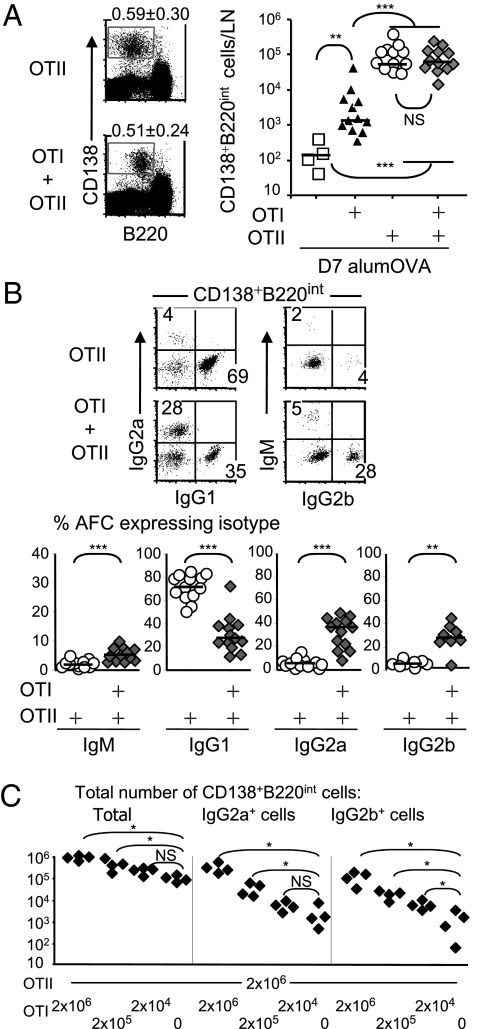
The limiting effect of T-cell help on the development of the antibody response to alumOVA is apparent from Fig. 2A. Thus, at 7 d after immunization with alumOVA, the number of CD138+B220Int antibody-forming cells (AFC) produced in LN of chimeras constructed with OTII cells was 400 times the number in WT mice. AFC numbers generated in the response of OTII cell chimeras to alumOVA has previously been shown to peak 7 d after immunization (11). As expected (1, 11, 31) the response assisted by OTII cells alone predominantly induced switching to IgG1 (Fig. 2B). Somewhat surprisingly, CD8 T cells also promoted AFC production. Thus chimeras constructed with OTI cells had approximately eightfold more AFC than WT controls. Overall the cotransfer of CD8 (OTI) with CD4 (OTII) cells did not greatly alter the number of AFC produced compared with the number of AFC generated in OTII cell chimeras (Fig. 2A). The Ig classes produced by AFC in the mixed chimeras were strikingly different from those produced in chimeras receiving OTII cells only. The proportions (Fig. 2B) and absolute numbers (Fig. S2A) of IgG1-producing cells were greatly reduced, and many AFC now produced IgG2a or IgG2b.
Fig. 2.
OTII cells responding to alumOVA induce a Th2 CSR pattern that is altered to Th1 type CSR in the presence of increased number of OTI cells. Chimeras were constructed and immunized as in Fig. 1. (A) Seven days after alumOVA, AFC were quantified in the popliteal LN cell suspensions as CD138+B220int cells. Numbers in quadrants indicate percentage ± SD of CD138+B220int cells. (B) In AFC population, proportions of cells expressing cytoplasmic IgM, IgG1, IgG2a, or IgG2b were assessed by intracellular FACS staining. Data are derived from two to five independent experiments with eight to 13 mice in each group. Each symbol represents data from the two pooled popliteal LN of one mouse. **P < 0.01, ***P < 0.001. (C) Chimeras were constructed with 2 × 106 OTII and with 10-fold dilutions of OTI cells starting from 2 × 106, or no OTI cells, and immunized as in Fig. 1. The total numbers of AFC, IgG2a+ AFC, and IgG2b+ AFC were assessed 8 d after immunization as a function of the number of OTI cells transferred. Data are derived from two independent experiments with four mice in each group. Each symbol represents data from the two pooled popliteal LN of one mouse. NS, not significant, *P < 0.05. Differences between groups were calculated using the two-tailed nonparametric Mann–Whitney test.
