Abstract
Germ cell-deficient fish usually develop as phenotypic males. Thus, the presence of germ cells is generally considered to be essential for female gonadal differentiation or the maintenance of ovarian structure. However, little is known of the role of germ cells in the determination of the sexual fate of gonadal somatic cells. We have established an inducible germ cell deficiency system in the loach (Misgurnus anguillicaudatus, Cypriniformes: Cobitidae), a small freshwater fish, using knockdown of the dead end gene with a morpholino antisense oligonucleotide. Interestingly, loach lacking germ cells could develop as either phenotypic males or females, as characterized morphologically by the presence or absence of bony plates in the pectoral fins, respectively. The phenotypic males and females had testicular and ovarian structures, respectively, but lacked germ cells. Gene expression patterns in these male and female germ cell-deficient gonads were essentially the same as those in gonads of normal fish. Our observations indicate that sexually dimorphic gonads can develop in germ cell-deficient loach. In contrast to the situation in other model fish species, the gonadal somatic cells in phenotypic females autonomously differentiated into ovarian tissues and also played a role in the maintenance of gonadal structure. On the basis of our observations, we propose two possible models to explain the role of germ cells in sex determination in fish.
Keywords: dead end, gonadal development, sex differentiation
Teleost species inhabit a wide range of aquatic environments, exhibit diverse life histories, and show an array of sex determination mechanisms (1). Although most fish species lack distinguishable heteromorphic sex chromosomes, they nevertheless have genetically based sex determination, based on single gene or polygenic systems (1). In some species, however, environmental conditions, such as temperature, population density, pH, or social status, influence sex determination rather than a genetic system as in most gonochoristic species (2). Moreover, phenotypic sex in some fish species can be modified by exogenous hormonal treatment before sex differentiation (1).
What factors determine gonadal sex? The model species zebrafish is categorized as an undifferentiated gonochorist. In this species, the gonads first differentiate into ovaries in all individuals after proliferation of germ cells, and then the process of sex differentiation occurs after a period of juvenile hermaphroditism (3). In medaka, the sexes are determined by the sex determining gene DMY (4) and embryos show sexual differentiation before hatching; this differentiation is the result of proliferation of germ cells (5). Furthermore, gonadal somatic cells of genetically XY male medaka can induce sex reversal in genetically XX females, suggesting that primary gonadal sex differentiation is susceptible to influence by gonadal somatic cells (6). However, germ cells themselves are important for the development of sexual dimorphism in medaka and zebrafish: embryos lacking germ cells develop into fish appearing phenotypically male and the gonads of germ cell-deficient fish differentiate into tube-like structures that express male-specific genes (7–9). The results of germ cell depletion experiments in mammals also suggest that germ cells are necessary for the complete differentiation of an ovary, although the early stages of ovarian differentiation can occur in the absence of germ cells (10). Overall, the available data indicate that germ cells are essential for female gonad differentiation or the maintenance of ovarian structure, although little is known of the role of the germ cells in determining the sexual fate of gonadal somatic cells. However, this conclusion on the necessity of germ cells for complete ovarian differentiation is based on results from a small number of experimental species. Given the enormous biodiversity of sex differentiation systems among teleosts, it is unlikely that a single model for gonad differentiation will be universally applicable.
In this study, we used the loach Misgurnus anguillicaudatus (Cypriniformes: Cobitidae), a gonochoristic species, as the experimental material. Loaches are distributed across a wide area of the temperate zone of East Asia and live in rivers, lakes, ponds, and paddy fields. Sex determination in the loach was concluded to be a male-heterozygous XX–XY system because only female loaches were produced after induced gynogenesis in which a paternal contribution to the zygotes was eliminated (11). However, it has also been reported that sex ratio in the loach is influenced by high temperature during the sex differentiation period, and that female-to-male sex reversal frequently occurs (12). Male loaches take about 6 mo to achieve sexual maturity under our artificial rearing conditions whereas females take 1 y. The time taken to reach maturity in loach is higher than that in model species such as zebrafish and medaka. In the present study, we successfully induced germ cell deficiency in loach by knockdown of the dead end (dnd) gene using a morpholino antisense oligonucleotide (MO). This gene normally functions in primordial germ cell (PGC) migration and maintenance during embryogenesis (13). We then investigated gonadal development and gonadal structures in the resulting germ cell-deficient fish.
