Abstract
In T cell-mediated autoimmune diseases, self-reactive T cells with known antigen specificity appear to be particularly promising targets for antigen-specific induction of tolerance without compromising desired protective host immune responses. Several lines of evidence suggest that delivery of antigens to antigen-presenting dendritic cells (DCs) in the steady state (i.e., to immature DCs) may represent a suitable approach to induce antigen-specific T-cell tolerance peripherally. Here, we report that anti-DEC205–mediated delivery of the self-peptide proteolipid protein (PLP)139–151 to DCs ameliorated clinical symptoms in the PLP-induced SJL model of experimental autoimmune encephalomyelitis. Splenocytes from treated mice were anergized to PLP139–151, and IL-17 secretion was markedly reduced. Moreover, we show directly, using transgenic CD4+ Vβ6+ TCR T cells specific for PLP139–151, that, under the conditions of the present experiments, these cells also became anergic. In addition, evidence for a CD4+ T cell-mediated suppressor mechanism was obtained.
Keywords: DEC205, multiple sclerosis, anergy, monophosphoryl lipid A, T cells
Multiple sclerosis is a T cell-mediated autoimmune disease characterized by immune cell infiltration, inflammatory demyelination of neuronal axons, and axonal loss in the human central nervous system (1, 2). Studies of multiple sclerosis are facilitated by the animal model experimental autoimmune encephalomyelitis (EAE) that recapitulates many aspects of the human disease (3). Active induction of EAE is accomplished by stimulation of T cell-mediated immunity to myelin, the insulating phospholipid layer surrounding the neuronal axons, through immunization with myelin proteins or synthetic peptide antigens derived from myelin and then emulsified in adjuvant (4). This treatment leads to activation of autoreactive myelin-specific CD4+ T cells that circulate in the periphery of naïve animals. Activated autoreactive T cells will cross the blood–brain barrier (5). Within the central nervous system, local and infiltrating antigen-presenting cells, such as dendritic cells (DCs) derived from microglia, present MHC class II molecule-associated myelin peptides to infiltrating T cells in the context of costimulation. Myelin-specific CD4+ T cells are reactivated, initiating a cascade of neuroinflammatory responses that ultimately leads to demyelination in the central nervous system and neurodegeneration. EAE can also be passively induced by adoptive transfer of preactivated myelin-specific T cells (6).
Although T helper 1 (Th1) cells secreting IFN-γ were considered to be the primary mediators of EAE, T helper 17 (Th17) cells recently were shown to exhibit greater pathogenicity, suggesting that they play a more decisive role in mediating severe tissue damage (7, 8). However, both Th1 and Th17 cells, generated with kinetic differences and/or involved at different stages, may be involved in development of EAE (9). In fact, the relative contribution of both Th subsets was recently suggested to affect the anatomical location of lesion distribution between brain and spinal cord parenchyma (10).
Self-reactive T cells with known antigen specificity, which can be found in T cell-mediated autoimmune diseases such as multiple sclerosis, appear particularly promising targets for antigen-specific tolerance induction without compromising host immunity to infectious insults. Various protocols have been used to interfere with unwanted immunity using peptide-induced tolerance (11), including the administration of antigens over extended periods of time via osmotic minipumps (12, 13). In addition, peptide antigens can also be directly delivered to antigen-presenting cells via targeting approaches. In particular, antigens delivered to different subsets of DCs after fusion with antibodies to the endocytic receptors DEC205 (αDEC205) or 33D1 are efficiently processed and presented by MHC class I and class II molecules (14). This route of antigen delivery to murine (15) or human (16) DCs is several orders of magnitude more efficient than free peptides and in conjunction with maturation stimuli represents an effective method for inducing strong T-cell responses, i.e., vaccination. By contrast, targeting antigen to immature DCs in the steady state has been described as promoting immunological tolerance but through different mechanisms in different studies (15, 17–20). It may lead to deletion of antigen-specific T cells with residual cells becoming immunologically unresponsive, a mechanism that in one study increased CD5 expression on activated T cells (17). In addition, delivering minute amounts of peptides via αDEC205 fusion proteins to steady-state immature DCs can lead to the de novo generation of antigen-specific Foxp3+ Treg in vivo (18, 21).