We next studied the minimum number of antigen-specific CD8 T cells that could affect the CD4 T cell-dependent switching pattern of B cells. Chimeras were constructed where a constant number of OTII cells were transferred together with 10-fold dilutions of OTI cells. The mice were then immunized as before, and the numbers of AFC producing different IgG classes were determined 8 d later. The results show a progressive reduction in the numbers of IgG2a- and IgG2b-producing cells with reduced numbers of OTI cells (Fig. 2C). In addition, 2 × 105 OTI cells cotransferred with 2 × 106 OTII cells induced significantly more plasma cells switched to IgG2a (P = 0.02; median 3.1 × 104 vs. 1.4 × 103) and IgG2b (P = 0.02; median 1.9 × 104 vs. 1.1 × 103) than the control chimeras that received 2 × 106 OTII cells only. Even when 2 × 104 OTI cells were transferred with 2 × 106 OTII cells, there were significantly more IgG2b cells than in controls receiving 2 × 106 OTII cells only (P = 0.02; median 5.1 × 103 vs. 1.1 × 103), and there was a trend for skewing to IgG2a (P = 0.15; median 5.0 × 103 vs. 1.4 × 103). Thus, modest numbers of responding CD8 cells can modify CD4-dependent CSR. These data make it plausible that this effect of CD8 T cells specifically responding to alum-precipitated protein in certain circumstances could occur physiologically.
IFN-γ Production in Mixed Chimeras Is Largely Responsible for Loss of CSR to IgG1 and Induction of CSR to IgG2a and IgG2b.
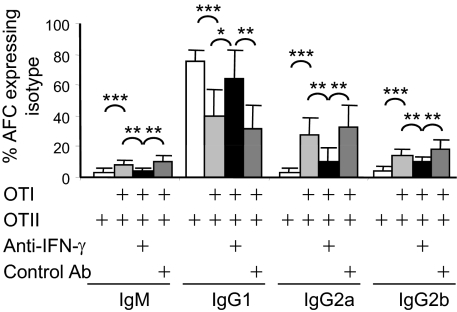
IFN-γ can suppress CSR to IgG1 or IgE and can promote CSR to IgG2a in vivo (21, 22, 32). Consequently, we next tested whether the effect of OTI cells on CSR in the mixed chimeras was due to IFN-γ production. Chimeras were constructed and immunized as before with 2 × 106 OTI and OTII cells, but neutralizing antibody against IFN-γ, or isotype control antibody, was given at the time of transfer and 3 d after immunization. Neutralizing anti–IFN-γ antibody in large part reverses the effect of cotransferring OTI with OTII cells (Fig. 3). In proportion, CSR to IgG1 is restored, and there are significantly fewer AFC producing IgG2a and IgG2b (Fig. 3). This loss of IgG2a- and IgG2b-producing cells caused by neutralizing IFN-γ also applies when the absolute number of AFC is considered, although neutralizing anti–IFN-γ also slightly reduced the total number of AFC produced (Fig. S2B). Thus, CD8 T cell-directed CSR to IgG2a and IgG2b are largely dependent upon IFN-γ.
Fig. 3.
Neutralizing IFN-γ inhibits OTI-dependent induction of IgG2a and IgG2b and suppression of IgG1. Chimeras were constructed and immunized with alumOVA as in Fig. 1. At the time of the immunization and again 3 d after, chimeras were given either neutralizing anti–IFN-γ or isotype control Ab. Classes of cytoplasmic IgM, IgG1, IgG2a, or IgG2b expressed by AFC produced in draining LN were assessed 7 d after immunization, using flow cytometry with staining and gating as illustrated in Fig. 2. Bar chart shows mean percentage and SD of CD138+B220int cells producing indicated Ig isotypes. Data are derived from three independent experiments with eight mice in total. *P < 0.05, **P < 0.01, ***P < 0.001.
T-bet Is Required for IFN-γ–Induced CSR to IgG2a but Is Redundant Both for CSR to IgG2b and Suppression of CSR to IgG1.
