Abstract
Understanding cooperation is a central challenge in biology, because natural selection should favor “free-loaders” that reap benefits without reciprocating. For interspecific cooperation (mutualism), most approaches to this paradox focus on costs and benefits of individual partners and the strategies mutualists use to associate with beneficial partners. However, natural selection acts on lifetime fitness, and most mutualists, particularly longer-lived species interacting with shorter-lived partners (e.g., corals and zooxanthellae, tropical trees and mycorrhizae) interact with multiple partner species throughout ontogeny. Determining how multiple partnerships might interactively affect lifetime fitness is a crucial unexplored link in understanding the evolution and maintenance of cooperation. The tropical tree Acacia drepanolobium associates with four symbiotic ant species whose short-term individual effects range from mutualistic to parasitic. Using a long-term dataset, we show that tree fitness is enhanced by partnering sequentially with sets of different ant symbionts over the ontogeny of a tree. These sets include a “sterilization parasite” that prevents reproduction and another that reduces tree survivorship. Trees associating with partner sets that include these “parasites” enhance lifetime fitness by trading off survivorship and fecundity at different life stages. Our results demonstrate the importance of evaluating mutualism within a community context and suggest that lifespan inequalities among mutualists may help cooperation persist in the face of exploitation.
Keywords: Acacia drepanolobium, cooperation, plant defense, life history theory, ant-plant
Cooperative partnerships between species (mutualisms) are among the most widespread (1) and economically important (2) species interactions. Equally widespread are species that exploit these partnerships: rhizobia that use plant sugars but fail to fix nitrogen (3), cleaner fish that consume tissue but ignore ectoparasites (4), and caterpillars eat the broods of their ant defenders (5). Because natural selection should favor such free-loaders if they can reap benefits without reciprocating, the persistence of mutualisms is a central puzzle in biology (6).
Most theoretical studies of mutualism evolution have focused on strategies for deterring or excluding exploiters while rewarding good partners (e.g., refs. 6–8). These approaches generally calculate the costs and benefits of interacting with a given partner species independent of an individual's life stage and its interactions with other partner species. In nature, however, mutualists often occur within species-rich networks (9), and longer-lived species often interact with a variety of shorter-lived partners at different stages of their lives (10–12). However, we know little about how such successive interactions might cumulatively and nonadditively influence the lifetime fitness of long-lived mutualists (13, 14). Considering such ontogenetic variability may enhance our general understanding of how species interactions evolve (15) and, for mutualisms, how cooperation persists. Because so much of the world's biodiversity (e.g., coral-reef and tropical-forest communities) and agricultural production (many plants and their root symbionts) rests on mutualisms (16, 17), understanding the dynamics of these relationships is of practical significance as well.
Our study focuses on a long-lived (>100 y) obligate ant plant, Acacia drepanolobium, and four specialized ant symbionts. In most ant–plant mutualisms, multiple ant species compete for housing and/or food provided by host plants in exchange for protecting those plants from herbivores, pathogens, or encroaching vegetation (18). In A. drepanolobium, as in many other ant–plant systems, the quality of services provided by individual ant associates is variable: Some species appear to exploit plants by taking up residence while providing little or no protection (19–21), and others sterilize their hosts (22–25).
Acacia drepanolobium is widely distributed throughout East Africa. Plants provide housing (swollen-thorn domatia) and food (extrafloral nectar) for resident ants. At our site in central Kenya, ants compete in a dominance hierarchy (Crematogaster sjostedti > Crematogaster mimosae > Crematogaster nigriceps > Tetraponera penzigi) for exclusive occupancy of host trees (26). Tradeoffs among ant species in colonization and competitive ability help maintain coexistence in this guild (27) and produce a stereotypical succession of ant occupants as trees age (28). Transitions between ant species on individual host plants are frequent, occurring on 8–10% of trees per year (26). Each ant species differs in the short-term benefits it provides and costs it imposes upon its host (Table 1). Notably, C. mimosae and C. nigriceps aggressively defend host plants from herbivores, whereas T. penzigi and C. sjostedti are moderately and weakly aggressive toward herbivores, respectively (29). Finally, both C. sjostedti and C. nigriceps appear to be “parasites” within this mutualist network: C. sjostedti actively facilitates attack on host plants by cerambycid beetles and is associated with high host-plant mortality (20), whereas C. nigriceps sterilizes host plants while in residence by destroying floral meristems throughout the canopy (23).
Table 1.
Variation among ant species in benefits provided to and costs imposed on A. drepanolobium host plants
| Ant species | Dominancerank* | Colonization rank† | Avg. no. trees per colony (± SEM) | Host plant defense‡ | Percent shoots browsed§ | Sterilization of host plant? | Extrafloral nectar use¶ | Beetle damage** |
| Cs | 1 | †† | 22.0 (4.8) | Low 1.3 (0.3) | 8.0 (1.1) | No | Low 0.1 (0.1) | 6.6 (1.3) |
| Cm | 2 | 3 | 4.4 (0.3) | High 17.6 (1.7) | 3.3 (0.4) | No | High 2.0 (0.4) | 1.4 (1.0) |
| Cn | 3 | 2 | 2.5 (0.2) | High 15.0 (1.5) | 2.5 (0.7) | Yes | High 3.8 (0.4) | 0.44 (1.2) |
| Tp | 4 | 1 | 1.3 (0.3) | Medium 5.4 (0.7) | 3.8 (0.6) | No | None | 4.5 (1.1) |
Note that the most competitively dominant ant species appears to be a relatively ineffective host-tree defender. Cs, C. sjostedti; Cm, C. mimosae; Cn, C. nigriceps; Tp, T. penzigi.