Previous studies indicated that αDEC205-mediated targeting of an encephalogenic peptide of the myelin oligodendrocyte glycoprotein (MOG), a minor myelin component, to DCs in vivo prevents EAE induction by subsequent injection of the same peptide in complete Freund's adjuvant (CFA) in C57BL/6 mice (17). In this model, pretreatment with large doses of the free peptide in the absence of adjuvants also leads to protection from subsequent challenge. Here, we report experiments with αDEC205-mediated targeting of the autoantigen of the proteolipid protein peptide (PLP139–151) (derived from a major myelin constituent) in the EAE model in SJL mice, which is much more prone to disease and in which free peptide administration does not lead to protection. This model represents a second example in which targeting of DCs in the steady state with nanogram amounts of a peptide that generates autoimmunity efficiently ameliorates disease by promoting tolerance. In the present case, the amelioration of disease results both from induction of T-cell anergy and by generation of suppressor T cells.
Results
Dendritic Cell Targeting of Proteolipid Protein-Derived Peptide via αDEC205 Fusion Antibodies.
To target the encephalogenic antigen to DCs, recombinant proteins consisting of amino acids 139–151 of proteolipid protein (PLP139–151) fused either to the C terminus of the Ig heavy chain of cloned αDEC205 (αDEC205/PLP) or to the GL117 isotype control antibody (GL117/PLP) were produced. To confirm that the antigenic peptide delivered by the αDEC205 fusion antibody was properly processed and presented, purified splenic CD11c+ DCs from SJL mice were incubated for 3 h with various concentrations of either αDEC205/PLP or GL117/PLP control antibodies. After unbound antibodies were removed by extensive washing, DCs were cocultured with antigen-specific CD4+ Vβ6+ T cells from PLP139–151-specific Vβ6+ TCR transgenic mice (22, 23). 3H-thymidine incorporation at day 4 of the culture demonstrated that DCs preincubated with αDEC205/PLP fusion antibody induced vigorous proliferation of these transgenic T cells compared with GL117/PLP isotype control antibody or in the absence of a specific antigen (Fig. S1A).
In addition, a PLP139–151-specific T-cell line was established by immunizing SJL mice with PLP139–151 and restimulating splenocytes from the immunized mice with the same peptide three times at 2-wk intervals in vitro. The CD4+ T-cell line obtained exhibited an activated surface marker phenotype (CD25+, CD69+, CD45+, CD30+, GITR+, CTLA4+, CD71low, or CD62Llow) and secreted high amounts of IL-17 (10,300 pg/mL) along with IL-6 (1,300 pg/mL), IL-5 (772 pg/mL), GM-CSF (2,960 pg/mL), and TNF-α (278 pg/mL). Coculture of the PLP139–151 T-cell line with CD11c+ DCs preincubated with 1 μg of αDEC205/PLP fusion antibody substantially enhanced T-cell proliferation in a dose-dependent manner (Fig. S1B). In contrast, preincubation of DCs with either GL117/PLP isotype control antibody or αDEC205 antibody fused to an irrelevant antigen [peptide 107–119 of hemagglutinin (HA), αDEC205/HA] induced little proliferation. Proliferation was accompanied by an ∼10-fold increase in IFN-γ secretion only after treatment with αDEC205/PLP (Fig. S1C).
Immunization or Preimmunization with αDEC205/PLP Ameliorates EAE Induced by Either Adoptive Transfer of a PLP139–151-Specific T-Cell Line or by Immunization with PLP139–151.
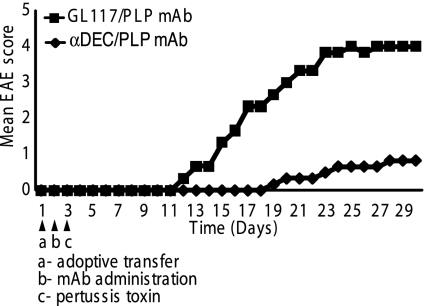
The PLP139–151-specific splenic T-cell line discussed above was adoptively transferred into naïve SJL mice to passively induce EAE, and then the mice were injected with either 1 μg of αDEC205/PLP or control GL117/PLP fusion antibodies, equivalent to ∼20 ng of PLP139–151. All recipients were injected with pertussis toxin (PT) the following day. As expected, mice that received PLP139–151-specific T cells and the GL117/PLP isotype control antibody rapidly developed severe EAE with a maximal mean score of 4 on day 28 of this experiment (Fig. 1). In contrast, mice that received PLP139–151-specific T cells followed by αDEC205/PLP exhibited a substantially delayed onset of disease with a low maximal mean score of 1 on day 28. Thus, αDEC205-mediated targeting of nanogram amounts of PLP139–151 efficiently interfered with the passive induction of EAE by adoptive transfer of highly encephalitogenic T cells with the same antigen specificity.
Fig. 1.