Results
Induction of Germ Cell-Deficient Loach.
To test the ability of dnd-specific morpholino antisense oligonucleotide (dnd-MO) to knock down the target gene and to determine an optimum dosage of dnd-MO to induce germ cell deficiency in fish, we injected 5–10 nL of various concentrations of dnd-MO solution. We then carried out a histological analysis for the presence or absence of PGCs in each hatched larva that had been injected with dnd-MO (Table S1). In addition, we analyzed the morphological features of 13-mo-old fish grown from each experimental group (Table S2).
More than 90% of the intact control larvae and larvae injected with the standard control morpholino oligonucleotide (control MO) hatched normally. Histological analysis found a mean number of 48.5 and 50.5 PGCs in intact control larvae and control MO larvae, respectively. For larvae injected with dnd-MO, an increased concentration of dnd-MO was correlated with reduced numbers of PGCs in hatched larvae. Larvae injected with more than 1,000 pg dnd-MO showed a complete absence of PGCs at the genital ridge. The hatching rates of dnd-MO embryos were not significantly decreased.
Adult fish that developed from control larvae showed morphologically normal ovaries and testes. By contrast, two types of morphologically unusual gonads were observed in fish derived from dnd-MO–injected embryos (hereafter, dnd morphants). In one group, a testis developed on one side of the body, whereas on the other, an atypical gonad was present that resembled the germ cell-deficient gonads described in previous studies (8, 9). The proportion of dnd morphants with a normal gonad decreased with increase in dnd-MO dosage, whereas the rate with an atypical gonad increased. All dnd morphants injected with 2,000–4,000 pg dnd-MO had atypical gonads. These results indicate that germ cell-deficient fish could be reliably induced by injection of 2,000–4,000 pg dnd-MO, and in all subsequent analyses, we adopted this injection regime.
Migration of PGCs During Embryogenesis in dnd Morphants.
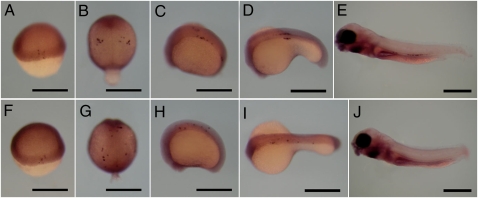
The migration of primordial germ cells (PGCs) was investigated in wild-type and dnd-MO injected (2,000–4,000 pg/embryo) embryos from the embryonic shield stage to the larval stage. Whole mount in situ hybridization was used to determine the positions of PGCs stained for vasa gene expression. vasa is an useful molecular marker for tracing PGCs during embryogenesis in many organisms (14). At the embryonic shield stage, vasa-positive cells were located at the marginal region of the blastoderm in both wild-type embryos and dnd morphants (Fig. 1 A and F). At the 2- to 4-somite stage, vasa-positive cells formed clusters in the near-anterior somite of wild-type embryos, but were distributed along the body axis in dnd morphants (Fig. 1 B and G). At the 20-somite stage, many vasa-positive cells in wild-type embryos were clustered on both sides of the first to fifth somites; later, at the 30-somite stage, the clusters were located at the base of the yolk extension region (Fig. 1 C and D). However, in dnd morphants at 20- and 30-somite stages, vasa-positive cells were found in the embryonic body at positions where PGCs are never located in wild-type embryos (Fig. 1 H and I). In 5-d postfertilization (dpf) wild-type larvae, vasa-positive cells were observed at the body cavity, whereas no vasa-positive cells were detected in dnd morphants (Fig. 1 E and J).
Fig. 1.
Migrating primordial germ cells (PGCs) were identified by vasa expression using whole mount in situ hybridization of wild-type and dnd-MO injected (2,000–4,000 pg/embryo) embryos at the embryonic shield to larval stages. (A–E and F–J) PGCs of wild-type and dnd morphants, respectively. (A and F) Embryonic shield stage. (B and G) Two- to four-somite stage. (C and H) Twenty-somite stage. (D and I) Thirty-somite stage. (E and J) Larvae at 5 d postfertilization. (Scale bars, 500 μm.)
