Abstract
The pressing need for broad-spectrum antivirals could be met by targeting host rather than viral processes. Cholesterol biosynthesis within the infected cell is one promising target for a large number of viral systems, including hepatitis C virus (HCV), hepatitis B virus (HBV) and HIV. Liposomes developed for intracellular, endoplasmic reticulum (ER)-targeted in vivo drug delivery have been modified to include polyunsaturated fatty acids that exert an independent antiviral activity through the reduction of cellular cholesterol. These polyunsaturated ER liposomes (PERLs) have greater activity than lovastatin (Mevacor, Altoprev), which is clinically approved for lowering cholesterol and preventing cardiovascular disease. Treatment of HCV, HBV, and HIV infections with PERLs significantly decreased viral secretion and infectivity, and pretreatment of naïve cells reduced the ability of both HCV and HIV to establish infections because of the decreased levels of plasma membrane cholesterol. Direct competition for cellular receptors was an added effect of PERLs against HCV infections. The greatest antiviral activity in all three systems was the inhibition of viral infectivity through the reduction of virus-associated cholesterol. Our study demonstrates that PERLs are a broadly effective antiviral therapy and should be developed further in combination with encapsulated drug mixtures for enhanced in vivo efficacy.
Keywords: antivirals, endoplasmic reticulum targeting, liposome
A number of viruses depend on cholesterol to maintain a certain level of fitness. Thus, drugs that target this process should be useful in treating a broad variety of viral infections. Here we focus on three important human pathogens—hepatitis C virus (HCV), HIV, and hepatitis B virus (HBV)—which together exact a heavy toll on public health. All three viral infections require associations with cholesterol during at least one stage of their life cycle. Most of the viral life cycle of HCV is closely associated with lipid and cholesterol metabolism in host cells; this association includes entry into naïve cells (1), RNA replication (2), viral assembly (3), and infectivity (4). HIV relies heavily on lipid rafts for entry, assembly/secretion, and infectivity (5). The HBV viral envelope requires cholesterol for proper infection of naïve cells (6), and more recently a dependence on caveolin-1 (located within plasma membrane caveolae) for cellular entry has been established (7).
The clinical use of cholesterol-lowering statins [inhibitors of 3-hydroxy-3-methyl-glutaryl (HMG) CoA reductase] to treat viral infections is limited and has been tested against HCV with little to no success (8, 9). One study including HIV/HCV-coinfected patients even found an increase in HCV titers following in vivo treatment (10), probably because of increased expression of cell-surface receptors [(i.e., LDL receptors (LDLr)], a direct effect of this type of cholesterol inhibition.
Liposomes capable of entering cells for endoplasmic reticulum (ER)-targeted drug delivery have been developed and are superior to other liposome-based systems for the delivery of both hydrophilic and hydrophobic cargo (11). Here we use polyunsaturated ER-targeting liposomes (PERLs) in the absence of encapsulated drugs to treat HCV-, HIV-, and HBV-infected target cells and demonstrate a resulting decrease in cholesterol levels within both infected cells and secreted virions for all three viral systems. Lowering cholesterol levels in this manner leads to significant antiviral activity in all three systems and suggests that PERLs may be useful as an in vivo therapy to treat a broad range of cholesterol-dependent viral infections and coinfections, either as monotherapy or in encapsulated drug mixtures.
Results
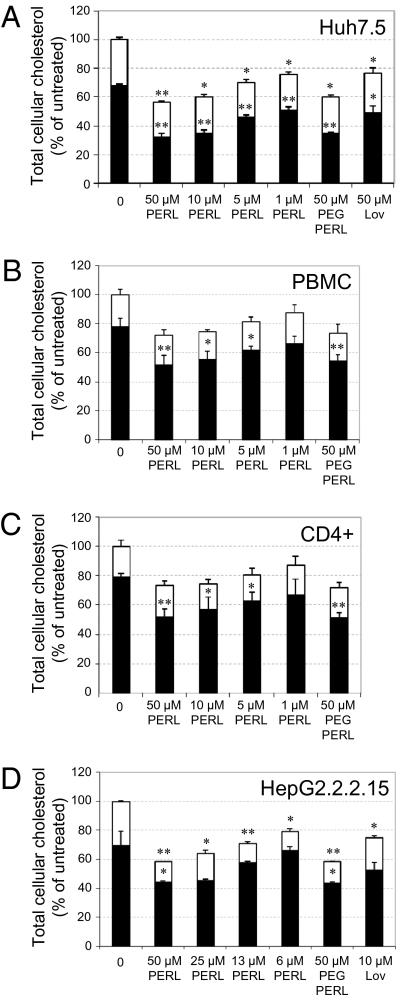
