Abstract
The pancreatic secretagogue cholecystokinin (CCK) is widely thought to stimulate enzyme secretion by acinar cells indirectly via activation of the vagus nerve. We postulate an alternative pathway for CCK-induced pancreatic secretion. We hypothesize that neurally related pancreatic stellate cells (PSCs; located in close proximity to the basolateral aspect of acinar cells) play a regulatory role in pancreatic secretion by serving as an intermediate target for CCK and secreting the neurotransmitter acetylcholine (ACh), which, in turn, stimulates acinar enzyme secretion. To determine whether PSCs (i) exhibit CCK-dependent ACh secretion and (ii) influence acinar enzyme secretion, primary cultures of human and rat PSCs were used. Immunoblotting and/or immunofluorescence was used to detect choline acetyltransferase (ACh synthesizing enzyme), vesicular ACh transporter (VAChT), synaptophysin, and CCK receptors 1 and 2. Synaptic-like vesicles in PSCs were identified by EM. ACh secretion by PSCs exposed to 20 pM CCK was measured by LC-MS/MS. Amylase secretion by acini [pretreated with and without the muscarinic receptor antagonist atropine (10 μM) and cocultured with PSCs] was measured by colorimetry. PSCs express ACh synthesizing enzyme, VAChT, synaptophysin, and CCK receptors; exhibit CCK-dependent ACh secretion; and stimulate amylase secretion by acini, which is blocked by atropine. In conclusion, PSCs express the essential elements for ACh synthesis and secretion. CCK stimulates ACh secretion by PSCs, which, in turn, induces amylase secretion by acini. Therefore, PSCs may represent a previously unrecognized intrapancreatic pathway regulating CCK-induced pancreatic exocrine secretion.
Keywords: acinar cells, cholecystokinin, pancreas
The pancreas is the major source of the body's digestive enzymes (1). The functional unit of the exocrine pancreas that produces these enzymes is the acinus, composed of individual acinar cells surrounding a central lumen. Acinar cells are capable of synthesizing and secreting as many as 10 million protein (enzyme) molecules per day. The process of enzyme synthesis and secretion at the acinar cell level is highly regulated and involves enzyme synthesis on the rough endoplasmic reticulum, posttranslational changes in the Golgi apparatus, and packaging into zymogen granules. Enzyme secretion is accomplished by the movement of zymogen granules to the apical pole of the acinar cell, fusion of the zymogen granule membrane with the apical plasma membrane followed by discharge of zymogen granule contents into the lumen, a process termed exocytosis (2).
Postprandial secretion of pancreatic enzymes is predominantly mediated by regulatory peptides such as cholecystokinin (CCK) and by the vagovagal reflex, which activates cholinergic postganglionic neurons in the gland (3). CCK is known to be a major secretagogue and a trophic factor for the pancreas (4). It is synthesized in I cells of the small intestine and is released into the circulation following a meal. CCK is also present in neurons of the gastrointestinal tract and in the brain (5). Like many other gastrointestinal regulatory peptides, CCK exists in multiple isoforms ranging from 8 to 58 aa in length. The physiologic functions of CCK are mediated via two related receptors: CCK1 and CCK2 (6). These receptors have 50% homology and are coupled to the same basic intracellular signaling pathways via the activation of guanine-nucleotide binding proteins (G proteins).
Whether CCK acts directly on human pancreatic acinar cells to stimulate digestive enzyme secretion has been a matter of some debate in the literature. Previous studies had reported that, in contrast to rodent acinar cells, human acinar cells lacked functional CCK receptors (7–9). However, these findings have been recently challenged by Murphy et al. (10), who have demonstrated that CCK, at physiological concentrations, directly stimulates amylase release by isolated human pancreatic acinar cells. In contrast to the debate over the direct effects of CCK on acinar cells, there is wider acceptance of the concept that CCK-mediated postprandial digestive enzyme secretion occurs at least in part via an indirect pathway, whereby CCK stimulates receptors on vagal afferent fibers, leading to signals being transmitted via vagal efferent fibers to intrapancreatic neurons. These in turn, release neurotransmitters such as acetylcholine (ACh), which act on muscarinic receptors on acinar cells and stimulate digestive enzyme secretion.