αDEC205/PLP ameliorates EAE induced by adoptive transfer of pathogenic PLP139–151-specific T cells. PLP139–151-specific T-cell lines were generated as described in Materials and Methodsand 5 × 106 cells were adoptively transferred into naïve SJL/J mice i.v. into the tail veins. One day later, mice were immunized i.p. with either 1 μg of αDEC205/PLP mAb (n = 5) or GL117/PLP mAb (n = 5), and then PT (200 ng) was injected i.v. on day 3. Mice were monitored for 30 d. αDEC205/PLP-treated mice were protected, whereas the GL117/PLP-treated mice developed severe disease (P < 0.02 at 30 d).
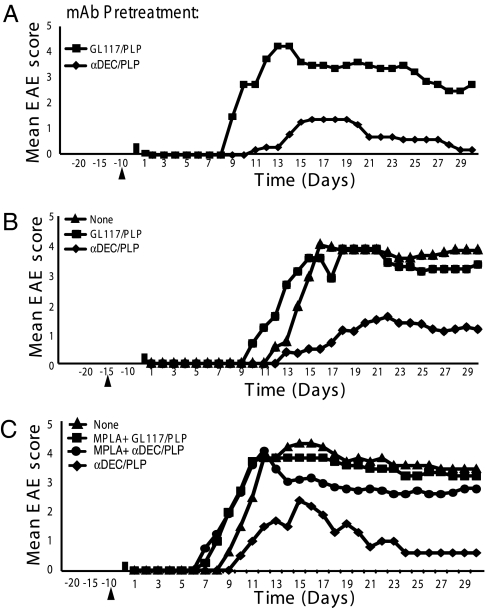
To determine whether preimmunization of SJL mice with αDEC205/PLP also ameliorated disease induced in mice immunized with unconjugated PLP139–151, SJL mice were either left untreated or treated with a single injection of 1 μg of αDEC205/PLP or GL117/PLP isotype control antibody at day minus 10 or minus 15. EAE was induced on day 0 by injection of 75 μg PLP139–151 in CFA followed by 200 ng PT (PLP139–151/CFA/PT) the next day, and mice were monitored daily for 30 d for clinical signs of EAE (Fig. 2 A–C). When EAE was induced in naïve SJL mice (i.e., without pretreatment), all of the mice developed clinical symptoms between days 9 and 10 and rapidly progressed to severe EAE with mean maximum scores of 3.8–4.4 by days 16–18 (Fig. 2 A and B). Similarly, pretreatment with the GL117/PLP control resulted in severe EAE with scores of 3.6–4.4 on days 16–18. Deaths of 40–60% of the mice occurred in these experiments. Thus, pretreatment with GL117/PLP control antibody did not result in an amelioration of disease progression and severity and was comparable to non-pretreated mice. Other control antibodies, αDEC205 itself (NLDC-145), and recombinant αDEC205/HA107-119 also had no significant effect on the disease course.
Fig. 2.
Effect of preadministration of αDEC205/PLP on disease course. (A and B) EAE was induced in SJL/J mice by immunizing with PLP 139–151 with or without preadministration of 1 μg of fusion mAbs (αDEC205/PLP mAb, αDEC205/HA mAb, αDEC205 mAb, or GL117/PLP mAb) in sterile PBS on either (A) day minus 10 or (B) day minus 15. Mice were then immunized with 75 μg of PLP139–151 in CFA s.c. on day 0 followed by PT (200 ng) i.v. on day 1. Appearance of clinical signs of EAE was monitored daily, and disease severity was scored as described in Materials and Methods. Mean EAE scores for 5–10 mice in each group are shown. The majority of mice in groups immunized with PLP139–151 that had received αDEC205 mAb, αDEC205/HA mAb, or GL117/PLP mAb were dead by day 12 whereas disease was ameliorated in those that received αDEC205/PLP. (A) On day 15, αDEC205/PLP mAb (n = 5) vs. GL117/PLP mAb (n = 5) (P < 0.01). (B) On day 15, αDEC205/PLP mAb (n = 13) vs. GL117/PLP mAb (n = 9) (P < 0.001). All scoring was performed double blind. The data shown are representative of three to six separate experiments. (C) Effect of preimmunization with fusion mAbs together with MPLA. MPLA (10 μg) was administered together with either αDEC205/PLP mAb (n = 8) or GL117/PLP (n = 5) i.p. 10 d before induction of EAE with PLP139–151 in CFA s.c. and PT (200 ng) i.v. as above. Mice that received MPLA + αDEC205/PLP mAb were not significantly different from controls (P > 0.05). A representative of two independent experiments is shown. All scoring was performed double blind.