The toxicity of PERLs against cells used for propagation of viral infections was measured, and the highest concentration associated with minimal toxicity was 50 μM in medium (Fig. S1). At this concentration Huh7.5 cells used for propagation of HCV in cell culture (HCVcc) demonstrated mean decreases in both free and esterified cholesterol of 53% (SD 2.7) (P < 0.001) and 25% (SD 1.1) (P < 0.001), respectively (Fig. 1A). When treated with 50 μM PERLs, peripheral blood mononuclear cells (PBMCs) used for HIV assays had a mean decrease of 33% (SD 6.3) (P < 0.001) in free cholesterol (Fig. 1B). Similar activity was observed for CD4+ T cells (Fig. 1C), the specific subset of PBMCs infected by HIV. HepG2.2.2.15 cells required for HBV propagation and secretion assays showed mean decreases in both free and esterified cholesterol levels of 36% (SD 0.8) (P = 0.05) and 54% (SD 0.05) (P < 0.001), respectively (Fig. 1D). Reduction in the levels of cellular cholesterol could be a result of sphingomyelinase activation in the presence of increased levels of unsaturated fatty acids within the cells, as suggested by the dose-dependent increase in enzymatic activity observed in all cell lines following treatment (Fig. S2). Lovastatin at nontoxic concentrations was used as a control in both Huh7.5 and HepG2.2.2.15 cells to compare its cholesterol-lowering activity with that of PERLs and was found to be inferior in both cell lines, with no significant effect on sphingomyelinase activity. Lovastatin could not be used in PBMCs because the concentrations necessary for cholesterol inhibition are toxic. A PEGylated version of PERLs [including a PEG-phosphoethanolamine (PE) lipid at a molar concentration of 3%], which increases in vivo stability, demonstrated activity similar to that of the non-PEGylated version. Although there is no direct evidence that the ER-targeting capabili-ties of PERLs are necessary for cholesterol inhibition, treatment of cells with unsaturated pH-sensitive liposomes, used for the cytosolic delivery of encapsulated cargo, did not have a similar outcome (Fig. S3). Therefore, treatment with PERLs leads to significant decreases in free or esterified cellular cholesterol levels, or both, depending on the cell lines, and is more effective than lovastatin, which currently is in clinical use.
Fig. 1.
PERL-treated cells decreased cholesterol levels following a 4-d treatment period. Free (black) and esterified (white) cholesterol levels were quantified by kinetic spectrofluorometric assays on cells, and results were normalized to total protein levels within the same samples. Cells assayed include (A) Huh7.5 cells, (B) PBMCs, (C) CD4+ cells purified from pooled PBMCs, and (D) HepG2.2.2.15 cells. PEGylated (PEG) PERLs are PERL liposomes in which 3% of the lipids have been replaced with a PEG-PE 2000 lipid for increased in vivo stability. Lovastatin (Lov) was used as a control for both Huh7.5 and HepG2.2.2.15 cells. Data represent the mean and SD three independent experiments performed in triplicate and are presented as a percentage of the untreated samples. Significant differences from this value are denoted by *P < 0.05 and **P < 0.001 (t test).
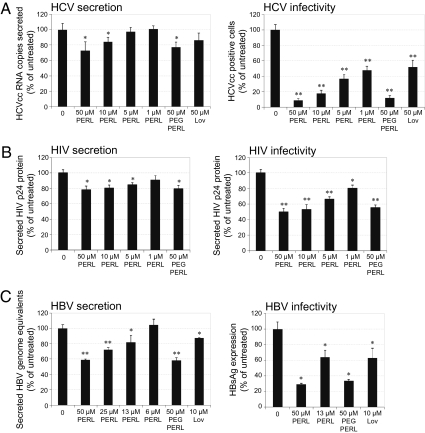
Single-round secretion and infectivity assays were performed with HCV-, HIV-, and HBV-infected cells. In all three viral systems, treatment with 50 μM PERLs led to significant decreases in both secretion and infectivity. Mean HCV secretion and infectivity were decreased by 27% (SD 11.3) (P = 0.01) and 91% (SD 2.2) (P < 0.001), respectively (Fig. 2A). Similar treatment with lovastatin led to no significant inhibition in HCV secretion and a mean decrease in infectivity of 48% (SD 8.7) (P < 0.001). ER liposomes were modified to include both 20:4 and 18:1 phospholipids and were used in single-round HCV secretion and infectivity assays. Contrary to previous findings (2), both 20:4 and 18:1 phospholipids led to increased HCV secretion, although viral infectivity still was decreased significantly with both formulations (Fig. S4). Of the compositions tested, 22:6 PERLs were the best for treating HCV infections. When 22:6 PERLs were used to treat HIV-infected PBMCs, mean viral secretion was suppressed by 22% (SD 4.6) (P = 0.004), and mean viral infectivity was decreased by 50% (SD 4.6) (P < 0.001) (Fig. 2B). Treatment of HBV-infected HepG2.2.2.15 cells led to decreases in both mean secretion and mean infectivity of 41% (SD 1.3) (P = 0.001) and 71% (SD 1.3) (P = 0.002), respectively (Fig. 2C). In all three viral systems PERL treatment had no significant effect of on intracellular viral replication and protein expression (Fig. S5 A–F); therefore any decrease in viral secretion probably results from the inhibition of particle assembly and/or budding from cells.