Embryos after whole mount in situ hybridization were categorized as “normal,” “normal and ectopic,” and “ectopic” on the basis of the distribution pattern of vasa-positive cells. vasa-positive cells in wild-type embryos and larvae were distributed normally except for four embryos at 30-somite stage (Table S3). In contrast, most vasa-positive cells in dnd morphants were ectopically positioned after the 2- to 4-somite stage and finally could not be detected at 5-dpf larvae (Table S3).
Sex Differentiation in Germ Cell-Deficient Loach During Gonadal Development.
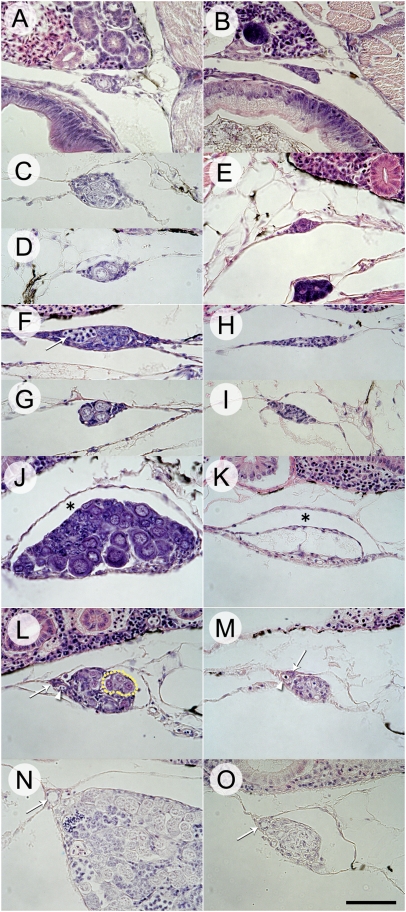
Development of the gonads was examined to determine whether gonadal sex differentiation occurs in the same manner in dnd morphants as in wild-type fish. At 33 d posthatching (dph), the germ cells of wild-type fish were surrounded by gonadal somatic cells and protruded into the coelomic cavity (Fig. 2A). At 55 dph, wild-type fish showed two gonadal types that could be distinguished with respect to the number of germ cells: in one type, germ cells had proliferated within a cyst (Fig. 2C); the second type had a few germ cells and no cyst (Fig. 2D). The distal edge of the gonads was attached to the coelomic wall at this stage. By contrast, the gonadal tissue was devoid of germ cells and protruded into the coelomic cavity at 33 dph in dnd morphants (Fig. 2B). As in the wild type, the distal edge of the gonads was attached to the coelomic wall at 55 dph in dnd morphants (Fig. 2E). At 70 dph, two gonadal types were seen in both groups. In the wild type, one of the gonadal types showed elongation from the dorsal to lateral coelomic wall and also proliferation of germ cells, which had entered meiosis, within a cyst (Fig. 2F). The second gonadal type had a club-like shape with only a few germ cells, and no meiotic cells were present (Fig. 2G). In the dnd morphants, one gonadal type showed elongation from the dorsal to lateral coelomic wall (Fig. 2H). The second was not elongated but exhibited a club-like shape and protruded from the dorsal coelomic wall (Fig. 2I). At 97–120 dph, female and male gonads in wild type could be discriminated clearly by their external appearances and by histological observation (Fig. 2 J and L). Oocytes were apparent in the ovaries of wild-type fish, and an ovarian lumen was formed between the oocytes and the coelomic wall (Fig. 2J). In the testes, seminal lobules had begun to form, and a blood vessel and a sperm duct anlage were present at the dorsal region where the gonad was attached (Fig. 2L). In the dnd morphants, ribbon-like and tube-like gonads could be discriminated by external appearance. The ribbon-like gonads had an ovarian lumen between the coelomic wall and the empty space in which oocytes should have been present (Fig. 2K). Stromal tissue was also present ventrally. The tube-like gonads had a blood vessel and a sperm duct anlage at the same position as wild-type testes (Fig. 2M). At 150 dph, spermatogenesis was observed in the testes of the wild type (Fig. 2N). A fully developed sperm duct was present in both wild-type testes and tube-like gonads (Fig. 2 N and O).
Fig. 2.