One resident cell type in the pancreas that has commanded considerable attention during the past decade is the pancreatic stellate cell (PSC), located in close proximity to the basolateral aspect of acinar cells (11). In health, PSCs are in their quiescent phenotype and contain abundant lipid droplets in their cytoplasm. Interestingly, PSCs express both mesenchymal markers such as desmin and α-smooth muscle actin (α-SMA) and neuroectodermal markers such as GFAP, nestin, NGF, BDNF, and neurotrophin 45, raising questions about their embryonic origin (i.e., mesenchymal vs. neuroectodermal). Functions that would be expected of a mesenchymal cell are now well established for PSCs, such as an ability to synthesize and secrete ECM proteins as well as matrix degrading enzymes (12). PSCs play a central role in maintenance of regular ECM turnover during health, and during pancreatic injury they are responsible for the deposition of excessive fibrous tissue leading to the prominent fibrosis seen in diseases such as chronic pancreatitis and pancreatic cancer. However, the functions of PSCs from the perspective of a neural origin have not yet been examined. This study hypothesizes that PSCs play a regulatory role in exocrine pancreatic secretion by serving as an intermediate target for CCK. It is postulated that PSCs respond to CCK by secreting the neurotransmitter ACh, which acts on muscarinic receptors on acinar cells to stimulate enzyme release. Therefore, the aims of this study were to examine (i) whether PSCs secrete ACh, and if so, whether this ACh secretion is stimulated by CCK; and (ii) whether PSCs influence enzyme secretion by acini.
Results
PSCs Express Choline Acetyltransferase, Vesicular ACh Transporter, Synaptophysin, and Synaptic-Like Vesicles.
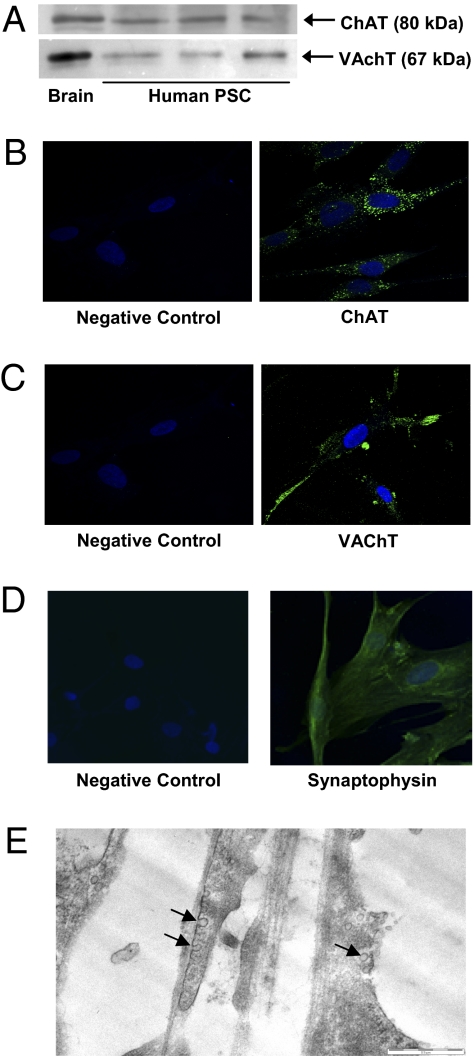
The synthesis and secretion of ACh requires certain cellular systems, including the ACh synthesizing enzyme choline acetyltransferase (ChAT) (13), vesicular ACh transporter (VAChT; the protein responsible for transport of ACh into the synaptic vesicles), synaptic-like vesicles, and proteins integral to synaptic vesicles such as synaptophysin (a synaptic vesicle membrane protein responsible for docking of synaptic vesicles to the cell membrane). Western blotting of human PSC lysates demonstrated the presence of both ACh synthesizing enzyme and VAChT (Fig. 1A) corresponding to the molecular weight of the bands observed in the positive control (brain homogenate). Abundant expression of ChAT and VAChT was found in the cytoplasm of human PSCs by immunofluorescence and confocal microscopy (Fig. 1 B and C). Negative controls incubated with the same concentration of isotype control did not display any signal. Synaptophysin expression was detected in human PSCs by immunofluorescence (Fig. 1D). Interestingly, EM identified the presence of synaptic-like vesicles in cultured human PSCs (Fig. 1E). The vesicles had translucent cores and were approximately 50 nm in size, both characteristic features of synaptic vesicles.