Fig. 2.
PERL treatment significantly decreases the secretion and infectivity of HCV, HBV, and HIV virus particles. (A) Huh7.5 cells were infected with Jc1 HCVcc at a MOI of 0.5 and were treated for 4 d. Secretion of HCVcc particles after the treatment period was quantified by real-time PCR of RNA within cell-culture supernatant, and infectivity of secreted particles was determined by reinfecting naïve Huh7.5 cells with cell supernatant normalized to RNA levels and measuring the level of infection 2 d later by HCV core protein immunofluorescence. (B) PBMCs were infected with HIV-1 primary isolate, LAI, at TCID50 = 100. Infected cells were treated for 4 d, at which time secreted HIV particles were quantified in the cell-culture supernatant by capture p24 ELISA. Infectivity was determined following reinfection of naïve PBMCs with normalized cell supernatant and measuring HIV secretion after a 3-d incubation period by capture p24 ELISA. (C) HepG2.2.2.15 cells stably transfected with two copies of the HBV genome were treated for 4 d; then HBV viral particles were purified and quantified by real-time PCR. Infectivity assays were performed by normalizing virus particles and infecting differentiated HepaRG cells. Infected HepaRG cells were harvested 9 d postinfection, and intracellular viral antigens were quantified to determine the level of infection. Data represent the mean and SD of three independent experiments performed in triplicate and are presented as a percentage of the untreated samples. Significant differences from this value are denoted by *P < 0.05 and **P < 0.001 (t test).
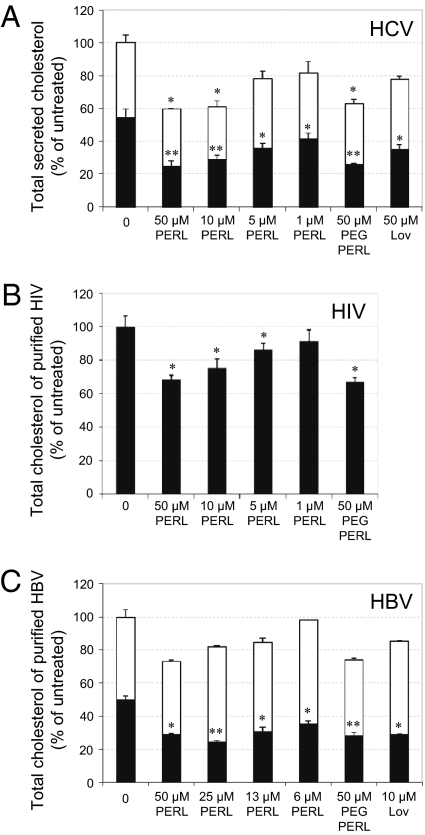
The effect of PERL treatment on secreted HCV, HIV, and HBV particles was assessed by measuring cholesterol levels in virus secreted from treated cells. HCV particles could not be fully purified from secreted lipoproteins; however, mean free and esterified cholesterol levels within this mixture were decreased by 55% (SD 3.7) (P < 0.001) and 25% (SD 0.5) (P = 0.02), respectively (Fig. 3A). To confirm that cholesterol reduction affected the viral particle itself, exogenous cholesterol was added back into the mixture and used to infect naïve cells, where infection levels were restored to almost normal levels (Fig. S6). Recent studies showing that mature HCV particles are rich in cholesterol (4) and that depletion leads to decreased infectivity explain the loss of infectivity observed in Fig. 2A. HIV-1 budding from the host cell occurs at lipid rafts, resulting in high cholesterol levels within the viral envelope (12, 13), and reduction in viral envelope cholesterol renders the virus incompetent for cell entry (14, 15). HIV particles secreted from PERL-treated PBMCs were significantly deficient in free cholesterol, so that levels were a mean of 32% (SD 2.7) (P = 0.008) lower than those in untreated particles (Fig. 3B). HBV particles also require cholesterol in the viral envelope for efficient infectivity (6), and PERL treatment led to a mean decrease in free cholesterol by 42% (SD 0.8) (P = 0.004) (Fig. 3C); this decrease probably accounts for the lower infection levels observed in Fig. 2C.
Fig. 3.
HCV, HIV, and HBV particles secreted from PERL-treated cells have significantly less cholesterol in their viral envelope. (A) Quantification of free (black) and esterified (white) cholesterol within the secreted material of liposome-treated, Jc1-infected Huh7.5 cells (MOI = 0.5). Cells were incubated 4 d in the presence of 22:6 ER liposomes, washed, and incubated in serum-free medium for a further 48 h. Supernatant corresponding to 107 cells was concentrated and used for cholesterol assays followed by normalization to total protein levels. (B) Secreted HIV-1 particles were harvested from cell supernatant following a 4-d treatment period. HIV-1 particles were purified by ioxidanol gradient ultracentrifugation, assayed for total cholesterol levels, and normalized to HIV p24 protein levels within the purified material. (C) HBV particles were purified by ultracentrifugation from cell supernatant following a 4-d treatment period. Purified virus was used in cholesterol assays to quantify both free (black) and esterified (white) cholesterol levels in samples normalized to total protein levels. Data represent the mean and SD of two independent experiments performed in duplicate and are presented as a percentage of the untreated samples. Significant differences from this value are denoted by *P < 0.05 and **P < 0.001 (t test).