By contrast, mice pretreated with 1 μg αDEC205/PLP showed consistently delayed onset of disease by up to 5 d, with maximal scores of 1.4–1.7 on days 16–23 (Fig. 2 A and C) (only two mortalities were observed). This reduction was seen when αDEC205/PLP was administered 10 or 15 d before induction of EAE (Fig. 2 A and B) but in one experiment appeared less effective when administered at day 20. Thus, the treatment prevented disease when administered 23 d before disease onset in controls. However, administration of αDEC205/PLP at the same time as immunization with PLP139–151/CFA/PT did not interfere with onset or severity of EAE, possibly due to the rapid conversion of immature to mature DCs by immunization. Moreover, coadministration at day 10 of 1 μg αDEC205/PLP with 10 μg monophosphoryl lipid A (MPLA), a low-toxicity derivative of LPS with potent proinflammatory activity that leads to DC maturation and activation (24), completely abrogated the beneficial effect of αDEC205/PLP alone on PLP139–151/CFA/PT-induced EAE (Fig. 2C).
Effect of αDEC205-Mediated Targeting on Pathogenic IL-17–Producing T Cells.
To determine whether αDEC205/PLP-mediated targeting interfered with early antigen-specific T-cell induction, SJL mice were either left untreated or treated with a single injection of 1 μg of αDEC205/PLP or GL117/PLP control mAb 10 d before immunization with PLP139–151/CFA/PT. Total splenocytes that contained both antigen-presenting cells and T cells isolated from mice at day 17, either without pretreatment or pretreated with GL117/PLP control antibody, proliferated vigorously to various PLP139–151 concentrations in vitro, whereas little proliferation was seen after pretreatment with αDEC205/PLP even in response to nonphysiologically high peptide concentrations (Fig. 3A). Thus, αDEC205 targeting in vivo reduced either the numbers of antigen-specific T cells or their proliferative capacity tested in vitro.
Fig. 3.
Effect of αDEC205/PLP on splenocyte proliferation and number of IL-17–producing cells. (A) SJL/J mice were preimmunized with 1 μg of different fusion antibodies. After 10 d, mice were immunized with PLP139–151 followed by i.v. PT as described in Fig. 1. Seventeen days after disease induction, splenocytes were removed and challenged with a titration of PLP139–151. On day 4 of the proliferation assay, cells were pulsed with 3[H]-thymidine; 16 h later, proliferative response was measured as cpm. (B) IL-17 ELISPOT analysis of mouse splenocytes isolated on day 17. Splenocytes were plated onto precoated plates as described in protocols from eBioscience’s IL-17 ELISPOT kit and stimulated with 10 μg/mL PLP139–151. Unstimulated wells were used as controls. A representative of two independent experiments is shown. (C) Quantification of IL-17 ELISPOT. Statistics: none vs. αDEC205/PLP mAb (P < 0.02); αDEC205/PLP mAb vs. GL117/PLP mAb (P < 0.006). Spots per million were calculated by multiplying the average of triplicate wells (2 × 105 cells) by 5-fold.
To address this question in more detail, the number of pathogenic IL-17–secreting cells in splenocytes from SJL mice that were either left untreated or pretreated with a single injection of 1 μg of recombinant αDEC205/PLP, GL117/PLP control, or irrelevant αDEC205/HA fusion mAb followed by PLP139–151/CFA/PT immunization 10 d later, was determined. ELISPOT analysis at day 17 using total splenocytes and overnight restimulation with varying concentrations of PLP139–151 in vitro showed that αDEC205/PLP resulted in an ∼2- to 3-fold reduction in the number of cells secreting IL-17 compared with mice that were not pretreated (P < 0.02) or were pretreated with αDEC205/HA (P < 0.03) (Fig. 3 B and C). Pretreatment with GL117/PLP seemed to increase the number of IL-17 secreting cells in the spleen (P < 0.004).
CD4+ T Cells from αDEC205/PLP-Pretreated Mice Control EAE Induction After Adoptive Transfer.