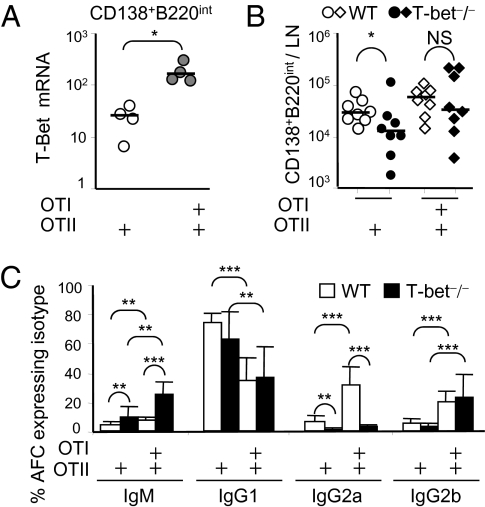
The transcription regulator T-bet is induced within cells activated by IFN-γ through their IFN-γR (29, 33). Thus, in combination with B-cell receptor (BCR) cross-linking, T-bet up-regulation by IFN-γ in B cells leads to CSR to IgG2a (26, 29). We found that AFC induced by alumOVA in chimeras with mixed OTII plus OTI cells had levels of T-bet mRNA six times that in AFC induced in chimeras with OTII cells only (Fig. 4A). To test whether the IFN-γ–induced CSR change is dependent upon T-bet activity in B cells, chimeras were constructed as before, but T-bet–sufficient OTII cells or OTII plus OTI cells were transferred into T-bet–deficient recipients as well as WT recipients. Seven days after alumOVA, T-bet deficiency in recipients had no significant effect of on the total numbers of AFC induced in the different chimeras (Fig. 4B). In chimeras constructed in WT recipients, CD8 T cells again reduced CSR to IgG1 and increased CSR to IgG2a and IgG2b. By contrast, in chimeras constructed in T-bet–deficient mice, CSR to IgG2a was largely lost, whereas OTI cells still suppressed the proportion of cells undergoing CSR to IgG1 and induced CSR to IgG2b (Fig. 4C and Fig. S3A).
Fig. 4.
Non–T-cell T-bet is required for IFN-γ–induced IgG2a, but not for plasmablast formation or IFN-γ–induced IgG2b. (A) Chimeras were constructed and immunized as in Fig. 1. Seven days later, CD138+B220int AFC populations from draining LN were FACS sorted, and levels of T-bet mRNA were assessed by real-time RT-PCR. Data are derived from two independent experiments with four mice in each group. (B) Chimeras were constructed by transfer of OTII cells or OTI plus OTII cells, all of which are T-bet+/+, into either WT C57BL/6 or congenic T-bet−/− mice. Chimeras were immunized with alumOVA, and 7 d later the number of CD138+B220int AFC in draining LN suspensions was assessed as in Fig. 2A. (C) Proportions of CD138+B220int cells that expressed cytoplasmic IgM, IgG1, IgG2a, or IgG2b. Bar chart shows mean percentage and SD. Open bars show data from WT recipients; filled bars show data from T-bet−/− recipients. Data are derived from two independent experiments with eight mice in each group. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
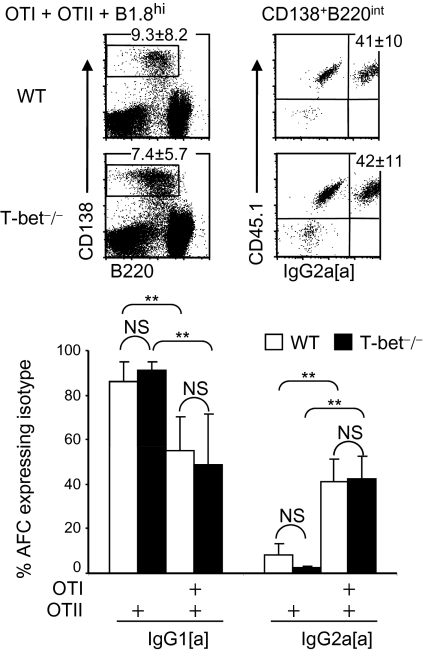
Recently T-bet expression in dendritic cells has been found to optimize polarization of naive T cells into IFN-γ–producing Th1 effectors (34). To test whether T-bet in non-B non-T cells was affecting CSR, we cotransferred T-bet–sufficient B cells, specific for the hapten (4-hydroxy-3-nitrophenyl) acetyl (NP), from B1.8hi mice with either OTII or OTII plus OTI cells into WT C57BL/6 or congenic T-bet−/− recipients. Seven days after immunization with alumNP-OVA, the proportions (Fig. 5) and numbers (Fig. S3B) of IgG2a B1.8hi cells obtained from the T-bet−/− chimeras constructed with OTII plus OTI cells were comparable to those seen in WT recipients. Thus, in this system, T-bet in dendritic cells or other non-B non-T cells is dispensable for CD8 T-cell–derived IFN-γ–induced IgG2a. These data indicate that IFN-γ induction of T-bet activity in B cells is a key regulator of CSR to IgG2a in mixed chimeras and show that a different CSR pathway(s) regulate(s) IFN-γ–dependent CSR to IgG2b and suppression of CSR to IgG1.