Sex differentiation in wild-type fish and dnd morphants during gonadal development. (A) Wild-type gonad at 33 d posthatch (dph). (B) dnd morphant gonad at 33 dph. (C) Wild-type gonad with proliferating germ cells within a cyst at 55 dph. (D) Wild-type gonad with only a few germ cells and no cyst formation at 55 dph. (E) dnd morphant gonad at 55 dph. (F) Laterally elongated gonad with proliferating germ cells within a cyst in a wild-type gonad at 70 dph. Arrow indicates germ cells at the zygotene stage of meiosis. (G) Club-shaped gonad with a few germ cells in a wild-type gonad at 70 dph. (H) Laterally elongated gonad in dnd morphant at 70 dph. (I) Club-shaped gonad in dnd morphant at 70 dph. (J) Ovary with oocytes in wild type at 97 dph. Asterisk indicates ovarian lumen. (K) Ribbon-like gonad of dnd morphant at 120 dph. Asterisk indicates ovarian lumen. (L) Testis with undifferentiated germ cells in a wild-type gonad at 97 dph. Arrow and arrowhead indicate sperm duct anlage and blood vessel, respectively. Dotted line indicates seminal lobules. (M) Tube-like gonad of dnd morphant at 120 dph. Arrow and arrowhead indicate sperm duct anlage and blood vessel, respectively. (N) Testis with germ cells undergoing spermatogenesis in a wild-type gonad at 150 dph. Arrow indicates sperm duct. (O) Tube-like gonad of dnd morphant at 150 dph. Arrow indicates sperm duct. (Scale bar, 50 μm.)
External Sexual Characteristics and Gonadal Structure of Germ Cell-Deficient Loach.
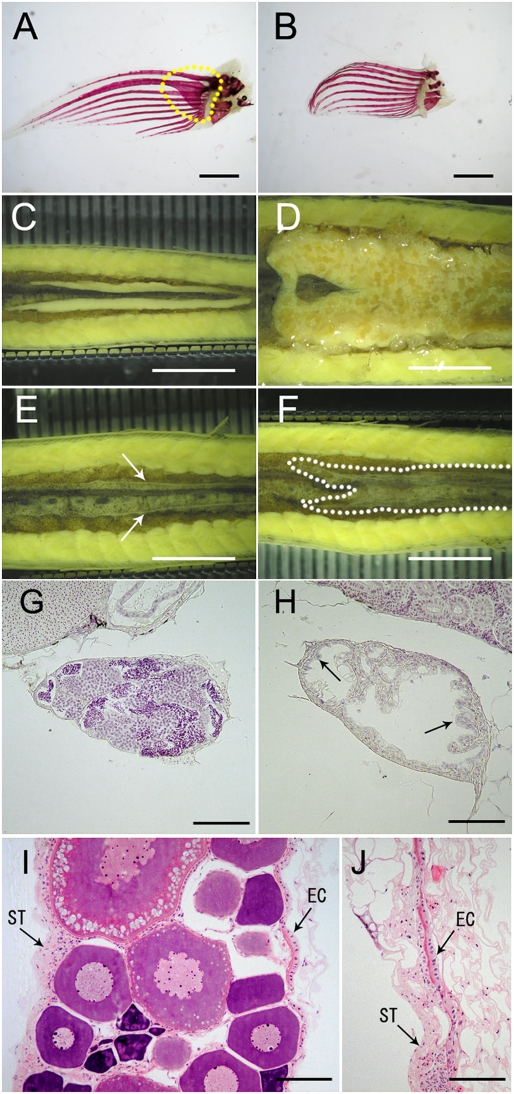
In sexually mature wild-type loach, males and females can be characterized phenotypically by the presence or absence, respectively, of bony plates in the pectoral fins (Fig. 3 A and B). Two-year-old wild-type males and females have testes and ovaries, respectively (Fig. 3 C and D and Table 1). By contrast, phenotypic males and females of 2-y-old dnd morphants have tube-like and ribbon-like gonads, respectively, except in one case (Fig. 3 E and F and Table 1). The tube-like gonads of phenotypic males of dnd morphants consisted of presumptive testicular tubules that contained interstitial tissue but lacked germ cells, but otherwise resembled the testes of wild-type fish (Fig. 3 G and H). In the dnd morphants, a sheet of epithelial cells lined the inside of the tubules, and the epithelial cells were hypertrophied and ciliated (Fig. 3H). By contrast, the ribbon-like gonads of phenotypically female dnd morphants contained stromal tissue on the ventral side and epithelial cells lining the coelomic wall, similar to the ovaries of wild-type females (Fig. 3 I and J). Overall, the structure of the ribbon-like gonads resembled the normal ovary quite remarkably except for the lack of oocytes.