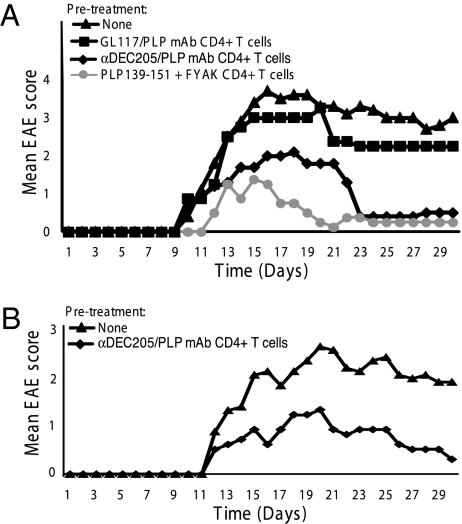
Did αDEC205/PLP-mediated targeting also result in induction of regulatory T cells (Treg)? To address the question, SJL mice were either untreated or pretreated with either 1 μg αDEC205/PLP or GL117/PLP (Fig. 4A). In one of the experiments, as a positive control, an additional group of SJL mice was coimmunized with 500 μg of the synthetic amino acid copolymer poly(F,Y,A,K)n, which has previously been shown to ameliorate PLP139–151-induced EAE by the generation of IL-10–secreting Tr1-like Tregs (25, 26). Disease was induced 10 d later by PLP139–151/CFA/PT administration. After an additional 10 d, splenic CD4+ T cells from all four groups were purified using magnetic beads, and 5 × 106 cells were i.v. transferred into naïve SJL mice. EAE was induced in recipients the following day by PLP139–151/CFA/PT immunization. Recipients adoptively transferred with 5 × 106 CD4+ T cells from mice without pretreatment or pretreated with GL117/PLP developed severe EAE with mean maximum scores of 3.2–3.6 on days 16–18 (Fig. 4). As expected, adoptive transfer of CD4+ T cells from poly(F,Y,A,K)n pretreated mice efficiently prevented EAE induction in recipient SJL mice. Similarly, CD4+ T cells from αDEC205/PLP-treated mice also significantly ameliorated EAE with a mean maximum score of 2.0 on days 16–18 (P = 0.003 compared with the control groups). Strikingly, symptoms ameliorated in the treated groups (but not in the untreated groups) so that, from day 23 onward, basically no signs of EAE were detectable (Fig. 4). Thus, the generation of regulatory CD4+ T cells also played a role in amelioration of EAE after administration of αDEC205/PLP.
Fig. 4.
Adoptive transfer (ATx) of CD4+ T cells from anti-DEC205/PLP139–151 mAb preimmunized mice ameliorates induction of PLP139–151-induced EAE. Two independent experiments are presented (A and B). (A) The 5 × 106 CD4 + T cells enriched from splenocytes from SJL mice preimmunized on day 10 i.p. with a 1 μg fusion mAbs (αDEC205 /PLP mAb or GL117/PLP mAb) were adoptively transferred i.v. into the tail veins, followed by immunization on day 1 with 75 μg of PLP139–151 in CFA and PT i.v. the following day. Controls received PBS injections. Mean disease scores of five mice/group are shown. At days 20–21, αDEC205 /PLP mAb ATx vs. GL117/PLP mAb ATx were compared (P < 0.02). (B) An additional identical experiment is shown in which SJL mice were preimmunized at day 10 i.p. with 1 μg of αDEC205/PLP mAb only. Mice were monitored for clinical signs of EAE for 30 d. All scoring was performed double blind.
Effects of αDEC205/PLP on Pathogenic Vβ6+ TCR Transgenic T Cells.
Splenocytes and lymph node cells from Vβ6+ TCR CD4+ T cells recognizing PLP139–151 obtained from 5B6 transgenic B10.S mice (22, 23) were adoptively transferred into rag−/− B10.S(I-As) mice. Mice were treated with 1 μg of either αDEC205/PLP or GL117/PLP. Splenocytes and lymph nodes were harvested 10 d later, and CD4+ T cells were separated using anti-CD4 magnetic beads. Cells from the mice that had been injected with αDEC205/PLP exhibited limited proliferation and reduced IL-17 production but unchanged IFN-γ production in response to in vitro restimulation, in comparison with PLP139–151-specific CD4+ T cells from GL117/PLP-treated recipients (P < 0.006) (Fig. 5 A–C). Thus, αDEC205/PLP targeting in vivo contributed to amelioration of EAE by reducing the number of antigen-specific pathogenic IL-17–producing T cells and their proliferative capacity in vitro. In addition, Foxp3+ cells in the CD4+ T cell populations were enumerated by FACS (Fig. 5 D). The percentage of Foxp3+ cells among CD4+ cells in αDEC205/PLP and GL117/PLP pretreated mice was 15% in each case under these conditions. Anti-DEC205/PLP did not result in detectable conversion of CD4+ Foxp3− T cells to Foxp3+ cells. The percentage of these cells in normal B10.S mice that have been shown to express a high level of CD4+ CD25+ Tregs (27) averaged 6.1%, and in B10.S mice bearing the Vβ6 TCR transgene, it averaged 8.3%. Thus, homeostatic expansion of Foxp3+ CD4+ T cells (28, 29) in the rag−/− background likely accounts for the increased numbers found in both αDEC205/PLP- and αGL117/PLP-treated mice. A smaller specific conversion to Foxp3+ CD4+ T cells induced by αDEC205/PLP treatment (18) would not have been detected. CD5 was found to be expressed in a previous study of αDEC205/MOG35–55 treatment in an EAE model in C57BL/6 mice (17). However, no CD5 was expressed on the isolated anergized Vβ6+ CD4+ T cells from αDEC205/PLP-treated mice shown here.