Fig. 5.
T-bet deficiency in innate cells and nonresponding lymphocytes does not prevent IFN-γ–induced CSR to IgG2a in T-bet–sufficient B cells. Chimeras were constructed by transfer of NP-specific B1.8hi B cells and OTII cells or both OTI plus OTII cells, all of which are CD45.1+, either into CD45.2+ WT C57BL/6 or congenic T-bet−/− mice. Chimeras were immunized in both footpads with alumNP-OVA, and 7 d later popliteal LN cell suspensions were prepared as in Fig. 2. Percentage of IgG1 and IgG2a-containing B1.8hi AFC (CD138+B220intCD45.1+ cells) in these suspensions was assessed by flow cytometry. CSR of B1.8hi cells was assessed by using specific anti-IgG1[a] and IgG2a[a] allotypes antibodies. Gating is shown in dot plots; mean percentage ± SD of IgG2a[a]+ B1.8hi cells are in top right quadrants of right-hand dot plots. Bar chart shows mean percentage and SD of B1.8hi cells containing IgG1[a] or IgG2a[a]. Open bars show data from WT recipients; filled bars show data from T-bet−/− recipients. Data are derived from two independent experiments with six mice in each group. NS, not significant, **P < 0.01.
Discussion
The current study shows that the presence of antigen-specific CD8 T cells during T-dependent antibody responses to alum-precipitated proteins can skew B-cell CSR from IgG1 to IgG2a and IgG2b. IFN-γ produced by the activated CD8 T cells plays a major part in redirecting the response of CD4 T cells and B cells from Th2 to Th1. At this stage of the study, it is unclear when and where the influence of the activated CD8 T cells occurs. Indeed, it may involve more than one mechanism and may occur in at least two different sites. There could be a direct effect in the outer T zone where responding CD8 T cells mingle with B cells that have taken up antigen. The B-cell T-bet dependence of switching to IgG2a seen in our experiments indicates a direct effect of T-cell–derived IFN-γ on the B cells, but this IFN-γ might be derived from CD4 or CD8 T cells or both.
It is unlikely that the CD8 T cells directly affect switching in the follicles, as these cells typically are not found in the follicles (35), and when they are they seem to be detrimental to the antibody response (36). Consistent with this, OTI cells do not acquire T follicular helper cell features including expression of CXCR5 and PD-1 (15). The finding of IFN-γ–producing TFh cells in mixed chimeras indicates how CD8 T cells can indirectly modify CSR in follicles; Th1 cells have been previously been shown to migrate into germinal centers (37). The other responding OTII cells generated in the mixed chimeras were shown to produce IFN-γ, indicating that OTI cells may influence CSR indirectly in the outer T zone.