Fig. 3.
Sexual dimorphism in 2-y-old wild-type fish and dnd morphants. (A) Pectoral fin of male with bony plate (framed by dotted line). (B) Pectoral fin of females without bony plate. (C) Testes in wild-type male. (D) Ovary in wild-type female. (E) Tube-like gonad (indicated by arrows) in a phenotypically male dnd morphant. (F) Ribbon-like gonad (indicated by dotted line) in a phenotypically female dnd morphant. (G) Testis of wild-type male. (H) Tube-like gonad of male dnd morphant. (I) Ovary of wild-type female. (J) Ribbon-like gonad of female dnd morphant. EC, epithelial cells; ST, stromal tissue. [Scale bars, 1 mm (A and B), 5 mm (C–F), and 50 μm (G–J).]
Table 1.
Gonadal structures and morphological sexual characteristics of pectoral fins in 2-y-old wild-type fish and dnd morphants
| Wild-type | dnd morphant | ||||
| Bony plates at pectoral fin | Presence | Absence | Presence | Absence | |
| Typical gonad | Testis | 27 | 0 | 2 | 0 |
| Ovary | 0 | 15 | 0 | 1 | |
| Atypical gonad | Tube | 3 | 0 | 31 | 0 |
| Ribbon | 1 | 2 | 1 | 22 | |
Gene Expression in Germ Cell-Deficient Gonad.
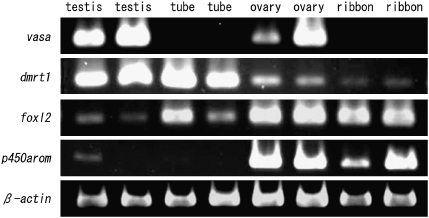
RT-PCR analyses were used to determine the expression patterns of the following genes: vasa, dmrt1, foxl2, p450 aromatase (p450arom), and β-actin. Two replicate RT-PCR experiments were performed using independent gonadal samples from 2-y-old adult loaches (Fig. 4). β-actin was used as the control. Analysis of gene expression patterns in germ cell-deficient gonads showed that vasa was not expressed in either phenotypically male or female dnd morphants (Fig. 4). For the other genes examined, the tube-like gonads showed a similar gene expression pattern to the wild-type male, except for p450arom, which showed a low level of expression in one wild-type testis (Fig. 4). Overall, the ribbon-like gonads of phenotypically female dnd morphants had the same expression pattern as wild-type ovaries (Fig. 4).
Fig. 4.
Patterns of expression of vasa, dmrt1, foxl2, p450arom, and β-actin in gonads of wild-type fish and dnd morphants. Two replicate RT-PCR analyses were performed using independent samples from each gonadal type.
Discussion
In wild-type fish, we identified sex-related variation in gonadal differentiation during development (Fig. 2 C and D). Two gonadal types were distinguished: one showed early proliferation of germ cells within a cyst and entry of the germ cells into meiosis (Fig. 2 C and F); the other had a small number of germ cells and no meiotic cells (Fig. 2 D and G). Sexual dimorphism in the proliferation of germ cells has been described previously in many species of teleosts (15). In general, presumptive ovaries have more germ cells than presumptive testes during early gonadal development, and the entry of germ cells into meiosis occurs earlier in ovaries than in testes (15). Therefore, we assume that the gonads that showed greater proliferation of germ cells within a cyst, lateral elongation, and also evidence of meiosis were likely to develop into ovaries in the adult, whereas the gonads with only a few germ cells and with a club-like shape are presumed to develop into testes in the adult. In light of the sex differentiation process in the wild type, the elongated gonads and club-shaped gonads observed here in dnd morphants at 70 dph are presumed to develop into the ribbon-like and tube-like gonads, respectively, of the adult (Fig. 2 H and I). In addition to similar patterns of gonadal sex differentiation, we found that the sex ratio, based on gonadal structures, was similar between wild-type and dnd morphants. Therefore, we conclude that gonadal sex differentiation in germ cell-deficient loach occurs in the same manner as in the wild type.