*Dominance ranks for interspecific competition among mature colonies for nest sites, taken from ref. 26.
†Colonization ability ranks taken from ref. 27.
‡Numerical data shown are mean number of workers recruiting in response to simulated disturbance (± SEM), from ref. 29.
§Percentage of total shoots (± SEM) with mammalian browsing damage from randomly selected size-matched trees [ANOVA (F3,92 = 10.6; P < 0.0001)], from ref. 29.
¶Numerical data shown are mean number of workers tending nectaries (± SEM) for 50 scans of different host plants occupied by each species. Note that T. penzigi does not use extrafloral nectar, because this species destroys all host-plant nectaries.
**Number of new cerambycid beetle scars (± SEM) accumulating on host plants over an 18-mo period, from ref. 20.
††C. sjostedti colonies do not appear to colonize new host plants via aerial dispersal.
To determine how successive interactions with multiple ant partners cumulatively determine lifetime plant fitness, we monitored annual survival, growth, reproduction, and ant occupancy of 1,750 Acacia drepanolobium (0.1–6.5 m in height) over 8 y. Using this long-term dataset, we constructed demographic models of Acacia growth, reproduction, and survival as functions of tree size, ant identity, and size-specific ant-transition probabilities. Specifically, we asked: (i) Does the inclusion of putative “free-loader” ant species within the mutualist network weaken the lifetime benefits of ant association relative to what they would be if these ants were not present? (ii) Are the fitness benefits to the longer-lived mutualist (trees) of interacting with a particular short-lived partner (one ant species) independent of interactions with other short-lived partner species?
Results and Discussion
Contrasting Effects of Different Ant Partners on Survival and Reproduction of Trees.
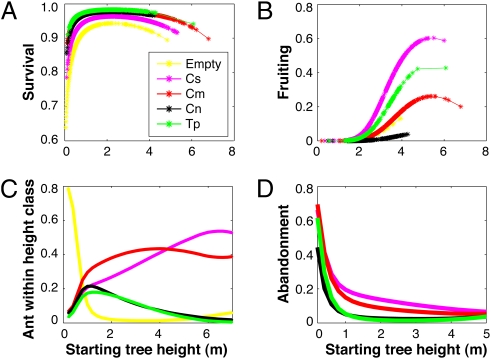
Our demographic models revealed that none of the four symbiotic ant species is a “perfect” partner, with different species having contrasting effects on Acacia survival and reproduction throughout ontogeny (Fig. 1). We first considered each ant species independently, finding that occupancy by any ant species increased survival of acacias of all sizes relative to plants that lacked ants (Fig. 1A), and, with the exception of the sterilizing symbiont C. nigriceps, the same was true of fruiting (Fig. 1B). However, the ranking of ants’ effects on survival vs. reproduction differed, with T. penzigi and C. nigriceps producing highest survival rates and C. sjostedti producing the greatest fruiting frequencies.
Fig. 1.
(A) Best-fit probabilities for survival of different-sized Acacia trees occupied by each of the four ant symbionts or unoccupied. (B) Best-fit fruiting probabilities for different-sized Acacia trees occupied by colonies of each of the four ant symbionts; (C) Best-fit probabilities of occupation by different ant species for different-sized Acacia trees. Cs = C. sjostedti, Cm = C. mimosae, Cn = C. nigriceps, Tp = T. penzigi. (D) Best-fit probabilities for abandonment of different-sized Acacia trees occupied by colonies of each of the four ant symbionts.
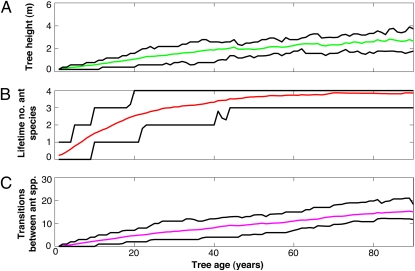
As mentioned above, individual host trees associate with multiple ant species and typically undergo many transitions between ant partners during ontogeny (Fig. 2). Importantly, the probabilities of specific ant transitions depended on both tree size and the identity of the current ant occupant. As trees grew, the most probable ant partner changed in a predictable, quasi-successional way, from strongly colonizing species with small colonies (T. penzigi and C. nigriceps) to competitively dominant species with larger colonies (C. mimosae and C. sjostedti) (Fig. 1C and Table 1) (see also refs. 26 and 28). Thus, by the time a tree reached an age of 54 y, it had a 90% chance of having partnered with three of the four ant species, and a >50% chance of having partnered with all four species (Fig. 2B). At that age, 90% of trees can expect to have had six or more transitions in ant occupancy (median, 10 transitions; Fig. 2C).