Fig. 1.
Expression of ACh synthesizing systems by culture-activated human PSCs. (A) Representative Western blots for ChAT and VAChT in human PSC lysates (50 μg and 100 μg of total protein was loaded onto the gel for ChAT and VAChT, respectively). The positive control was rat brain homogenate. (B) A representative immunofluorescent confocal micrograph of human PSCs demonstrates strong positive staining for ChAT in the cytoplasm, whereas the negative control (rabbit IgG) showed no staining (magnification 600×). (C) A representative immunofluorescent confocal micrograph of human PSCs demonstrated strong positive staining for VAChT in the cytoplasm, whereas the negative control (rabbit IgG) showed no staining (magnification 600×). (D) A representative immunofluorescent confocal micrograph of human PSCs demonstrates strong positive staining for synaptophysin in the cytoplasm, whereas the negative control (rabbit IgG) showed no staining (magnification 600×). (E) A representative electron micrograph of cultured PSCs shows synaptic-like vesicles (arrows) on a cytoplasmic extension. (Scale bar, 0.5 mm.)
PSCs Secrete ACh.
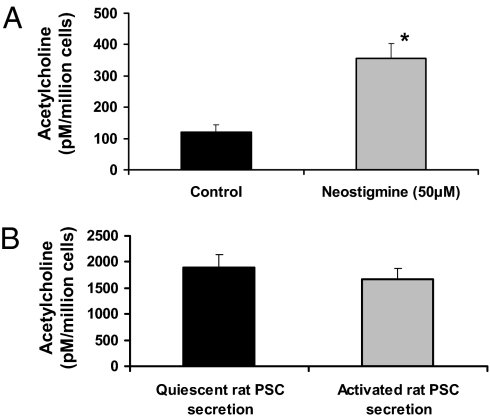
To determine whether PSCs could synthesize and secrete ACh, LC-MS/MS was used. Stable isotope 16H2-labeled ACh (parent ion m/z, 162) and internal standard coeluted with PSC extracted ACh (parent ion m/z, 146) and was observed at an LC retention time of 7.7 min (Fig. S1A). Quantification was achieved using peak area ratios of collision-induced decay (CID) fragment ions m/z of 94.2 and 87.2, respectively, and reference to an ACh calibration curve (0–400 fmol/10 μL on column concentration range; Fig. S1B). Culture-activated human PSCs secreted ACh (Fig. 2A). In the presence of the ACh esterase inhibitor neostigmine, ACh secretion was significantly increased [Fig. 2A; mean ACh concentrations ± SE: control, 120.3 ± 22.7 pM/million cells; neostigmine, 355.4 ± 47.0 pM/million cells (P < 0.03); n = 4 separate PSC preparations]. This suggests that PSCs also synthesize ACh esterase. To confirm that the observed ACh production by human PSCs was not dependent on an activated phenotype, ACh secretion by quiescent and activated rat PSCs (from the same cell preparation) was examined. Rat PSCs were also found to secrete ACh, albeit at levels that were higher than in human PSCs (Fig. 2B). The reason for this difference is unknown at present, but could be related to species differences. Importantly, culture activation did not significantly alter the amount of ACh secreted by rat PSCs (Fig. 2B), indicating that ACh can be secreted by quiescent PSCs and this secretion is not dependent on cell activation. From these data, the amount of ACh secreted by human PSCs can be estimated on the basis of the calculated mean basal secretion of ACh by PSCs (without neostigmine), which was 120.26 pM/million cells, equivalent to 12 μM/g PSC lysate protein (SI Experimental Procedures describes calculations).
Fig. 2.