In adult dnd morphants, histological analysis showed that the tube-like gonads of phenotypic males had testicular structures, whereas the ribbon-like gonads of phenotypic females had ovarian structures (Fig. 3 H and J). The tube-like gonads of germ cell-deficient loach consisted of hypertrophied and ciliated cells, which lined the tubules. The gene expression patterns in these tube-like gonads were similar to those of testes of wild-type males. Overall, tubule structure and gene expression patterns were similar to those previously reported in the germ cell-deficient gonads of zebrafish and medaka (8, 9). We suggest that the somatic cells of the tube-like gonads of phenotypic males are responsible for the formation of the testicular structures typical of male sexual differentiation.
The most notable characteristic of the germ cell-deficient gonads of phenotypic females was the presence of ciliated and hypertrophied epithelial cells, which lined the coelomic walls of the ribbon-like gonads. It was previously reported that hypertrophic epithelial cells in the ovarian lumen were present in advance of ovarian maturation in medaka (16). Further, it was proposed that these cells played a role in both the maintenance of ovulated eggs and their transport through the oviduct for spawning. We suggest that the somatic cells of the ribbon-like gonads of phenotypic females differentiate into ovarian structures. In conclusion, germ cell-deficient gonads in loach can follow either the male or female differentiation pathway and can maintain the gonadal somatic structures of the testis or ovary.
We confirmed here that dnd morphants had complete ablation of germ cells using expression of the marker gene, vasa. Our RT-PCR analysis showed expression of vasa in testes and ovaries of wild type, but not in the tube-like gonads or ribbon-like gonads of the dnd morphants.
This study also showed sex-related variation in the expression of dmrt1, foxl2, and p450arom in the germ cell-deficient gonads. It was previously shown that dmrt1 is expressed in testicular Sertoli cells before and during sex differentiation and is involved in the regulation of testicular differentiation (17). Here, we found a high level of dmrt1 expression in wild-type testes and tube-like gonads of dnd morphants. Expression was also detected in ovaries and ribbon-like gonads, but the signal was weak when compared with that in the testes and tube-like gonads. These results indicate that a high level of dmrt1 expression is necessary for testicular differentiation.
We examined the expression patterns of two marker genes for ovarian differentiation in vertebrates: foxl2, which belongs to the forkhead gene family, and p450arom, which encodes an enzyme that converts testosterone to estradiol-17β (18). Because estradiol-17β is crucial for directing the initial steps of ovarian differentiation, regulation of p450arom expression has a key role in sex differentiation (17). A recent study in tilapia showed that foxl2 likely binds directly to the promoter of p450arom and interacts with Ad4BP/SF-1 to form a heterodimer that also enhances transcription of p450arom (19). Here, we found that expression of foxl2 was detectable not only in ovaries and ribbon-like gonads but also in testes and tube-like gonads. However, a high level of p450arom expression was detected only in ovaries and the ribbon-like gonads. Expression of p450arom was not detected in the germ cell-deficient gonads of zebrafish or medaka (8, 9). It is noteworthy that expression of both foxl2 and p450arom was present in both ovaries and ribbon-like gonads of loach. This result strongly suggests that endogenous estrogen might give rise to ovarian differentiation in germ cell-deficient gonads. On the other hand, in zebrafish and medaka, germ cells are necessary for ovarian differentiation, although exogenous estrogen induced feminization in zebrafish lacking the germ cells (7). Thus, in these species, germ cells might control the expression of ovarian genes such as foxl2 and p450arom. On the basis of our observations, we suggest that the dimorphic structures of germ cell-deficient gonads are maintained by expression of the same set of genes as normal testes or ovaries.
In conclusion, our study demonstrates that germ cell-deficient gonads in the loach can develop into testicular and ovarian structures and that gene expression patterns in these organs are similar to those of wild-type testes and ovaries. Therefore, it is no exaggeration to say that sexual dimorphism is evident in germ cell-deficient gonads in the loach. Thus, germ cells are not necessary for ovarian differentiation in the loach, indicating that germ cells do not control the expression of ovarian genes such as foxl2 and p450arom. On the basis of this conclusion, two of the models that have been proposed for the role of germ cells in sex determination can be examined. In the first model, germ cells are indispensable for differentiation of gonadal somatic cells into ovarian tissues. The second model envisages that gonadal somatic cells autonomously differentiate into ovarian tissues and also play a role in their maintenance. Clearly, the available evidence indicates that gonadal differentiation in zebrafish, medaka, and mammals follows expectations of the first model, whereas, the present study shows that the loach is consistent with the second model. Comparative studies of the two models should help to elucidate the key factors controlling the differences in interactions between germ cells and somatic cells.