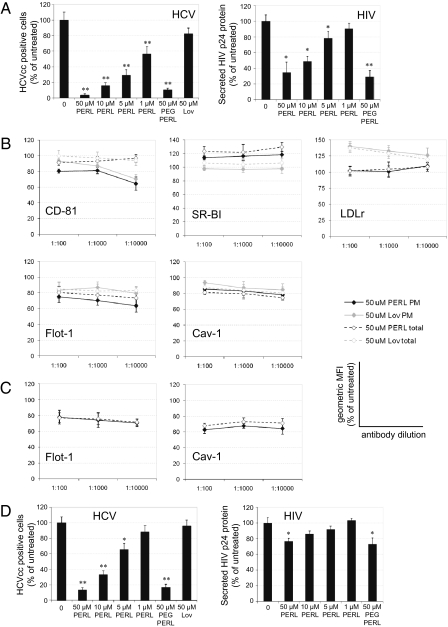
Next, the effects of cholesterol depletion by pretreating cells with PERLs were determined for both HCV and HIV infections. (These experiments could not be done in the HBV system, because pretreatment of HepaRG cells is incompatible with conditions necessary for cellular differentiation before infection.) Huh7.5 cells and PBMCs were treated with PERLs before infection with HCV or HIV, respectively. In both systems, pretreatment led to a significant reduction in the ability of viral particles to infect these cells. As shown in Fig. 4A, HCV infection was decreased by a mean of 94% (SD 1.7) (P < 0.001), and HIV infection was decreased by a mean of 64% (SD 13.2) (P = 0.004). The decrease in HCV infection as a result of lovastatin pretreatment was not significant.
Fig. 4.
Pretreatment of cells with PERLs prevents infection by HCV and HIV. (A) Uninfected Huh7.5 cells and PBMCs were treated for 4 d before infection with Jc1 HCVcc (MOI = 0.5) or LAI HIV-1 (TCID50 = 100), respectively. Viral infectivity was quantified as previously described. (B) Flow cytometric analysis of uninfected Huh7.5 cells treated for 4 d. For analysis of plasma membrane (PM) expression of viral receptors (CD-81, SR-BI, and LDLr), lipid rafts (Flot-1), and caveolae (Cav-1), cells were neither fixed nor permeabilized before detection and were gated to exclude cells positively stained with propidium iodide. Total protein expression (total) was measured after cells were fixed and permeabilized. (C) Flow cytometric analysis of uninfected CD4+ T cells purified from PBMCs and treated for 4 d. Both plasma membrane and total expression of Flot-1 and Cav-1 were determined as described. All flow cytometry data are presented as the geometric mean fluorescent intensity (MFI) of each sample and represent the mean and SD of triplicate samples from two independent experiments. (D) Addition of PERLs to viral stocks of HCVcc or HIV during infection periods of 1 h and 16 h, respectively. The ability of PERLs to neutralize the HCVcc and HIV infections was monitored by HCV core protein immunofluorescence assay or by p24 capture ELISA, respectively, following a 2-d incubation. Unless stated otherwise, all data represent the mean and SD of three independent experiments performed in triplicate. Data are presented in relation to the untreated control (100%), and significant differences from this value are denoted by *P < 0.05 and **P < 0.001.
Localization to cholesterol-enriched plasma membrane microdomains (or lipid rafts) has been demonstrated for both CD81 and SR-BI, two main receptors for HCV entry. SR-BI is associated with caveolae (16), whereas CD81 interacts physically with cholesterol (17). Although the dependence of HCV infection on cellular cholesterol is currently unknown, HCV pseudoparticle fusion with liposomes is enhanced by the presence of cholesterol in the target membrane (18). To determine whether PERL treatment suppresses the ability of HCV to infect cells by decreasing either plasma membrane cholesterol or cellular receptors associated with cholesterol, flow cytometry was performed on pretreated Huh7.5 cells to measure both plasma membrane and total protein expression of the cholesterol markers flotilin-1 and caveolin-1, as well as HCV receptors CD81, SR-BI, and LDLr. Although the total expression of CD81 was not affected by treatment, the cell-surface localization was reduced with both PERL and lovastatin treatments (Fig. 4B). Both total and plasma membrane expression of SR-BI increased with PERL treatment, whereas LDLr expression increased with lovastatin treatment (Fig. 4B), as is consistent with the inhibition of HMG CoA reductase. The increase in SR-BI expression with PERL treatment could result from these liposomes’ use of scavenger receptors for entry into cells: Continuous treatment might lead to increased expression. SR-BI is known to be involved in cholesterol efflux (19); therefore increases in SR-BI expression caused by PERL treatment may be another factor leading to reduced cholesterol levels. Decrease in cellular cholesterol was confirmed within total cells and specifically at the plasma membrane through reduction in both flotilin-1 and caveolin-1 expression (Fig. 4B). Together, these results suggest the inhibition of HCV entry following PERL treatment is caused by decreases in both cellular receptors (CD81) and plasma membrane cholesterol.