ACh secretion by PSCs. All PSC secretions were collected after 15 min for measurement of ACh. (A) Quantitative analysis of the ACh peaks (obtained from the LC-MS/MS traces for culture activated human PSC secretions) was achieved using peak area ratios of CID fragment ions m/z of 94.2 and 87.2, respectively, and reference to an ACh calibration curve. Analysis of all traces (n = 4 separate PSC preparations) showed that ACh levels in the secretions were significantly increased (*P = 0.0234) in the presence of neostigmine (ACh esterase inhibitor, 50 μM). (B) Quantitative analysis of ACh secreted by quiescent and activated rat PSCs from the same cell preparation (n = 3 separate cell preparations). All secretions were collected in the presence of neostigmine (50 μM). Culture activation had no effect on the amount of ACh secreted by rat PSCs.
PSCs Express CCK Receptors and Respond to CCK.
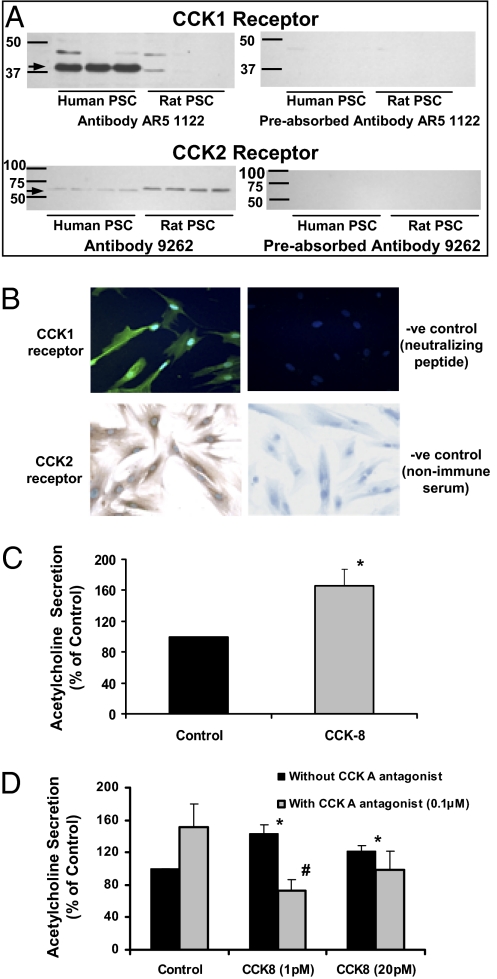
Given that our hypothesis was that PSCs may be an intermediate target for CCK-induced acinar secretion, we examined the expression of CCK receptors on PSCs using immunofluorescence and immunoblotting studies. Western blotting of human and rat PSC lysates using specific anti-CCK1 and anti-CCK2 receptor antibodies demonstrated the presence of both the CCK1 receptor (a 37-kDa band significantly more prominent in human PSCs) and the CCK2 receptor (at 60 kDa, suggestive of a variant isoform) in the cells (Fig. 3A). Furthermore, preincubation of the CCK-receptor antibodies with the appropriate neutralizing peptides resulted in the nonappearance of CCK1 and CCK2 bands, confirming the specificity of the antibodies used to detect both receptors. Immunofluorescence/immunocytochemistry of cultured PSCs confirmed expression for CCK1 and CCK2 receptors (Fig. 3B).
Fig. 3.
CCK receptor expression by PSCs. (A) Representative Western blots demonstrating the presence of CCK1 and CCK2 receptors in both human and rat PSCs. Preincubation of the CCK-receptor antibodies with the appropriate neutralizing peptides resulted in the absence (nonappearance) of the CCK1 and CCK2 receptor bands. (B) A representative immunofluorescent confocal micrograph (Upper) of human PSCs demonstrates strong positive staining for the CCK1 receptor, whereas the negative control showed no staining (magnification 600×). Lower: Representative immunostain of human PSCs demonstrates strong positive staining for the CCK2 receptor, whereas the negative control showed no staining (magnification 600×). (C) Quantitative analysis of ACh secreted by human PSCs incubated in the presence of neostigmine (50 μM) ± 20 pM CCK-8 for 15 min (n = 5 separate cell preparations; *P < 0.04). (D) ACh secreted by human PSCs incubated with two doses of CCK8 (1 and 20 pM) in the presence and absence of the CCK1 receptor antagonist (lorglumide, 0.1 μM). *P < 0.05 vs. control; #P < 0.05 vs. CCK (1 pM) without antagonist; n = 3.