A role for T-bet in B cells was first shown in murine systemic lupus erythematosus, where IgG2a-mediated autoimmune disease involves T-independent and T-dependent mechanisms (26, 38). These mice, as well as humans with this pathology, have a range of antinuclear antibodies (39, 40). T-bet seems to be necessary for the T-independent induction of IgG2a associated with binding of unmethylated CpG DNA to TLR9 on B cells (41–43). This pathway of T-bet activation does not require STAT-1, a transcription factor acting downstream of IFN-γR in cells exposed to IFN-γ (41). By contrast, studies in vitro indicate that T-bet in B cells is dispensable for Th1 type CSR to IgG2a driven by CD40 ligation plus IFN-γ (28, 44). Gerth et al. reported that CSR to IgG2a induced by soluble hapten-protein antigen was similar in T-bet−/− and in WT B cells (28). This response, as one would expect, only had low levels of CSR to IgG2a in the WT controls. Furthermore, the absence of T-bet from the T and B cells would be expected to reduce IFN-γ production and to lower responsiveness to IFN-γ (27, 45). Consequently the low level of CSR to IgG2a in the Gerth et al. study probably operated through an IFN-γ–independent mechanism. Type I IFNs, for example, have been reported to induce IgG2a in T-bet−/− B cells stimulated with LPS (26). The existence of such a Type I IFN-dependent induction of IgG2a in vivo has been confirmed recently (46). This mechanism is distinct from the high level of IFN-γ– and T-dependent CSR to IgG2a reported in this paper, where T-bet induction is likely to occur through the synergic activation of the B cells via their BCR and IFN-γ receptors. The latter is likely to be acting through phosphorylation of STAT-1, a direct regulator of the T-bet promoter (29, 33).
Our results show that IFN-γ–dependent CSR to IgG2b induced by the CD8 T cells does not require T-bet activity in B cells. To our knowledge, no target gene downstream of IFN-γR has been identified that induces CSR to IgG2b. TGF-β has been identified as a specific inducer of CSR to IgG2b (47, 48). Perhaps IFN-γ induces TGF-β production by another cell and so indirectly brings about CSR to IgG2b. IFN-γ-dependent CSR may act by redirecting Th2 cell differentiation into regulatory T cells (49, 50), for regulatory T cells are recognized producers of TGF-β. Against this possibility, our preliminary studies in mixed OTII plus OTI chimeras indicate neither the level of TGF-β nor Foxp3 mRNA increases in total LN cell suspensions during responses to alumOVA (Fig. S1B). Recent studies show that B cell deficiency of the transcription factor Ikaros (51) alters CSR induced by LPS in the presence of IL-4 from IgG1 to IgG2b and IgG2a. In addition, B cell lack of Gfi1 also suppresses CSR to IgG1 but only favors CSR to IgG2b (52). Whether IL-4 and/or IFN-γ signals in B cells regulate the activity of these DNA-binding regulatory factors remains to be tested.
The present report shows heterogeneity in the mechanisms of CSR induced by IFN-γ. Changes in the balance of the responses of antigen-specific B cells, CD4 T cells, and CD8 T cells can profoundly influence the outcome of the response to an alum-precipitated protein vaccine. Although aluminum adjuvants typically induce a Th2 response through Toll-like receptor (TLR) signaling-independent pathways (53–56), our data show that recruitment of antigen-specific CD8 T cells bypasses the need for TLR agonists to achieve B-cell CSR to the Ig isotypes associated with Th1 cells. This mechanism offers an option for modulating B-cell responses induced by vaccination protocols using alum adjuvant.
Material and Methods
Mice, Adoptive Transfer, Immunization, and IFN-γ Blockade.