Fig. 5.
Effect of αDEC205/PLP on adoptively transferred CD4+ Vβ6+ TCR 5B6 transgenic (tg) T cells. Splenocytes were isolated from B10.S mice that carry a transgenic TCR 5B6 recognizing PLP139–151 presented on I-As. Splenocytes were enriched for Vβ6+ CD4+ tg T cells using Miltenyi CD4-positive selection kits (purity ∼89%). (A and B) The 10 × 106 T cells were injected i.v. into naïve B10.S rag−/− mice along with 1 μg i.p. of fusion antibodies (αDEC205/PLP mAb or GL117/PLP mAb). Splenocytes (A) and axillary lymph nodes (B) were removed 10 d later. Single cell suspensions were stimulated with PLP139–151 for 4 d, and 3H-thymidine incorporation was measured. The αDEC205/PLP mAb-treated Vβ6+ CD4+ tg T cells did not proliferate in response to PLP139–151 peptide, whereas Vβ6+ CD4+ tg T cells treated with GL117/PLP mAb proliferated (P < 0.03). (C) Vβ6+ TCR 5B6 tg CD4+ T cells were stimulated by cross-linking using plate-bound CD3 and CD28 mAb coated overnight to detect cytokine production. Supernatants from the proliferation assay were removed 3 d after stimulation, and cytokines were measured by Luminex assay as described in Materials and Methods. IL-17 was significantly reduced upon administration of 1 μg of αDEC205/PLP mAb compared with a control group treated with 1 μg of GL117/PLP mAb (P < 0.005). (D) Splenocytes used were obtained in A. FACS analysis of gated CD4+ cells stained for intracellular Foxp3 was carried out using CD4-FITC and Foxp3-PE.
Discussion
Lack or loss of tolerance to several self-molecules that have been identified as target antigens in autoimmune diseases is one of the key events promoting autoimmunity such as multiple sclerosis or type I diabetes. Despite many studies in both rodents and humans to stimulate tolerogenic mechanisms using various protocols of antigen administration with antigens in different pharmaceutical forms (e.g., peptides or whole antigens) and testing diverse administration routes, robust data demonstrating clinical benefits are not yet available (18). Recent studies in mice have also indicated that repeated administration of free antigens can induce fatal autoimmune responses (30). In this context, the ability to target minute amounts of antigens to steady-state immature DCs in vivo has promise as an approach to obtain antigen-specific immunological tolerance.
In earlier studies of immunological tolerance induced by targeting of peptides to immature DCs by fusion to αDEC205, several different mechanisms have been reported. In earlier studies using an artificial system in which HA was the target antigen, the induction of immunological unresponsiveness by deletion of autoreactive T cells or by anergization was emphasized (15, 17, 20). Later studies, however, focused on the generation of regulatory T cells as an important mechanism in induction of antigen-specific tolerance (18, 21). In the only previous study using a known autoantigen, MOG35–55-induced EAE in C57BL/6 mice was ameliorated by pretreatment at day minus 7 with αDEC205/MOG35–55 (17). In the present experiment, αDEC205/PLP139–151 fusion mAb was synthesized and used to prevent EAE in the model in which disease is induced by PLP139–151 in SJL mice. Anti-DEC205–mediated targeting of low nanogram amounts of the immunodominant PLP139–151 efficiently ameliorated EAE induced either by immunization with PLP139–151 or by adoptive transfer of PLP139–151-specific T cells (Fig. 2). It is important to note that, in the PLP139–151-induced EAE model in SJL mice, pretreatment with large doses of free peptide in the absence of adjuvants does not lead to protection from disease induced by subsequent challenge with peptide/CFA/PT, in contrast to the MOG35–55-induced EAE model in C57BL/6 mice (17). Thus, the fact that αDEC205 targeting is several magnitudes more efficient in inducing T-cell responses compared with free peptide administration does not explain the tolerogenic effect of small amounts of αDEC205/PLP fusion antibodies in the PLP-induced EAE model.
In an attempt to define the mechanism of PLP139–151-induced tolerance, we showed that αDEC205-mediated targeting interfered with early antigen-specific T-cell induction in peripheral lymphoid organs upon active EAE induction, reflected by reduced numbers of pathogenic antigen-specific IL-17–producing T cells (Fig. 3). In addition, and consistent with previous reports (15), the remaining cells exhibited an anergic phenotype upon restimulation in vitro. It is likely that both deletion and induction of an anergic phenotype in pathogenic T cells contributed to αDEC205/PLP-mediated amelioration of EAE.