Of particular relevance to this study (given that PSCs had been found to possess CCK receptors) was our observation that human PSCs respond to CCK8 stimulation by an increase in ACh secretion (Fig. 3C). Use of the CCK1 receptor antagonist inhibited CCK-induced ACh secretion by PSCs (Fig. 3D), suggesting that the CCK1 receptor may play a role in mediating CCK-induced ACh secretion by PSCs. CCK1 receptor antagonist alone in the absence of CCK increased (albeit statistically insignificantly) ACh secretion by PSCs. With regard to the CCK2 receptor antagonist (L-365260), this compound is only soluble in DMSO. We performed studies to examine the effect of the vehicle on ACh measurements. Cells incubated with DMSO alone yielded substantially increased ACh readings in the LC-MS/MS assay [mean ± SE, 160.6 ± 38.8% of control (PSCs in Hepes buffer without DMSO and CCK), n = 4]. This result made it difficult to demonstrate an induction of ACh release in the presence of 20 pM CCK8 [mean ACh secretion ± SE, 113.0 ± 19.1% of control (PSCs in Hepes buffer plus DMSO), n = 4]. The confounding effects of DMSO on ACh measurements meant that we could not pursue the CCK2R antagonist studies.
PSCs Stimulate Acinar Cell Secretion.
In view of our findings that PSCs secrete ACh, it was important to determine whether PSCs influence acinar secretion. As a first step, adequate functionality of isolated rat pancreatic acini was confirmed by the response of these cells to ACh. At a concentration of 100 nM, ACh stimulated amylase secretion; this ACh-induced amylase release was blocked by pretreatment of acini with atropine (a muscarinic receptor antagonist; 1 and 10 μM; Fig. S2) confirming previously published reports (14, 15).
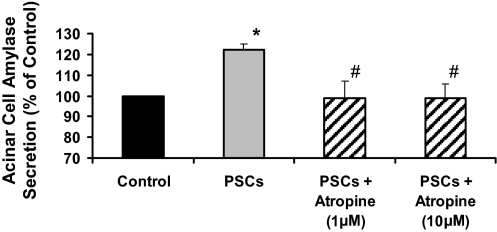
Coculture of rat acini (2 million acinar cells) with human PSCs (150,000 cells) significantly stimulated amylase secretion by acini (Fig. 4). The mean basal amylase release (as a percentage of total cellular amylase content) was 8.66 ± 1.32%. Based on our measurements of ACh secreted by 1 million PSCs in 15 min (Fig. 2), we estimate that the amount of ACh secreted by 150,000 cells into 1 mL of culture medium over a period of 30 min is at least 52.5 pM. To further strengthen the observed induction of amylase secretion by PSCs, we assessed the effect of 50 pM ACh on acinar amylase release and found that this concentration of ACh stimulated acinar amylase secretion to 142.2 ± 29.8% of control. Importantly, PSC-induced acinar secretion was blocked in the presence of the muscarinic receptor antagonist atropine, indicating that the observed stimulation of amylase secretion by acini in the presence of PSCs was a result of the ACh secreted by the latter (Fig. 4).
Fig. 4.
Amylase secretion by rat pancreatic acini is stimulated by coculture with human PSCs. Rat pancreatic acini with or without pretreatment with atropine were cocultured with human PSCs for 30 min. Acini amylase secretion was significantly stimulated (n = 5 cell preparations; *P < 0.05 PSCs vs. control) in the presence of human PSCs. PSC-induced amylase secretion was blocked in the presence of atropine (#P < 0.05, atropine vs. PSCs).
CCK Did Not Influence MAPK Activity, Cell Proliferation, or α-SMA Expression in PSCs.
Given the known trophic effects of CCK on the pancreas and the fact that PSCs express CCK receptors, the effect of CCK (at concentrations ranging from physiological to supraphysiological) on PSC functions such as proliferation and activation (as assessed by α-SMA) was examined. Furthermore, as PSC proliferation and activation are known to be regulated by the MAPK pathway, the effect of CCK on MAPK activation in PSCs was also assessed. CCK8 did not influence human PSC proliferation (Fig. S3A) or α-SMA expression (Fig. S3B). Not surprisingly, CCK did not affect MAPK activity (Fig. S3C) in human PSCs.