WT CD45.2+ C57BL/6J mice were purchased from HO Harlan OLAC Ltd. OTII mice transgenic for αβTCR specific for 323–339 OVA-peptide in the context of H-2 I-Ab, and OTI mice transgenic for αβTCR specific for SIINFEKL OVA-peptide in the context of H-2Kb (Charles River) were crossed to CD45.1+ C57BL/6J congenic mice (Jackson Laboratory). B1.8hi mice were kindly supplied by Michel C. Nussenszweig (Rockefeller University, New York, NY); these mice carry a prerearranged VHDJH genes specific for the hapten 4-hydroxy-3-nitrophenyl acetyl (NP) when combined to Igλ light chain (57). CD45.1+ B1.8hi cells were obtained by crossing CD45.1+ mice deficient for Igκ light chain with B1.8hi mice. For adoptive transfer, CD4 T cells from LN of OTII mice were purified using anti-CD4 MACS microbeads; CD8 T cells from LN of OTI mice were purified using anti-CD8 MACS microbeads; and B cells from B1.8hi Igκ−/− mice were purified using anti-B220 MACS microbeads (Miltenyi Biotec). In some experiments, OTI or OTII cells were stained with CFSE (Cambridge Bioscience) before injection into recipient mice as described elsewhere (31). The different types of chimeras were constructed by injecting i.v. 2 × 106 purified CD45.1+OTI (unless specified otherwise), 2 × 106 purified CD45.1+OTII, and 106 purified CD45.1+B1.8hi cells into WT or T-bet−/− (Jackson Laboratory) congenic CD45.2+ recipient mice. The chimeras were immunized with alumOVA or, where stated, with alum-precipitated NP-conjugated ovalbumin (alumNPOVA), respectively prepared by mixing endotoxin-free OVA protein (Hyglos) or NP-conjugated ovalbumin (Biosearch Technologies) with a 9% aluminum potassium sulfate (Sigma-Aldrich) solution. Either 10 μg alumOVA or alumNP-OVA in a final volume of 10 μL in PBS was injected s.c. into both footpads. In vivo IFN-γ neutralization was achieved by giving two i.v. injections of 1 mg rat anti–IFN-γ antibody clone XGM1.2 (BIO X CELL): at the time of immunization and 3 d later. Control mice were similarly given Rat IgG1 clone HRPN antibody (BIO X CELL). All animals were maintained under standard animal house conditions following local and Home Office regulations.
Ex Vivo Restimulation of OTI and OTII Cells.
Day 7 after immunization, popliteal LN cells were incubated at 107 cells/mL in 24-well plates with 10 μM free SIINFEKL peptide or 323 to 339 OVA-peptide, respectively recognized by OTI cells and OTII cells (Alta Bioscience) for 5 h before cytokine detection. Intracellular FACS staining was performed using cytofix/cytoperm kit (Becton Dickinson). Anti–IFN-γ-PE (XMG1.2) (eBioscience), anti–IL-4-PE (11B11) (BD PharMingen).
Flow Cytometry Analysis and FACS Cell Sort.
LN single-cell suspensions prepared in RPMI medium containing 5% FCS and 0.15 mg/mL DNase I (Sigma-Aldrich) were incubated for 5min with 10 mM EDTA (Sigma-Aldrich). Cells were then treated for 15 min on ice with supernatant from 2.4G2 hybridoma culture and 5% normal mouse serum in FACS buffer (2 mM EDTA-PBS supplemented with 0.1% FCS). The antibodies used for surface and intracellular staining are listed in Table S1. Intracellular staining was performed using Cytofix/cytoperm kit (Becton Dickinson). IgG1[a] and IgG2a[a] allotypes for B1.8hi cells were detected with specific biotinylated antibodies (Table S1). Cells were sorted using a MoFlo cell sorter (Dako), and analysis by flow cytometry of LN cell suspensions or purity assessment of sorted cells was done using a FACScalibur (Becton Dickinson). Final analysis and graphical output were performed using FlowJo software (Treestar). The number of AFC per LN node was calculated by counting manually the total number of cells per LN and reporting this number to the percentage of AFC found in the LN.
Real-Time RT-PCR.
mRNA extraction and gene expression by real-time RT-PCR has been previously described (11). PCR were performed on ABI 7900 using TaqMan chemistry (Applied Biosystems). TaqMan probes and primers were designed by using Primer Express computer software (Applied Biosystems) and synthesized by Eurogenetec. Standard reaction conditions for the TaqMan PCR were used. Primers and probes are detailed in Table S2. Relative quantification of target gene mRNA was calculated by referring to the β-actin or β2-microglobulin mRNA levels, quantified in a duplex PCR. When not compatible in duplex, target and reference genes were measured in simplex real-time PCR run simultaneously for the same sample.