Fig. 2.
Results from 10,000 stochastic simulations showing the 10th, 50th (median), and 90th percentiles for (A) height of surviving trees at different ages; (B) the total number of unique ant species (of four) ever occupying trees for surviving trees of different ages; and (C) the total number of transitions between different ant species on surviving trees at different ages. Results are shown up to 90 y, encompassing the usual range of ages for A. drepanolobium. A 1-y-old seedling has an average future life expectancy of 19 y and a 5% chance of reaching 40 y of age. A small but established plant (age 10 y) has a total life expectancy of 34 y and a 5% chance of reaching age 75 y.
Our models assumed that ant occupants influence the demographic traits (e.g., growth, fruiting) of acacias rather than responding to preexisting differences in quality between trees. We used two approaches to validate this assumption. First, we experimentally switched the identities of ant occupants on host plants and then measured growth of these plants over 18 mo relative to control plants where resident-ant identity was not changed. This experiment demonstrated that different ant species exerted strong and contrasting effects on tree growth rate that were consistent with the correlations we observed between ant occupancy and plant growth in our 8-y demographic study (SI Text and Table S1). Second, we conducted three retrospective analyses of the 8-y demographic data. First, we added a variable (“tree growth over the previous annual transition”) to each of our 15 multinomial logistic models for ant-transition probabilities (Table S2) to see if past growth rates (an indicator of vigor) influenced ant transitions; in no case did this new variable improve model fit (SI Text). Second, we evaluated the causal direction of the strong correlation between C. sjostedti occupation and host-plant fruiting. Adding “fruiting (yes/no) in prior year” to our models for ant-transition probabilities did not increase their predictive power, nor did it improve the fit of a subset of models predicting only the takeover of large trees by C. sjostedti (SI Text). Finally, we used logistic regression to establish that occupation by C. sjostedti in prior years strongly predicted fruiting probability in future years (SI Text). Collectively, these results demonstrate that occupancy by different ant species strongly and differentially drives plant demographic traits, rather than vice versa (Table S2 and Materials and Methods for details).
Lifetime Acacia Fitness Benefits of Associating with Sets of All Four Ant Species (Including Putative Free-Loaders).
To determine how key components of Acacia fitness (survival and reproduction) were influenced by the contrasting effects of each ant species, we used our 8-y dataset to simulate A. drepanolobium demography in the presence of all four ant associates and then again with the simulated removal of one-or-more ant species. For these “reduced-community models,” the tree size- and ant-specific probabilities of transition to the ant species being removed were proportionally reallocated to the remaining possible occupancy states. In SI Text we discuss the realism of this assumption and explore alternative models that yielded similar results.
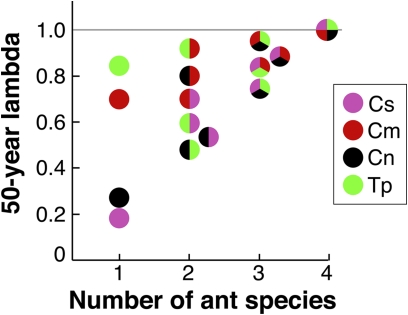
Our most surprising finding is that acacias sequentially associating with partner sets that included both the sterilizing C. nigriceps and the poorly defending C. sjostedti had higher expected lifetime fitness than did acacias partnering with any single ant species—even when that single species was C. mimosae, the one member of this guild that is both a very aggressive defender and a nonsterilizer. Acacia fitness was highest when trees partnered with all four ant species over their lifetimes and typically declined with the removal of one, two, or three ant species (Fig. 3). Our results show that the lifetime fitness of A. drepanolobium cannot be evaluated by summing the independent effects of associations with different ant partner species: Rather, fitness is a complex function of the duration, contrasting benefits and costs, and ontogenetic timing of a plant's interactions with each partner ant species. Likewise, the net benefits to trees of interactions with a given ant species depend in part on the suite of other ant species with which the plant interacts (e.g., ref. 30).
Fig. 3.
Long-term Acacia population growth rates (λ50) for simulated communities consisting of one, two, three, or four ant species. Species abbreviations are as in Fig. 1.
Dependence of Tree Fitness on the Timing and Duration of Association with Different Ant Partners.
Although the four ant species differ markedly in their defensive aggression (Table 1), the longer-term survival benefits to plants also depended on the timing and year-to-year consistency of occupation by each ant partner. In total absence of ants, host-plant survival was very low at all sizes (whether a small sapling or a larger abandoned plant) (Fig. 1A). Although C. nigriceps sterilizes host plants and T. penzigi is only moderately aggressive, these species conferred high survival rates to host plants (Fig. 1A), in part because of their high year-to-year reliability. Both these rapidly colonizing species tend to occupy younger trees (Fig. 1C and Table 1), for which survival elasticities are highest (Fig. S1), and they rarely abandon their hosts (Fig. 1D), probably because their colonies are restricted to only one or a few host plants (Table 1). In contrast, small acacias with little nesting space are less desirable for the competitively dominant C. sjostedti and C. mimosae (31), both of which are highly polydomous, with single colonies typically occupying multiple host trees (Table 1). Although these two species will colonize and occupy smaller host plants, they more frequently abandon both small and large trees (Fig. 1D).