Discussion
This study describes a previously unknown function of PSCs relevant to their possible neuroectodermal origin. We have provided evidence that PSCs (i) possess the cellular systems required for the synthesis of ACh, including ChAT, VAChT, synaptophysin, and synaptic-like vesicles; (ii) synthesize and secrete ACh; (iii) stimulate acinar amylase secretion; (iv) express CCK receptors; and (v) respond to CCK via stimulation of ACh secretion.
The finding that PSCs can synthesize and secrete ACh is important because it suggests that, in addition to their well documented role in pancreatic fibrogenesis, these cells have the potential to play a role in normal pancreatic function by stimulating enzyme secretion from acinar cells via the production of the neurotransmitter ACh. Our observations also suggest that vagally controlled neurons within the pancreas may not be the only source of cholinergic stimulation of acinar cells. In this regard, it is interesting to note a report by Chariot and colleagues (16) demonstrating that, even after extrinsic denervation of rat pancreas (via bilateral truncal vagotomy), cholinergic mechanisms mediating basal pancreatic secretion persisted in the gland.
In PSCs, as in neurons, the synthesis of ACh is likely to involve several steps including the uptake of choline from the extracellular compartment and then formation of ACh via cytoplasmic enzymes. In support of this, the enzyme responsible for ACh synthesis (13) was identified in PSCs. In neurons, cytoplasmic ACh is then shuttled by the VAChT into vesicles from which it is released via exocytosis (17). Synaptic vesicles are vesicular carriers highly specialized for the secretion of nonpeptide neurotransmitters and, until recently, were considered neuron-specific organelles (18). Synaptic vesicles are highly homogeneous in size (50 nm in diameter) and have a translucent core when visualized by conventional EM (18).
Our results indicate the presence of abundant VAChT in PSCs and our EM studies demonstrate the presence of synaptic-like vesicles, suggesting a possible mechanism for transport of ACh into synaptic vesicles. Interestingly, Cassiman et al. (19) have also previously identified the presence of electron translucent vesicles in cytoplasmic processes of human hepatic stellate cells (counterpart cells of the liver). However, to the best of our knowledge, there are no reports in the literature on ACh release by hepatic stellate cells.
PSCs also exhibited positive expression for synaptophysin, which is an abundant protein of synaptic vesicles and is often used as a marker of these organelles (18). This result concurs with data in hepatic stellate cells (HSCs), demonstrating that quiescent as well as activated human and rat HSCs expressed synaptophysin (19). Thus, our findings with human PSCs support the concept that the release of ACh from PSCs may occur in a manner similar to the well accepted events for neurotransmitter release by neuronal cells.
The amount of ACh secreted by human PSCs, calculated to be approximately 12 μM/g PSC protein, is similar to that reported to be produced by neuronal PC12 cells, 7 μM/g protein (20), but significantly higher than that released by nonneuronal oviductal epithelial cells, 33 to 587.5 pM/g protein (21). ACh secretion by human PSCs was significantly increased in the presence of the ACh esterase inhibitor (neostigmine), providing evidence that the cells synthesize ACh esterase. This finding concurs with a recent study by Anderson et al. (22) reporting that ACh esterase was highly expressed in fibroblasts.
Traditionally, the main source of ACh has been thought to be neuronal cells. However, recent studies indicate that ACh can be synthesized (as evidenced by anti-ChAT immunoreactivity or HPLC detection of ACh) by several human nonneuronal cells including epithelial cells, endothelial cells, immune cells, mesothelial cells, and mesenchymal cells. Our findings add another cell type to this list. Neuronal contamination of PSC preparations cannot explain our findings because (i) density gradient centrifugation was used for their isolation (this method relies on the vitamin A content of PSCs, which gives the cells a certain buoyancy, and vitamin A is not present in neuronal cells) and (ii) PSCs were cultured in medium (lacking specific growth factors required for nerve cells), which would not allow survival of neuronal cells.