Several studies have proposed a role for lipid rafts in HIV infection, because depletion of cholesterol inhibited HIV entry mediated by raft-associated and raft-excluded CD4 (20). None of the HIV receptors tested (CD4, CCR5, and CXCR4) demonstrated any change in expression with PERL treatment (Fig. S7). However, pretreatment of PBMCs with PERLs decreased levels of total and plasma membrane expression of flotilin-1 and caveolin-1 (Fig. 4C), suggesting that HIV infection is inhibited by the depletion of both lipid rafts and caveolae and not by the inhibition of cell-surface receptors.
ER-targeting liposomes use both lipoprotein and scavenger receptors for cell entry (11), which in the case of an HCV infection would cause these liposomes to compete with the virus for entry into naïve cells. When HCV particles were coincubated with PERLs during the 1-h infection period, infection rates decreased by a mean of 87% (SD 2.9) (P < 0.001) (Fig. 4D). This decrease probably explains why increases in SR-BI expression with PERL treatment did not increase HCV infection. In a similar experiment, PERLs were coincubated with HIV during a 16-h infection period. In this case, infection rates were decreased by a mean of 23% (SD 3.6) (P = 0.02) (Fig. 4D). However, this decrease probably is the result of the extended incubation period, which may have resulted in the reduction of plasma membrane lipid rafts necessary for infection, rather than direct competition for cellular receptors.
Discussion
The strategy presented for efficient down-regulation of cellular cholesterol levels using PERLs has been found to be antiviral in three important viral systems (HCV, HBV, and HIV) and to be more potent than a clinically approved cholesterol-lowering drug. The main mechanism for antiviral activity appears to be the secretion of cholesterol-deficient viral particles from PERL-treated cells, which have lower infectivity than untreated particles. However, PERL treatment also causes decreased secretion of viral particles and inhibits the entry of untreated virions when cells have been pretreated by lowering the levels of cell-associated cholesterol levels.
Cholesterol down-regulation within treated cells may be linked to sphingomyelinase activation (observed in Fig. S2), because removal of sphingolipids from the plasma membrane displaces cholesterol from lipid rafts, increasing the intracellular cholesterol pool, and this increase acts as a signal to down-regulate the mevalonate pathway (21). Direct inhibition of the mevalonate pathway is achieved through the use of statins; however, the cholesterol-lowering capability of lovastatin was inferior to that of PERLs in all cell lines tested. The additional effect of PERL treatment on cellular cholesterol levels may result from these nanoparticles competing with lipoproteins for entry into target cells by binding to both scavenger and lipoprotein receptors on the cell surface, another mechanism by which cells acquire cholesterol (11). Furthermore, the observed increase in SR-BI expression following PERL treatment also could lower intracellular cholesterol levels through its role in cholesterol efflux (19).
Reduced levels of cellular cholesterol led to the inhibition of viral secretion in all three viral systems. PERL treatment caused the specific depletion of free cholesterol at the plasma membrane (and a decrease in the number of lipid rafts) in CD4+ T cells, markedly and specifically reducing HIV-1 production (22). In HCV infections, decreased viral secretion may have more to do with lower levels of esterified cholesterol; the lower levels translate to fewer lipid droplets within the cell. Various studies have shown that introducing mutations impairing the association of HCV core protein with lipid droplets diminishes the production of infectious viruses (23, 24). Although there is no current evidence linking cholesterol metabolism to HBV assembly and secretion, it is conceivable that lipid droplets are involved, and therefore a similar effect would be seen in this system as well.
The reduced level of free cholesterol in pretreated cells also was shown to diminish the infection rate for both HIV and HCV. Plasma membrane lipid rafts are known entry points for HIV, and depletion of cholesterol already has been shown to inhibit HIV entry mediated by raft-associated and raft-excluded CD4 (20). The effect on HIV entry also could be indirect, via the inhibition of intermediates of the mevalonate cholesterol pathway, including those involved in protein prenylation. Reduction of prenylation of small G proteins leads to inhibition of Rho-GTPase and Rho-A activation, resulting in reduced virus entry (25). When PERLs were used for the pretreatment of Huh7.5 cells, the total amount of free cholesterol and lipid rafts was lowered, and so was the cell-surface expression of an important HCV receptor, CD81. This lowering could explain the observed reduction in viral infections even with the increased expression of another important receptor, SR-BI (an increase which probably is negated by the competition with PERL nanoparticles for entry into cells).