Materials and Methods
Molecular cloning, PCR conditions, and primer sets (Table S4) appear in SI Materials and Methods.
Ethics.
This study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals at Hokkaido University.
Experimental Fish and Embryos.
Wild-type loach M. anguillicaudatus was caught from a natural population in Iwamizawa, Hokkaido, Japan during the spawning season. The fish were reared as breeding stocks at Hokkaido University, Faculty of Fisheries Sciences. The collection of gametes and fertilization of eggs were performed as previously described (20).
dnd Knockdown to Induce Germ Cell-Deficient Loach.
The dnd-specific morpholino antisense oligonucleotide (dnd-MO, 5′-GATCTGCTCCTTCCATTGCGTTTGC-3′) including start codon was designed by and obtained from Gene Tools. Control MO was also obtained from Gene Tools. To determine optimum dosages of dnd-MO to induce germ cell-deficient fish, 250–500, 500–1,000, 1,000–2,000, and 2,000–4,000 pg of the dnd-MO diluted with 0.2 M KCl were injected at the central part of the blastodisc at one- to early two-cell–stage embryos. Control embryos were injected with 1,000–2,000 pg of control MO diluted with 0.2 M KCl.
After the injection treatment, germ cell deficiency was confirmed by a histological analysis to search for PGCs at the genital ridge in hatching fry and also by examination of the gonads of adult dnd morphants in each dosage group.
After hatching, fry were kept at 20–23 °C in 2-L containers at a density of 20–30 fish for 3 mo and, thereafter, were transferred to a 40-L tank. They were fed with brine shrimp and commercial food. Uninjected wild-type loaches were also kept under the same conditions.
Sexing of Fish on the Basis of Secondary Sexual Characteristics and Gonadal Appearance.
The sex of individual fish was established by screening for the presence or absence of a pectoral fin ray and analyses of the gonads. In loach, the male can be distinguished by the formation of a bony plate at the base of the dorsal segment of the second pectoral fin ray (21). To make the bony plate visible, the pectoral fin was removed from the body and fixed with 10% formalin solution. The fin was then cleared by immersion in 2% KOH for 2–4 h, and the bones were subsequently stained with 0.1% Alizarin red S in 2% KOH for 2 h. After staining, the fin was washed with 1% KOH for 1–2 h and then dehydrated in 50% ethanol for 2 h. The specimen was cleared and stored by transfer to 100% glycerol plus 0.025% thymol.
Histology.
Larvae at 5 dpf, young fish at 33–150 dph and undergoing sex differentiation, and 2-y-old adults were fixed overnight in Bouin's fixative. Fixed specimens were stored in 80% ethanol. The gonads of adult fish were dissected under a stereoscopic microscope and then the gonads and the stored young fish were dehydrated and infiltrated with and embedded in resin (Technovit 7100) or paraffin. Resin sections were cut at 2 μm and stained with hematoxylin and alcoholic eosin. Paraffin sections were cut at 6 μm and stained with hematoxylin and aqueous eosin.
Whole Mount in Situ Hybridization.
The migration of PGCs during embryogenesis was examined in dnd-MO injected embryos by detection of vasa mRNA using whole mount in situ hybridization. Embryos, at the embryonic shield stage, 2–4 somite, 20 somite, 30 somite, and 5 dpf, were fixed with 4% paraformaldehyde in PBS for 30 h and stored in 100% methanol at −20 °C. vasa expression was detected with an antisense vasa RNA probe labeled with digoxigenin; the probes were designed using ≈0.56-kb fragments from the 3′-UTR region of vasa cDNA in zebrafish (22). These probes were generated using an in vitro transcription kit according to the manufacturer's instructions (Roche). Hybridization, washing, and detection steps in whole mount in situ hybridization were carried out as previously described (23). Distribution pattern of vasa-positive cells at each stage was categorized as “normal,” “normal and ectopic,” and “ectopic.” Normal position of vasa-positive cells was in accordance with our previous study (23).