Although the poor survivorship of trees occupied by the poorly defending C. sjostedti is consistent with observations from prior studies (20), the relatively low lifetime fitness of host trees in simulated communities with only C. mimosae (the seemingly “best” mutualist) was unexpected. Although C. mimosae confers strong antiherbivore protection to its host trees, the resulting survival benefits are offset by this species’ propensity to abandon plants over longer time scales. These results emphasize that the fitness value of particular mutualists is conditioned by the temporal and/or spatial reliability of the services they confer, as noted for other mutualisms (32–36).
The effects of different ant species on lifetime Acacia reproductive output depended on the timing of their association with the plant. Predictably, reproductive elasticities were low for small plants and increased with plant height (Fig. S1). Although the sterilizing C. nigriceps has a direct negative impact on Acacia reproduction, it confers high survival and tends to occupy host plants early in their ontogeny, when reproduction is less important to fitness than survival. Thus, C. nigriceps exerted positive effects on overall Acacia fitness, provided that nonsterilizing ant species were available to colonize plants at later life stages (Fig. 3).
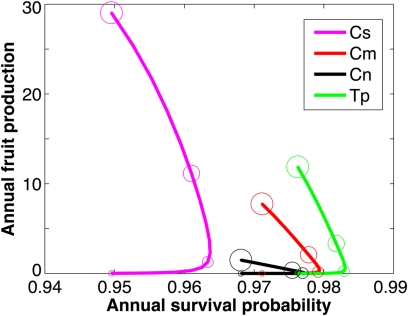
Conversely, an ant species that increases plant mortality may not necessarily diminish plant fitness if colonization occurs primarily during later life stages that have low survival elasticities. For example, although C. sjostedti negatively affects plant condition and survival (Table 1 and ref. 20), its presence was correlated with substantially increased reproduction (Figs. 1 and 4), possibly because of a tolerance response (37, 38) by plants to the high levels of herbivore and beetle damage associated with C. sjostedti occupation (e.g., ref. 39). (Because large trees occupied by C. sjostedti produced fewer, smaller swollen thorns and fewer active nectaries than similar-sized trees occupied by C. mimosae, C. sjostedti trees might have more energy available for reproduction.) We note that the reproductive benefits of C. sjostedti did not appear to hinge on the tempo of seedling recruitment into the host-tree population. Although most of our analyses assumed time-invariant recruitment probabilities, our results are robust even when we assumed episodic recruitment at intervals of 5, 10, or 20 y (Materials and Methods).
Fig. 4.
Predicted annual survivorship vs. fruit production for acacias occupied by each of the four ant species across different plant sizes. Increased bubble sizes around points on the line indicate increases in plant size in 1-m increments from 1 m (smallest bubbles) to 4 m (largest bubbles). Species abbreviations are as in Fig. 1.
Surprisingly, the single ant species predicted to have the strongest positive effects on host fitness is T. penzigi, which is an only moderately effective defender. This effect resulted from the high year-to-year reliability of T. penzigi combined with moderately high fruiting success of larger trees occupied by this species. Mature trees occupied by T. penzigi may have more resources to allocate to fruiting because they do not produce nectar (40).
Dependence of Ant Effects on a Host Plant's Association with Other Ant Species.
The dynamics of the ant–plant mutualism cannot be understood fully by comparing the costs and benefits of pairwise associations between the different partners. Rather, it is necessary to consider the entire guild of ant symbionts and their cumulative net effects on lifetime host fitness. Although both the sterilizing C. nigriceps and the weakly defending C. sjostedti were predicted to have strong negative impacts on host-plant fitness in the absence of other ants, each generally increased lifetime plant reproductive output as a member of a mutualist set that included other ant species (Fig. 3). This increase occurred because long-lived host plants can accrue complementary benefits from the different ant partners at different stages of their ontogeny. The more rapidly colonizing C. nigriceps and T. penzigi conferred strong survivorship, especially to vulnerable, small host plants, because of their high interannual reliability, whereas acacias occupied by the late-successional C. sjostedti invested heavily in reproduction, offsetting the low survivorship associated with occupation by this nondefending ant partner (Fig. 4).
Conclusions
The Importance of Integrating Multiple Partner Effects.
Our data and analyses show that the effects of ant symbionts in an intensively studied ant–plant mutualism can be understood meaningfully only by considering the timing, duration, and sequence of a plant's lifetime interactions with the entire set of its associated ants. Multiple partnerships are a common feature of mutualism, and other studies describe ontogenetic succession of different partners (e.g., refs. 41–43). It has long been known that characterizing the net effects of interactions in multispecies communities entails understanding the direct and indirect effects of those species on one another (44, 45). However, natural selection acts on lifetime fitness, and the fitness of long-lived mutualists is determined by the temporally integrated effects of multiple partners. Thus, even knowing the net effects of the entire community of ants on A. drepanolobium over limited time scales is insufficient to describe this mutualism.