Statistical Analysis.
Differences between two groups were assessed by the two-tailed nonparametric Mann–Whitney test.
Supplementary Material
Acknowledgments
We are grateful for the support from the Biomedical Services Unit at University of Birmingham. This work was funded by a program grant from the British Medical Research Council (to I.C.M.M.), a Leverhulme Trust Emeritus Research Fellowship (to I.C.M.M.), a Wellcome Trust award (to K.S.), and Mathematical Modelling of In Vivo Cell Dynamics in Germinal Centers, a New and Emerging Science and Technology project from the European Union (K.-M.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004879107/-/DCSupplemental.
References
- 1.Serre K, et al. Molecular differences between the divergent responses of ovalbumin-specific CD4 T cells to alum-precipitated ovalbumin compared to ovalbumin expressed by Salmonella. Mol Immunol. 2008;45:3558–3566. doi: 10.1016/j.molimm.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham AF, et al. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol. 2004;34:2986–2995. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]
- 3.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T-cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 4.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: Major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 5.Mastroeni P, et al. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–483. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer JM. (How) do aluminium adjuvants work? Immunol Lett. 2006;102:10–15. doi: 10.1016/j.imlet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: The role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 9.Toellner KM, et al. T helper 1 (Th1) and Th2 characteristics start to develop during T-cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–2066. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 11.Mohr E, et al. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182:2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Sad S, Kägi D, Mosmann TR. CD8Tc1 and Tc2 cells secrete distinct cytokine patterns in vitro and in vivo but induce similar inflammatory reactions. J Immunol. 1997;158:4152–4161. [PubMed] [Google Scholar]
- 13.Seder RA, et al. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992;148:1652–1656. [PubMed] [Google Scholar]
- 14.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 15.Serre K, et al. IL-4 directs both CD4 and CD8 T cells to produce Th2 cytokines in vitro, but only CD4 T cells produce these cytokines in response to alum-precipitated protein in vivo. Mol Immunol. 2010;47:1914–1922. doi: 10.1016/j.molimm.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyahara N, et al. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 17.Coyle AJ, et al. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med. 1995;181:1229–1233. doi: 10.1084/jem.181.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggi E, et al. Th2-like CD8+ T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J Exp Med. 1994;180:489–495. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akdis M, et al. Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J Immunol. 1999;163:466–475. [PubMed] [Google Scholar]
- 20.Uzonna JE, Joyce KL, Scott P. Low dose Leishmania major promotes a transient T helper cell type 2 response that is down-regulated by interferon gamma-producing CD8+ T cells. J Exp Med. 2004;199:1559–1566. doi: 10.1084/jem.20040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedgwick JD, Holt PG. Induction of IgE-secreting cells and IgE isotype-specific suppressor T cells in the respiratory lymph nodes of rats in response to antigen inhalation. Cell Immunol. 1985;94:182–194. doi: 10.1016/0008-8749(85)90095-4. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Sanchez D, Noble A, Staynov DZ, Lee TH, Kemeny DM. Elimination of IgE regulatory rat CD8+ T cells in vivo differentially modulates interleukin-4 and interferon-gamma but not interleukin-2 production by splenic T cells. Immunology. 1993;78:513–519. [PMC free article] [PubMed] [Google Scholar]
- 23.McKee AS, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillon SB, et al. Induction of protective class I MHC-restricted CTL in mice by a recombinant influenza vaccine in aluminium hydroxide adjuvant. Vaccine. 1992;10:309–318. doi: 10.1016/0264-410x(92)90369-u. [DOI] [PubMed] [Google Scholar]