Supplementary Material
Acknowledgments
We thank Mr. Sotozaki (Loach Farming Cooperation, Iwamizawa, Hokkaido, Japan) for the supply of loach samples. This study was supported by a Grant-in-Aid for the 21st Century Center of Excellence program (K-02, 2006–2008) for Graduate School of Fisheries Sciences of Hokkaido University from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and that for Promotion of Basic Research Activities for Innovative Biosciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the DDBJ database [accession nos. AB531493 (vasa), AB531494 (dead end), AB531495 (dmrt1), AB531496 (p450arom), and AB531497 (foxl2)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007032107/-/DCSupplemental.
References
- 1.Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. [Google Scholar]
- 2.Baroiller JF, D'Cotta H, Saillant E. Environmental effects on fish sex determination and differentiation. Sex Dev. 2009;3:118–135. doi: 10.1159/000223077. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi H. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull Fac Fish Hokkaido Univ. 1977;28:57–65. [Google Scholar]
- 4.Matsuda M, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda M. Sex determination in the teleost medaka, Oryzias latipes. Annu Rev Genet. 2005;39:293–307. doi: 10.1146/annurev.genet.39.110304.095800. [DOI] [PubMed] [Google Scholar]
- 6.Shinomiya A, Shibata N, Sakaizumi M, Hamaguchi S. Sex reversal of genetic females (XX) induced by the transplantation of XY somatic cells in the medaka, Oryzias latipes. Int J Dev Biol. 2002;46:711–717. [PubMed] [Google Scholar]
- 7.Slanchev K, Stebler J, de la Cueva-Méndez G, Raz E. Development without germ cells: The role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA. 2005;102:4074–4079. doi: 10.1073/pnas.0407475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurokawa H, et al. Germ cells are essential for sexual dimorphism in the medaka gonad. Proc Natl Acad Sci USA. 2007;104:16958–16963. doi: 10.1073/pnas.0609932104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegfried KR, Nüsslein-Volhard C. Germ line control of female sex determination in zebrafish. Dev Biol. 2008;324:277–287. doi: 10.1016/j.ydbio.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Guigon CJ, Magre S. Contribution of germ cells to the differentiation and maturation of the ovary: Insights from models of germ cell depletion. Biol Reprod. 2006;74:450–458. doi: 10.1095/biolreprod.105.047134. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki R, Oshiro T, Nakanishi T. Survival, growth and fertility of gynogenetic diploids induced in the cyprinid loach, Misgurnus anguillicaudatus. Aquaculture. 1985;48:45–55. [Google Scholar]
- 12.Nomura T, Arai K, Hayashi T, Suzuki R. Effect of temperature on sex ratios of normal and gynogenetic diploid loach. Fish Sci. 1998;64:753–758. [Google Scholar]
- 13.Weidinger G, et al. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol. 2003;13:1429–1434. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- 14.Raz E. The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 2000;1:1017.1–1017.6. doi: 10.1186/gb-2000-1-3-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura M, Kobayashi T, Chang X, Nagahama Y. Gonadal sex differentiation in teleost fish. J Exp Zool. 1998;281:362–372. [Google Scholar]
- 16.Yamamoto K. Cyclical changes in the wall of the ovarian lumen in the mekada, Oryzias latipes. Annot Zool Jpn. 1963;36:179–186. [Google Scholar]
- 17.Nagahama Y. Molecular mechanisms of sex determination and gonadal sex differentiation in fish. Fish Physiol Biochem. 2005;31:105–109. doi: 10.1007/s10695-006-7590-2. [DOI] [PubMed] [Google Scholar]
- 18.Ijiri S, et al. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol Reprod. 2008;78:333–341. doi: 10.1095/biolreprod.107.064246. [DOI] [PubMed] [Google Scholar]
- 19.Wang DS, et al. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol Endocrinol. 2007;21:712–725. doi: 10.1210/me.2006-0248. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto T, et al. Embryonic stages from cleavage to gastrula in the loach Misgurnus anguillicaudatus. Zoolog Sci. 2004;21:747–755. doi: 10.2108/zsj.21.747. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda H. On the sexual dimorphism and taxonomical status of some Japanese loaches. Zool Mag. 1936;48:983–994. [Google Scholar]
- 22.Yoon C, Kawakami K, Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto T, et al. Developmental stages and germ cell lineage of the loach (Misgurnus anguillicaudatus) Zoolog Sci. 2006;23:977–989. doi: 10.2108/zsj.23.977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.