Likewise, the effects of individual ant species on plant fitness are conditioned by a plant's prior and future interactions with other ant species; two identical guilds of ant partners can have vastly different effects on plant fitness if they differ in the timing, sequence, or duration of their association with the plant. Integrating the effects of multiple partners is necessary to establish how natural selection shapes the life-history strategies of species embedded within mutualist networks.
Sterilization “Parasites” as Mutualists.
Our work further demonstrates that some “parasites” are actually beneficial partners when considered within the broader context of the mutualist assemblage. In our system, as in others (22, 46), ant species identified as sterilizing “parasites” nonetheless can confer valuable protective benefits to host plants, often occupy host plants earlier in ontogeny, and are likely to be replaced by nonsterilizing ants as plants grow older. We show that when followed by nonsterilizing species later in ontogeny, these putative parasites can confer complementary benefits to plants. In other mutualisms, parasites have been shown to strengthen the relationship between mutualistic species (47, 48); in contrast, our results suggest that, in some cases, the persistent language of “mutualists” vs. “free-loaders” or “cheaters” may be misleading.
Effects of Lifespan Inequalities Within Mutualisms.
Temporal mismatches in the lifespan of interacting mutualist partners may play a critical and currently unappreciated role in the maintenance of interspecific cooperation in nature, potentially increasing the persistence of mutualist networks where short-term partner quality is variable. Exploiters of mutualisms often display r-selected life-history strategies, such as strong early-colonization ability (49, 50). Where mutualists have a long lifespan relative to their partners, longevity itself may serve to hedge bets against a lifetime association with a weak partner.
More broadly, these results suggest that demographic methods and concepts can help establish the conditions under which long-lived mutualists tolerate or even benefit from apparently antagonistic partners and also explain how the existence of multiple partners might drive the evolution of life histories in mutualistic species. First, demographic models suggest that greater longevity generally should be favored by unpredictable environments (51, 52). For mutualists, variation in partner quality is effectively variation in the environment. Thus, interacting with multiple partners of varying quality might favor increased longevity in mutualist species. Second, demographic-sensitivity analyses can predict the relative importance of different demographic rates through ontogeny (53–55), providing a framework for understanding the age- or size-specific costs and benefits of different partners. Finally, stochastic demographic models show that negative correlations between different demographic performance measures through time can ameliorate the negative effects of environmental variability or even increase fitness (56), suggesting how sets of partners that generate such negative correlations in mutualists’ vital rates might yield higher fitness than any single, seemingly optimal, partner. Such approaches and reasoning have been used recently to address the ontogeny of plant defense (15).
Implications for Mutualism Stability.
Efforts to explain the apparent paradox of mutualism stability have described a range of strategies to enforce or incentivize good partner behavior, such as partner choice, host sanctions, and partner-fidelity feedback (57). Such stabilizing mechanisms are not necessary to explain the stability of this mutualism; instead, tradeoffs between survival and reproduction over the multidecadal lifespan of the longer-lived partner enable cooperation to persist when the short-term effects of ant partners range from cooperative to antagonistic.
Materials and Methods
Natural History of Acacia drepanolobium and Its Ant Mutualists.
This study was conducted at the Mpala Research Centre (0°20′ N, 36°53′ E) on the Laikipia Plateau, Kenya. Rainfall is variable, averaging 550 mm/y. Our study site is underlain by heavy clay vertisols dominated by A. drepanolobium (mature individuals 1.5–7 m tall, >95% of woody cover).
Acacia drepanolobium’s population size structure is L-shaped, implying healthy recruitment (28). A pair of straight, sharp spines is produced at each node. Approximately 5–10% of nodes house ants (in domatia ≤5 cm diameter), which feed partly from nectaries at the leaf bases (58). Virtually all trees >1 m tall have a single resident ant colony, although a single colony may occupy multiple trees (31). A wide range of herbivores feed on A. drepanolobium, including elephants, giraffes, and other large mammals, along with many species of insect (29).
Long-Term Survey of A. drepanolobium and Acacia–Ant Dynamics.
In 1998 we established five permanently marked 200-m × 30-m belt transects. Along these transects we marked >1,750 A. drepanolobium, stratifying our sampling by host-plant size (five initial height classes: 0–0.50 m, 0.51–1.00 m, 1.01–1.5 m, 1.51–3.00 m, and >3.00 m) and ant occupant (five occupancy states: occupied by one of the four ant species or empty). Each tree was permanently tagged and scored for ant occupant and height (to the nearest 5 cm). We surveyed each tree annually from 1998 to 2007, recording vertical growth (to the nearest 1 cm), mortality, ant occupant, and number of fruits per tree.
Modeling Acacia drepanolobium Demography.