The reduction in cellular cholesterol as a result of PERL treatment led directly to a decrease in cholesterol in the secreted particles, resulting in diminished infectivity in all three viral systems. The importance of cholesterol for infectivity of HIV virions has been shown previously by experiments in which treatment of HIV particles with cholesterol-sequestering drugs, such as MβCD, led to disruptions of the virion lipid bilayer that could not be restored by the addition of exogenous cholesterol (14). Studies have shown that cholesterol and sphingolipid have important roles in HCV infection and virion maturation. Specifically, mature HCV particles are rich in cholesterol (4). Depletion of cholesterol from HCV or hydrolysis of virion-associated sphingomyelin results in a loss of infectivity (4). Moreover, the addition of exogenous cholesterol restores infectivity, as was confirmed in these experiments. HBV particles also require cholesterol for proper infection of target cells, although binding to the cell surface remains unaffected, suggesting that cholesterol within the viral envelope is required for a later step in viral fusion (6). In this system, PERL and lovastatin treatments had similar activity, and there was no significant difference in the amount of virus-associated esterified cholesterol, even though these cholesterol stores were more affected than free cholesterol following PERL treatments.
In conclusion, treatment of virus-infected cells with PERLs demonstrated effective antiviral activity in three different viral systems—HCV, HIV, and HBV—because of decreased levels of cellular and virus-associated cholesterol. In each experiment, PERL treatment was more effective than lovastatin and therefore may be better suited for in vivo antiviral therapy. Furthermore, the potential use of PERLs for in vivo treatment of viral infections, as well as the frequent coinfections with the viruses tested here, eventually should include the encapsulation of other antiviral therapies, leading to enhanced treatment efficacy. Because of the host cell-based antiviral mechanism of action, we expect all clades and genotypes of the investigated viruses to respond to this treatment and the likelihood of the emergence of resistant viral escape mutants to be substantially lower than with conventional antiviral treatments.
Materials and Methods
Lipids and Liposomes.
PERLs are composed of 1,2-didocosahexaenoyl-sn-glycero-3-phosphoethanolamine, 1,2-didocosahexaenoyl-sn-glycero-3-phosphocholine, l-α-phosphatidylinositol, and l-α-phosphatidylserine, with a molar ratio of 1.5:1.5:1:1. All lipids were purchased from Avanti Polar Lipids. Fresh liposomes were prepared before each experiment as previously described (11).
Cell Culture.
Huh7.5 cells (Apath, LLC) were grown in complete Dulbecco’s Modified Eagle Medium (DMEM) (100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine, 1× MEM nonessential amino acids, and 10% FBS). PBMCs from four uninfected donors were isolated using Histopaque density gradient centrifugation (Sigma-Aldrich), pooled, and stimulated with 5 μg/mL phytohemagglutinin for 48 h followed by 40 U/mL IL-2 for 72 h in complete RPMI (RPMI plus 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine) before starting experiments. CD4+ T cells were isolated from PBMCs using a CD4+ Dynabeads isolation kit (Invitrogen). HepG2 2.2.15 cells stably transfected with two copies of the HBV genome were grown in RPMI medium 1640 (Euroclone) containing 10% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM Glutamax, and 200 μg/mL G418 (Gibco). HepaRG cells were grown in William's E medium (Gibco) supplemented with 10% FCS, 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM Glutamax, 5 μg/mL insulin, and 5 × 105 M hydrocortisone hemisuccinate and differentiated in the presence of 2% DMSO, as previously described (26). All incubations were at 37 °C/5% CO2, unless stated otherwise.
Jc1 HCVcc Secretion and Infectivity Assays.
Huh7.5 cells were infected for 1 h at a multiplicity of infection (MOI) 0.5 using a viral stock of known titer. Unless stated otherwise, treatment started when >50% of cells tested positive for HCVcc infection as determined by HCV core protein immunofluorescence. Virus secretion analysis was performed by quantitative PCR on viral RNA extracted from cell supernatant using the QIAGEN QIAamp Viral RNA Purification Kit, following the manufacturer's protocol. Quantification of secreted viral RNA was done by first converting isolated RNA to cDNA using a reverse transcriptase reaction (TaqMan; ABI) with primer RC21 (5′-CTCCCGGGGCACTCGCAAGC-3′) followed by real-time PCR using an SYBR Green mix (QIAGEN) and both RC21 and RC1 primers (5′-ATGCCATGGCGTTAGTA-3′) directed against the HCV cDNA. HCV transcript levels were determined relative to a standard curve comprised of serial dilutions of HCV Jc1 cDNA. The infectivity of secreted HCV virions within the supernatant of treated Jc1-infected Huh7.5 cells was determined by infecting naïve Huh7.5 cells with sample supernatant normalized using RNA copy numbers. Supernatant was left to infect naïve Huh7.5 cells for 1 h before cells were washed twice and incubated in complete DMEM for 2 d. After the 2-d incubation, cells were washed, fixed in methanol/acetone (1:1, vol/vol), and probed with a mouse anti-HCV core antibody (Affinity BioReagents), followed by an FITC-labeled anti-mouse secondary antibody (Sigma-Aldrich). Cells were stained with DAPI, and fluorescent images were taken using a Nikon Eclipse TE2000-U microscope. The percentage of infected cells was calculated by counting the total number of cells infected with HCV (detected by the anti-HCV antibody) and dividing that number by the total number of cells in the assay (detected by DAPI staining).