- 25.Garulli B, Stillitano MG, Barnaba V, Castrucci MR. Primary CD8+ T-cell response to soluble ovalbumin is improved by chloroquine treatment in vivo. Clin Vaccine Immunol. 2008;15:1497–1504. doi: 10.1128/CVI.00166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci USA. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravindran R, Foley J, Stoklasek T, Glimcher LH, McSorley SJ. Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J Immunol. 2005;175:4603–4610. doi: 10.4049/jimmunol.175.7.4603. [DOI] [PubMed] [Google Scholar]
- 28.Gerth AJ, Lin L, Peng SL. T-bet regulates T-independent IgG2a class switching. Int Immunol. 2003;15:937–944. doi: 10.1093/intimm/dxg093. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Zhang JJ. Stat1-dependent synergistic activation of T-bet for IgG2a production during early stage of B cell activation. J Immunol. 2005;175:7419–7424. doi: 10.4049/jimmunol.175.11.7419. [DOI] [PubMed] [Google Scholar]
- 30.Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 31.Serre K, et al. Early simultaneous production of intranodal CD4 Th2 effectors and recirculating rapidly responding central-memory-like CD4 T cells. Eur J Immunol. 2009;39:1573–1586. doi: 10.1002/eji.200838922. [DOI] [PubMed] [Google Scholar]
- 32.Finkelman FD, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 33.Lighvani AA, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-gamma and antigen-specific T-cell activation by dendritic cells. Proc Natl Acad Sci USA. 2003;100:7749–7754. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLennan IC. Germinal centers still hold secrets. Immunity. 2005;22:656–657. doi: 10.1016/j.immuni.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Beishuizen CR, et al. Chronic CD70-driven costimulation impairs IgG responses by instructing T cells to inhibit germinal center B cell formation through FasL-Fas interactions. J Immunol. 2009;183:6442–6451. doi: 10.4049/jimmunol.0901565. [DOI] [PubMed] [Google Scholar]
- 37.Smith KM, Brewer JM, Rush CM, Riley J, Garside P. In vivo generated Th1 cells can migrate to B cell follicles to support B cell responses. J Immunol. 2004;173:1640–1646. doi: 10.4049/jimmunol.173.3.1640. [DOI] [PubMed] [Google Scholar]
- 38.Peng SL, et al. Pathogenesis of autoimmunity in alphabeta T cell-deficient lupus-prone mice. Clin Exp Immunol. 1998;111:107–116. doi: 10.1046/j.1365-2249.1998.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 40.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: Multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 41.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 42.Jegerlehner A, et al. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 43.Peng SL, Li J, Lin L, Gerth A. The role of T-bet in B cells. Nat Immunol. 2003;4:1041. doi: 10.1038/ni1103-1041a. author reply 1041. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimoto T, et al. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173:2479–2485. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 45.Szabo SJ, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 46.Swanson CL, et al. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med. 2010;207:1485–1500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo GY, Park SR, Kim PH. Analyses of TGF-beta1-inducible Ig germ-line gamma2b promoter activity: Involvement of Smads and NF-kappaB. Eur J Immunol. 2009;39:1157–1166. doi: 10.1002/eji.200838732. [DOI] [PubMed] [Google Scholar]
- 48.McIntyre TM, et al. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J Exp Med. 1993;177:1031–1037. doi: 10.1084/jem.177.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, et al. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouaked N, et al. Regulation of the foxp3 gene by the Th1 cytokines: The role of IL-27-induced STAT1. J Immunol. 2009;182:1041–1049. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- 51.Sellars M, Reina-San-Martin B, Kastner P, Chan S. Ikaros controls isotype selection during immunoglobulin class switch recombination. J Exp Med. 2009;206:1073–1087. doi: 10.1084/jem.20082311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Igwe E, et al. The zinc finger protein Gfi1 is implicated in the regulation of IgG2b production and the expression of Igamma2b germline transcripts. Eur J Immunol. 2008;38:3004–3014. doi: 10.1002/eji.200838251. [DOI] [PubMed] [Google Scholar]
- 53.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piggott DA, et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun H, Pollock KG, Brewer JM. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine. 2003;21:849–855. doi: 10.1016/s0264-410x(02)00531-5. [DOI] [PubMed] [Google Scholar]
- 57.Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat Immunol. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.