Using height, survival, reproduction (2004–2007 only), and ant-occupancy data on 1,750 trees recorded annually for some or all years between 2000 and 2007, we fit a series of statistical models for mean tree growth, variance in tree growth, and fruit number if fruiting (general linear models); fruiting and survival probabilities (logistic regressions); and transitions in ant occupancy (ordinal logistic regressions). Five to 15 models were fit for each dependent variable (Table S2). Results of these analyses supported a set of predictive models for each Acacia demographic rate, with ant species having well-supported effects on all demographic rates (Fig. 1 and Fig. S2). Similarly, transition probabilities between ant species were influenced by both current ant occupant and tree height (Fig S3).
We then used the best-supported model [determined using Akaike's information criterion (AIC)] for each demographic rate to construct a density-independent demographic model (first-order Markov chain) to describe Acacia growth, survival, reproduction, and ant-occupancy transitions as functions of tree size and ant occupancy state (Table S4). We used these regression models to estimate the demographic rates for trees in 35 height classes (0.2–7 m, in 0.2-m increments) times five ant states, for 175 combined categories. We created a final category for the seedlings, using data on trees <0.2 m from all years of our study to generate the initial frequencies of ant occupancy for the youngest and smallest size class (probabilities of 0.7637, 0.0673, 0.0810, 0.0563, and 0.0316, for empty, T. penzigi, C. nigriceps, C. m, and C. sjostedti occupancy, respectively). The result was a deterministic demographic model for A. drepanolobium with 176 stage classes.
The one parameter in this model for which we have no field estimate is survival from seed to seedling establishment, which we set to 0.101 to yield replacement-level average tree fitness (λ = 1) for the full ant community model. Thus, all our fitness measures (dominant eigenvalues for different demographic models) are relative to that of the current Acacia population, which we assume to be stable. We tested the sensitivity of our results to this assumption by using an alternative value for seed to seedling establishment derived from a separate 3-y empirical study (59) and found no change in the ranking of fitness for different ant communities.
We performed standard demographic analyses on our basic model, in which the ant community inhabiting and influencing trees includes all four species. To test the effects of different combinations of ant mutualists, we constructed modified reduced-community models with one or more of the four ants removed. Reduced-community models used all the estimated effects of the remaining ant species on Acacia performance (growth, reproduction, and survival) and on probabilities of transitioning from each ant species to all others. However, eliminating one or more ant species required us to modify the transitions between the remaining ant occupants to account for the missing probabilities of transitions to the now-missing ant species. To do so, we assumed that the probability of moving from any state (ant species × height stage) to states involving excluded ants should be reassigned to the remaining, still-possible transitions (that is, involving ants in the hypothetical reduced community) in proportion to the originally estimated values for each transition probability. This assumption allows for the fact that many of the effects that result in ant abandonment of host (e.g., reduced host quality resulting from herbivore damage or overgrowth of saplings by grasses) are likely to occur in a density-independent manner, but alternative methods for modeling reduced communities produced qualitatively similar results (SI Text, “Analyses of Long-Term Transect Data”). Having created each modified matrix, we estimated λ and the frequency of ant states for the remaining stages. As is common practice (55), we interpret λ as a measure of lifetime fitness for acacias and focus on the effects of ant communities on this measure of plant performance.
Supplementary Material
Acknowledgments
We thank J. Lemboi, S. Akwam, and R. Eraguy for assistance and the Government of Kenya for permission to conduct this research (MOEST 13/001/36C483). This study was funded by National Science Foundation Grants DEB-0444071 (to T.M.P., M.L.S., and T.P.Y.), DEB-0934734 (to T.M.P. and D.F.D.), and OISE-0852961 (to R.M.P.) and by the University of Florida.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006872107/-/DCSupplemental.