HIV Secretion, Purification, and Infectivity Assays.
PBMCs were infected with primary isolate stock [at a 50% tissue culture infectious dose (TCID50)] of HIV-1 (100 per 4 × 105 cells) for 16 h before the start of treatment. HIV secretion and infectivity assays were performed using cell supernatant for p24 capture ELISAs as previously described (27). The HIV-1 primary isolate LAI was obtained from the National Institute for Biological Standards and Control. HIV particles were purified by ioxidanol gradient ultracentrifugation using OptiPrep density gradient medium (Axis-Shield) following the manufacturer's protocol for purification of HIV-1 virions (OptiPrep application V06).
HBV Secretion, Purification, and Infectivity Assays.
HepG2.2.2.15 cells were left untreated or were treated with liposomes at the indicated concentrations for 4 d. Cell supernatants were clarified from cell debris by a brief centrifugation at 2,000 × g. Virus particles were pelleted by ultracentrifugation through a 20% sucrose cushion in an SW 41 Ti Beckman rotor at 36,000 rpm, for 4 h. The pellet was resuspended in PBS, and virus concentration (genome equivalents) was determined by real-time PCR, using serial dilutions of known amounts of a pTriExHBV1.1 vector as standard curve. Virus samples were adjusted with PBS to contain an equal number of genome equivalents and then were used to infect differentiated HepaRG cells. HepaRG cells were differentiated in collagen-coated plates and incubated for 16 h with HBV from the step above or were left untreated as negative control. Cells were harvested 9 d postinfection and lysed. Lysates were clarified by centrifugation at 10,000 × g for 15 min. The protein content in the supernatant was determined using the bicinchoninic acid (BCA) method. The sample volumes were adjusted to equal amounts of total protein, and the level of HBV antigen expression was determined using the Monolisa HBs Ag Ultra kit (Bio-Rad), according to the manufacturer's instruction. Results were obtained as ratios of signal to cutoff and were converted to percentage of hepatitis B surface antigen expression.
Quantification of Cholesterol Levels.
Cells and supernatant containing isolated viral particles were lysed in PBS/1% Triton X-100 and a mixture of protease inhibitors (Sigma). Cell lysates were clarified by centrifugation. Cholesterol content was determined in each sample using the Amplex Red assay kit (Invitrogen), according to the manufacturer's instructions. The values obtained were normalized using the total protein content, as measured by either the BCA (Pierce) or Bradford (Bio-Rad) assay systems.
Flow Cytometry.
Treated cells were isolated and washed in PBS/1% FBS. To quantify total protein expression, cells were fixed in 2% paraformaldehyde and permeabilized with 0.1% Triton X-100. For detection of proteins on the plasma membrane, cells were left untreated, and all following steps were carried out at 4 °C. Cells were incubated with primary antibodies for 1 h and with labeled secondary antibodies for 30 min before analysis on a FACSCalibur flow cytometer (Becton Dickinson). Results were analyzed using CellQuest (Becton Dickinson). For detection of plasma membrane proteins, dead cells were excluded from the analysis by staining cell samples with propidium iodide before analysis.
Antibodies.
Rabbit polyclonal anti-SR-BI, mouse monoclonal anti-CD81 (TAPA-1), rabbit polyclonal anti-LDLr, and rabbit polyclonal anti–flotillin-1 antibodies were purchased from Abcam. Rabbit polyclonal caveolin-1 antibody was purchased from New England Biolabs. Alexa Fluor 488-labeled anti-mouse and anti-rabbit secondary antibodies were purchased from Invitrogen.
Supplementary Material
Acknowledgments
We thank Takaji Wakita (Tokyo Metropolitan Institute for Neuroscience, Tokyo), Jens Bukh (National Institutes of Health, Bethesda), Charles M. Rice (The Rockefeller University, New York), and Ralf Bartenschlager (University of Heidelberg, Heidelberg) for reagents. This work is funded by the Oxford Glycobiology Endowment, the Canadian Institutes of Health Research (S.P.), and by a “Blue Skies” research grant from United Therapeutics Corp. N.Z. is a Senior Research Fellow of Linacre College, Oxford. The Consiliului National al Cercetarii Stiintifice din Invatamantul Superior provided support with Idei Grant ID_84 (to N.B.N.).