In addition, however, adoptively transferred CD4+ T cells from αDEC205/PLP-treated mice efficiently prevented EAE induction in recipients (Fig. 4 A and B). These data point toward an additional dominant T-cell suppressive mechanism of immunological tolerance promoted by αDEC205/PLP-mediated targeting. However, this experiment does not make clear to what extent de novo generation or expansion of preexisting Foxp3− expressing CD4+ Tregs or IL-10 secreting T cells, or conversion of pathogenic CD4+ Foxp3− cells mediated by αDEC205/PLP, contributes to disease amelioration.
To approach the latter possibility, pathogenic CD4+ Vβ6+ T cells were adoptively transferred to B10.S rag−/− mice. After treatment with αDEC205/PLP, splenocytes or lymph node cells were markedly anergic to PLP139–151 and had severely reduced IL-17 production but little or no change in IFNγ secretion. This experiment may reinforce the relative importance of IL-17 in the pathogenesis of EAE in this model system (31). A high level of Foxp3+ CD4+ Vβ6+ T cells was seen after treatment with control GL117 mAb, and no further increase was found after treatment with αDEC205/PLP. Thus, no evidence of specific conversion could be detected under the conditions of the present experiment.
These experiments demonstrate that αDEC205/PLP139–151 ameliorates EAE induction mainly by inducing anergy in PLP139–151-specific T cells. In addition, evidence of T-cell suppression was obtained, although induction of neither IL-10 secretion nor Foxp3+ T cells was seen. In a previous study (17), MOG35–55 induced EAE was ameliorated by αDEC205/MOG35-55. In addition to these two autoantigens, MBP85–99 has also been shown to induce EAE, and all have been shown to be potentially important in multiple sclerosis (32, 33). Conceivably, a combination of these three αDEC205 fusion proteins could represent a therapeutic modality for this disease.
Materials and Methods
Mice.
Six- to 12-wk-old female SJL/J (H-2s) mice were purchased from the Jackson Laboratory. Vβ6+ PLP139–151-specific 5B6 TCR transgenic mice on the rag−/− B10.S (B10/I-As) background along with nontransgenic rag−/− B10.S mice were previously described (22). All animals were maintained at the animal facilities of Harvard University according to the animal protocol guidelines of Harvard University.
Recombinant Fusion Antibody Production.
Double-stranded DNA fragments coding for PLP139–151 with spacer residues on both sides were constructed using synthetic oligonucleotides as described previously (34) using the following oligonucleotides: PLP-1 forward, 5′-cta gcg aca tgg cca aga agg aga cag tct gga ggc tcg agg agt tcg gta ggt tca caa aca ggC AT; PLP-1 reverse, 5′-CAG GC Tat gcc tgt ttg tga acc tac cga act cct cga gcc tcc aga ctg tct cct tct tgg cca tgt cg; PLP-2 forward, 5′- AGC CTG GGC AAA TGG CTG GGC CAT CCG GAT AAA TTT tat tat gac ggt agg aca tga tag gc; PLP-2 reverse, 5′-ggc cgc cta tca tgt cct acc gtc ata ata AAA TTT ATC CGG ATG GCC CAG CCA TTT GCC (the PLP139–151 peptide-encoding nucleotide sequence split between the two sets of oligonucleotides is shown in uppercase letters). DNA fragments were added in-frame to the C terminus of the heavy chains of cloned NLDC-145 (αDEC205) and III/10 isotype control constant regions. To ensure the specificity of antigen targeting the rat IgG2a, constant regions of the original NLDC-145 and isotype control antibodies were replaced with mouse IgG1 constant regions, which carry point mutations interfering with Fc receptor binding (35). The plasmid vectors of the IgH chain cDNA of the cloned NLDC-145 (pDEC-IgH) and GL117 (GL117/10-IgH) and their respective IgL-k light chain cDNA (pDEC-IgL-k and pGL117/10-IgL-k) were kindly provided by M. C. Nussenzweig (The Rockefeller University, New York, NY). The plasmid vectors containing the cDNA of amino acids 107–119 of HA (HA107-119) added to the C terminus of cloned αDEC205 and III/10 control antibody have been described previously (18). Hybrid antibodies were produced using the FreeStyle MAX 293 expression system (Invitrogen) according to the manufacturer's recommendations. In brief, suspension cultures of FreeStyle 293-F cells were maintained in serum-free FreeStyle 293 expression medium and transiently transfected with plasmid vectors of the respective IgH chain and Igk chain cDNA using FreeStyle MAX reagent. The original anti-DEC205 antibody NLDC-145 (without peptide tag), which was included in some experiments as a control, was produced by hybridoma cells in serum-free Hybridoma medium (Invitrogen). All antibodies were purified on prepacked HiTrapTM Protein G HP columns (Amersham Biosciences). Protein concentrations were determined spectophotometrically by measuring the absorbance at 280 nm. The amount and the presence of full-length recombinant fusion protein were verified by SDS/PAGE with an IgG1/IgLκ antibody as a reference.