References
- 1.Stachowicz JJ. Mutualism, facilitation, and the structure of ecological communities. Bioscience. 2001;51:235–246. [Google Scholar]
- 2.Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 3.Kiers ET, Rousseau RA, West SA, Denison RF. Host sanctions and the legume-rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. [DOI] [PubMed] [Google Scholar]
- 4.Cote IM. Evolution and ecology of cleaning symbioses in the sea. Oceanography and Marine Biology: An Annual Review. 2000;38:311–355. [Google Scholar]
- 5.Thomas JA, Wardlaw JC. The capacity of a Mymica ant nest to support a predacious species of Maculinea butterfly. Oecologia. 1992;91:101–109. doi: 10.1007/BF00317247. [DOI] [PubMed] [Google Scholar]
- 6.West SA, Griffin AS, Gardner A. Evolutionary explanations for cooperation. Curr Biol. 2007;17:R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 8.Noë R, Hammerstein P. Biological markets: Supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav Ecol Sociobiol. 1994;35:1–11. [Google Scholar]
- 9.Bascompte J. Disentangling the web of life. Science. 2009;325:416–419. doi: 10.1126/science.1170749. [DOI] [PubMed] [Google Scholar]
- 10.Husband R, Herre EA, Turner SL, Gallery R, Young JPW. Molecular diversity of arbuscular mycorrhizal fungi and patterns of host association over time and space in a tropical forest. Mol Ecol. 2002;11:2669–2678. doi: 10.1046/j.1365-294x.2002.01647.x. [DOI] [PubMed] [Google Scholar]
- 11.Horvitz CC, Schemske DW. Spatiotemporal variation in insect mutualists of a neotropical herb. Ecology. 1990;71:1085–1097. [Google Scholar]
- 12.Dejean A, Djieto-Lordon C, Cereghino R, Leponce M. Ontogenetic succession and the ant mosaic: An empirical approach using pioneer trees. Basic Appl Ecol. 2008;9:316–323. [Google Scholar]
- 13.Stanton ML. Interacting guilds: Moving beyond the pairwise perspective on mutualisms. Am Nat. 2003;162(4, Suppl):S10–S23. doi: 10.1086/378646. [DOI] [PubMed] [Google Scholar]
- 14.Bronstein JL, Alarcón R, Geber M. The evolution of plant-insect mutualisms. New Phytol. 2006;172:412–428. doi: 10.1111/j.1469-8137.2006.01864.x. [DOI] [PubMed] [Google Scholar]
- 15.Boege K, Marquis RJ. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol Evol. 2005;20:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecol Lett. 2008;11:1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 17.Bascompte J. Mutualistic networks. Front Ecol Environ. 2009;7:429–436. [Google Scholar]
- 18.Davidson DW, McKey D. The evolutionary ecology of symbiotic ant - plant relationships. J Hymenopt Res. 1993;2:13–83. [Google Scholar]
- 19.Frederickson ME. Ant species confer different partner benefits on two neotropical myrmecophytes. Oecologia. 2005;143:387–395. doi: 10.1007/s00442-004-1817-7. [DOI] [PubMed] [Google Scholar]
- 20.Palmer TM, et al. Breakdown of an ant-plant mutualism follows the loss of large herbivores from an African savanna. Science. 2008;319:192–195. doi: 10.1126/science.1151579. [DOI] [PubMed] [Google Scholar]
- 21.McKey D. Interactions of the ant-plant Leonardoxa africana (Caesalpiniaceae) with its obligate inhabitants in a rainforest in Cameroon. Biotropica. 1984;16:81–99. [Google Scholar]
- 22.Izzo TJ, Vasconcelos HL. Cheating the cheater: Domatia loss minimizes the effects of ant castration in an Amazonian ant-plant. Oecologia. 2002;133:200–205. doi: 10.1007/s00442-002-1027-0. [DOI] [PubMed] [Google Scholar]
- 23.Stanton ML, Palmer TM, Young TP, Evans A, Turner ML. Sterilization and canopy modification of a swollen thorn acacia tree by a plant-ant. Nature. 1999;401:578–581. [Google Scholar]
- 24.Yu DW, Pierce NE. A castration parasite of an ant-plant mutualism. Proc R Soc Lond B Biol Sci. 1998;265:275–282. [Google Scholar]
- 25.Janzen DH. Pseudomyrmex nigripilosa: A parasite of a mutualism. Science. 1975;188:936–937. doi: 10.1126/science.188.4191.936. [DOI] [PubMed] [Google Scholar]
- 26.Palmer TM, Young TP, Stanton ML, Wenk E. Short-term dynamics of an acacia ant community in Laikipia, Kenya. Oecologia. 2000;123:425–435. doi: 10.1007/s004420051030. [DOI] [PubMed] [Google Scholar]
- 27.Stanton ML, Palmer TM, Young TP. Competition-colonization trade-offs in a guild of African Acacia-ants. Ecol Monogr. 2002;72:347–363. [Google Scholar]
- 28.Young TP, Stubblefield CH, Isbell LA. Ants on swollen-thorn acacias: Species coexistence in a simple system. Oecologia. 1997;109:98–107. doi: 10.1007/s004420050063. [DOI] [PubMed] [Google Scholar]
- 29.Palmer TM, Brody AK. Mutualism as reciprocal exploitation: Ant guards defend foliar but not reproductive structures of an African ant-plant. Ecology. 2007;88:3004–3011. doi: 10.1890/07-0133.1. [DOI] [PubMed] [Google Scholar]
- 30.Morris WF, et al. Direct and interactive effects of enemies and mutualists on plant performance: A meta-analysis. Ecology. 2007;88:1021–1029. doi: 10.1890/06-0442. [DOI] [PubMed] [Google Scholar]
- 31.Palmer TM. Wars of attrition: Colony size determines competitive outcomes in a guild of African Acacia-ants. Anim Behav. 2004;68:993–1004. [Google Scholar]