Footnotes
Conflict of interest statement: R.A.D. is a Director and Member of the Scientific Board of United Therapeutics Corp., which supported this work in part through a “Blue Skies” research grant. A patent covering cholesterol level-lowering ER-targeting liposomes has been filed.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009445107/-/DCSupplemental.
References
- 1.Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81:374–383. doi: 10.1128/JVI.01134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shavinskaya A, Boulant S, Penin F, McLauchlan J, Bartenschlager R. The lipid droplet binding domain of hepatitis C virus core protein is a major determinant for efficient virus assembly. J Biol Chem. 2007;282:37158–37169. doi: 10.1074/jbc.M707329200. [DOI] [PubMed] [Google Scholar]
- 4.Aizaki H, et al. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J Virol. 2008;82:5715–5724. doi: 10.1128/JVI.02530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV-1: From viral entry to assembly of progeny virions. J Clin Virol. 2001;22:217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 6.Bremer CM, Bung C, Kott N, Hardt M, Glebe D. Hepatitis B virus infection is dependent on cholesterol in the viral envelope. Cell Microbiol. 2009;11:249–260. doi: 10.1111/j.1462-5822.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 7.Macovei A, et al. Hepatitis B virus requires intact caveolin-1 function for productive infection in HepaRG cells. J Virol. 2009;84:243–253. doi: 10.1128/JVI.01207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forde KA, Law C, O'Flynn R, Kaplan DE. Do statins reduce hepatitis C RNA titers during routine clinical use? World J Gastroenterol. 2009;15:5020–5027. doi: 10.3748/wjg.15.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bader T, et al. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- 10.Milazzo L, et al. Does fluvastatin favour HCV replication in vivo? A pilot study on HIV-HCV coinfected patients. J Viral Hepat. 2009;16:479–484. doi: 10.1111/j.1365-2893.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 11.Pollock S, et al. Uptake and trafficking of liposomes to the endoplasmic reticulum. FASEB J. 2010;24:1866–1878. doi: 10.1096/fj.09-145755. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen DH, Hildreth JEK. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell SM, Crowe SM, Mak J. Virion-associated cholesterol is critical for the maintenance of HIV-1 structure and infectivity. AIDS. 2002;16:2253–2261. doi: 10.1097/00002030-200211220-00004. [DOI] [PubMed] [Google Scholar]
- 15.Guyader M, Kiyokawa E, Abrami L, Turelli P, Trono D. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J Virol. 2002;76:10356–10364. doi: 10.1128/JVI.76.20.10356-10364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graf GA, Connell PM, van der Westhuyzen DR, Smart EJ. The class B, type I scavenger receptor promotes the selective uptake of high density lipoprotein cholesterol ethers into caveolae. J Biol Chem. 1999;274:12043–12048. doi: 10.1074/jbc.274.17.12043. [DOI] [PubMed] [Google Scholar]
- 17.Charrin S, et al. A physical and functional link between cholesterol and tetraspanins. Eur J Immunol. 2003;33:2479–2489. doi: 10.1002/eji.200323884. [DOI] [PubMed] [Google Scholar]
- 18.Lavillette D, et al. Hepatitis C virus glycoproteins mediate low pH-dependent membrane fusion with liposomes. J Biol Chem. 2006;281:3909–3917. doi: 10.1074/jbc.M509747200. [DOI] [PubMed] [Google Scholar]
- 19.Gu X, Kozarsky K, Krieger M. Scavenger receptor class B, type I-mediated [3H]cholesterol efflux to high and low density lipoproteins is dependent on lipoprotein binding to the receptor. J Biol Chem. 2000;275:29993–30001. doi: 10.1074/jbc.275.39.29993. [DOI] [PubMed] [Google Scholar]
- 20.Popik W, Alce TM. CD4 receptor localized to non-raft membrane microdomains supports HIV-1 entry. Identification of a novel raft localization marker in CD4. J Biol Chem. 2004;279:704–712. doi: 10.1074/jbc.M306380200. [DOI] [PubMed] [Google Scholar]
- 21.Worgall TS, Johnson RA, Seo T, Gierens H, Deckelbaum RJ. Unsaturated fatty acid-mediated decreases in sterol regulatory element-mediated gene transcription are linked to cellular sphingolipid metabolism. J Biol Chem. 2002;277:3878–3885. doi: 10.1074/jbc.M102393200. [DOI] [PubMed] [Google Scholar]
- 22.Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci USA. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulant S, Targett-Adams P, McLauchlan J. Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J Gen Virol. 2007;88:2204–2213. doi: 10.1099/vir.0.82898-0. [DOI] [PubMed] [Google Scholar]
- 24.Miyanari Y, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 25.del Real G, et al. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med. 2004;200:541–547. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gripon P, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock S, Dwek RA, Burton DR, Zitzmann N. N-Butyldeoxynojirimycin is a broadly effective anti-HIV therapy significantly enhanced by targeted liposome delivery. AIDS. 2008;22:1961–1969. doi: 10.1097/QAD.0b013e32830efd96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.