Effect of Fusion Antibodies on the Induction of EAE.
For preimmunization, SJL/J mice were immunized with 1 μg i.p. of fusion antibodies (αDEC205/PLP mAb, αDEC205/HA mAb, αDEC205 mAb alone, or GL117/PLP mAb) either 10 or 15 d before inducing EAE. Six- to 10-wk-old female mice were immunized s.c. with 75 μg of PLP139–151 emulsified in CFA; 200 ng PT (List Biological Laboratories) was given i.v. on the day after immunization. The mice were monitored for clinical signs of EAE, and they were scored from 0 to 5: 1, limp tail; 2, hind limb paralysis; 3, complete hind limb paralysis; 4, four limbs paralyzed; 5, moribund. All scoring was performed double blind.
Details of the proliferation assay, cytokine measurements, and adoptive transfer experiments are included in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Nussenzweig (The Rockefeller University, New York) for providing the plasmid vectors of the IgH and respective Igκ light chain cDNA of cloned anti-DEC205 NLDC-145 and III/10 isotype control recombinant antibodies and T. Koenig (Kretschmer Laboratory) for excellent technical assistance in recombinant antibody production. This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI049524) and from the National Multiple Sclerosis Society (RG 3796A3/1).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010263107/-/DCSupplemental.
References
- 1.Hafler DA, Weiner HL. MS: a CNS and systemic autoimmune disease. Immunol Today. 1989;10:104–107. doi: 10.1016/0167-5699(89)90236-3. [DOI] [PubMed] [Google Scholar]
- 2.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 3.Wekerle H. Experimental autoimmune encephalomyelitis as a model of immune-mediated CNS disease. Curr Opin Neurobiol. 1993;3:779–784. doi: 10.1016/0959-4388(93)90153-p. [DOI] [PubMed] [Google Scholar]
- 4.Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- 5.Risau W, Engelhardt B, Wekerle H. Immune function of the blood-brain barrier: Incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol. 1990;110:1757–1766. doi: 10.1083/jcb.110.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 9.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 10.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 12.Verginis P, McLaughlin KA, Wucherpfennig KW, von Boehmer H, Apostolou I. Induction of antigen-specific regulatory T cells in wild-type mice: Visualization and targets of suppression. Proc Natl Acad Sci USA. 2008;105:3479–3484. doi: 10.1073/pnas.0800149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 15.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozzacco L, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 19.Mukhopadhaya A, et al. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci USA. 2008;105:6374–6379. doi: 10.1073/pnas.0802644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruder D, et al. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–3401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki S, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldner H, Collins M, Kuchroo VK. Activation of antigen-presenting cells by microbial products breaks self tolerance and induces autoimmune disease. J Clin Invest. 2004;113:990–997. doi: 10.1172/JCI19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchroo VK, et al. T cell response in experimental autoimmune encephalomyelitis (EAE): Role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- 24.Mata-Haro V, et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 25.Stern JN, et al. Amino acid copolymer-specific IL-10-secreting regulatory T cells that ameliorate autoimmune diseases in mice. Proc Natl Acad Sci USA. 2008;105:5172–5176. doi: 10.1073/pnas.0712131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern JN, et al. Amelioration of proteolipid protein 139-151-induced encephalomyelitis in SJL mice by modified amino acid copolymers and their mechanisms. Proc Natl Acad Sci USA. 2004;101:11743–11748. doi: 10.1073/pnas.0403832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy J, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 29.Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor-specified peripheral niche constraints. J Exp Med. 2010 doi: 10.1084/jem.20100205. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugliese A, et al. Self-antigen-presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. J Clin Invest. 2001;107:555–564. doi: 10.1172/JCI10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:3973–3984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettelli E, Baeten D, Jäger A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretschmer K, Heng TS, von Boehmer H. De novo production of antigen-specific suppressor cells in vivo. Nat Protoc. 2006;1:653–661. doi: 10.1038/nprot.2006.105. [DOI] [PubMed] [Google Scholar]
- 35.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.