- 32.Thompson JN. The Coevolutionary Process. Chicago: Univ of Chicago Press; 1994. [Google Scholar]
- 33.Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- 34.Horvitz CC, Schemske DW. Spatiotemporal variation in demographic transitions of a tropical understory herb: Projection matrix analysis. Ecol Monogr. 1995;65:155–192. [Google Scholar]
- 35.Palmer TM, Stanton ML, Young TP. Competition and coexistence: Exploring mechanisms that restrict and maintain diversity within mutualist guilds. Am Nat. 2003;162(4, Suppl):S63–S79. doi: 10.1086/378682. [DOI] [PubMed] [Google Scholar]
- 36.Herrera CM. Variation in mutualisms: The spatio-temporal mosaic of a pollinator assemblage. Biol J Linn Soc Lond. 1988;35:95–125. [Google Scholar]
- 37.Stowe KA, Marquis RJ, Hochwender CG, Simms EL. The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst. 2000;31:565–595. [Google Scholar]
- 38.Edwards DP. The roles of tolerance in the evolution, maintenance and breakdown of mutualism. Naturwissenschaften. 2009;96:1137–1145. doi: 10.1007/s00114-009-0559-0. [DOI] [PubMed] [Google Scholar]
- 39.Kozlowski TT, Pallardy SG. Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev. 2002;68:270–334. [Google Scholar]
- 40.Palmer TM, Young TP, Stanton ML. Burning bridges: Priority effects and the persistence of a competitively subordinate acacia-ant in Laikipia, Kenya. Oecologia. 2002;133:372–379. doi: 10.1007/s00442-002-1026-1. [DOI] [PubMed] [Google Scholar]
- 41.Fonseca CR, Benson WW. Ontogenetic succession in Amazonian ant trees. Oikos. 2003;102:407–412. [Google Scholar]
- 42.Feldhaar H, Fiala B, Hashim RB, Maschwitz U. Patterns of the Crematogaster-Macaranga association: The ant partner makes the difference. Insectes Soc. 2003;50:9–19. [Google Scholar]
- 43.Djieto-Lordon C, Dejean A, Gibernau M, Hossaert-McKey M, McKey D. Symbiotic mutualism with a community of opportunistic ants: Protection, competition, and ant occupancy of the myrmecophyte Barteria nigritana (Passifloraceae) Acta Oecol Int J Ecol. 2004;26:109–116. [Google Scholar]
- 44.Wilbur HM. Competition, predation and the structure of the Ambystoma-Rana sylvatica community. Ecology. 1972;53:3–21. [Google Scholar]
- 45.Wootton JT. Indirect effects and habitat use in an intertidal community: Interaction chains and interaction modifications. Am Nat. 1993;141:71–89. [Google Scholar]
- 46.Frederickson ME, Gordon DM. The intertwined population biology of two Amazonian myrmecophytes and their symbiotic ants. Ecology. 2009;90:1595–1607. doi: 10.1890/08-0010.1. [DOI] [PubMed] [Google Scholar]
- 47.Little AEF, Currie CR. Parasites may help stabilize cooperative relationships. BMC Evol Biol. 2009;9:124–132. doi: 10.1186/1471-2148-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn DW, et al. A role for parasites in stabilising the fig-pollinator mutualism. PLoS Biol. 2008;6:e59. doi: 10.1371/journal.pbio.0060059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clement LW, Köppen SCW, Brand WA, Heil M. Strategies of a parasite of the ant-Acacia mutualism. Behav Ecol Sociobiol. 2008;62:953–962. doi: 10.1007/s00265-007-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stat M, Morris E, Gates RD. Functional diversity in coral-dinoflagellate symbiosis. Proc Natl Acad Sci USA. 2008;105:9256–9261. doi: 10.1073/pnas.0801328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orzack SH, Tuljapurkar S. Population dynamics in variable environments 7: The demography and evolution of iteroparity. Am Nat. 1989;133:901–923. [Google Scholar]
- 52.Cole LC. The population consequences of life history phenomena. Q Rev Biol. 1954;29:103–137. doi: 10.1086/400074. [DOI] [PubMed] [Google Scholar]
- 53.Enright NJ, Franco M, Silvertown J. Comparing plant life-histories using elasticity analysis: The importance of life span and the number of life-cycle stages. Oecologia. 1995;104:79–84. doi: 10.1007/BF00365565. [DOI] [PubMed] [Google Scholar]
- 54.Benton TG, Grant A. Elasticity analysis as an important tool in evolutionary and population ecology. Trends Ecol Evol. 1999;14:467–471. doi: 10.1016/s0169-5347(99)01724-3. [DOI] [PubMed] [Google Scholar]
- 55.Caswell H. Matrix Population Models: Construction, Analysis and Interpretation. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 56.Doak DF, Morris WF, Pfister C, Kendall BE, Bruna EM. Correctly estimating how environmental stochasticity influences fitness and population growth. Am Nat. 2005;166:E14–E21. doi: 10.1086/430642. [DOI] [PubMed] [Google Scholar]
- 57.Foster KR, Wenseleers T. A general model for the evolution of mutualisms. J Evol Biol. 2006;19:1283–1293. doi: 10.1111/j.1420-9101.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 58.Hocking B. Insect associations with the swollen thorn acacias. Transactions of the Royal Entomological Society of London. 1970;122:211–255. [Google Scholar]
- 59.Goheen JR, Palmer TM, Keesing F, Riginos C, Young TP. Large herbivores facilitate savanna tree establishment via diverse and indirect pathways. J Anim Ecol. 2010;79:372–382. doi: 10.1111/j.1365-2656.2009.01644.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.