Our preliminary studies established that a minimum of 1 million cells was required to obtain reproducible measurements of ACh release by LC-MS/MS. As freshly isolated normal human PSCs do not achieve the required yield, PSCs were further cultured and passaged to obtain the number of cells necessary for ACh estimations. It is well known that subculture of freshly isolated PSCs on plastic leads to their activation to a myofibroblast-like phenotype. To confirm that ACh release was not only a function of activated PSCs, quiescent and activated rat PSCs (from the same cell preparation) were assessed for ACh synthesis. Our data demonstrated that both quiescent PSCs (as confirmed by the concomitant presence of lipid droplets and GFAP; Fig. S4) and activated PSCs secrete similar amounts of ACh, indicating that ACh synthesis and secretion is independent of cell activation. This lends further support to our hypothesis that PSCs may play a role in normal physiology in the pancreas.
There is evidence that CCK can act directly on pancreatic acinar cells or indirectly via receptors located on the vagus nerve to stimulate acinar cell secretion. At physiological concentrations of CCK, vagal afferent fibers are stimulated and transmit signals to efferent vagal fibers, which in turn activate intrapancreatic neurons to secrete ACh, which binds to muscarinic receptors located on acinar cells to induce secretion. In this study, we propose an additional pathway that may regulate acinar cell secretion. We have shown that coculture of rat acini with human PSCs significantly stimulated amylase secretion by acini. Our findings also demonstrate that the observed stimulation of acinar secretion by PSCs was a result of ACh secreted by PSCs, as stimulated amylase secretion was blocked by the muscarinic receptor antagonist atropine. Notably, ACh secretion by human PSCs was also increased following stimulation with physiological concentrations of CCK. These findings support our concept that ACh secreted by PSCs can influence acinar cell secretion, raising the reasonable (albeit speculative) contention that PSCs may represent a previously unrecognized intermediary cell in the CCK-regulated pancreatic secretory pathway.
The observation that PSCs possess the CCK receptors CCK1 and CCK2 explains the ability of the cells to respond to CCK. Use of the CCK1 receptor antagonist inhibited CCK-induced ACh secretion by PSCs, suggesting that the CCK1 receptor may play a role in mediating CCK-induced ACh secretion by PSCs. The observed increase in ACh levels in PSC secretions upon exposure to the CCK1 receptor antagonist but in the absence of CCK may be explained by a possible partial agonist effect of CCK receptor antagonists (23); although we acknowledge that the issue of “partial agonism” of CCK receptor antagonists remains unresolved. At this stage we cannot comment on the relative contribution of CCK2 receptor in the observed CCK-induced ACh secretion; as noted earlier, we were unable to conduct studies with the CCK2 receptor antagonist as a result of confounding effects of the vehicle on the ACh assay system.
It was particularly interesting to note that CCK did not affect PSC proliferation or α-SMA expression, or the signaling pathway (MAPK) known to regulate these functions. These findings lend strong support to our concept that nonactivated PSCs (in healthy pancreas) may play a role in physiological functions such as exocrine pancreatic secretion in response to CCK.
It is also reasonable to consider the possible implications of this function of PSCs in the setting of pancreatic injury. PSCs are known to be activated during pancreatitis and pancreatic cancer. Indeed, a bidirectional interaction has been demonstrated between PSCs and pancreatic cancer cells, which facilitates local tumor growth and distant metastases (24–26). In this regard, it is interesting to note that ACh has been shown to stimulate the growth of tumor cell lines (27, 28) and alter upstream signaling pathways that regulate numerous cell and tissue functions, by releasing growth factors, angiogenesis/metastasis factors, proinflammatory cytokines, and local neurotransmitters from cancer cells and their microenvironment (29). Thus, ACh secreted by activated PSCs could play an important role in the cross-talk between PSCs and cancer cells.
In conclusion, we described a previously unrecognized function (Fig. 5) for the PSC (a cell with a well-established role in fibrogenesis). We have provided evidence that PSCs secrete ACh, which can stimulate acinar cell amylase secretion. However, the relative contribution of ACh versus CCK on acinar cell enzyme secretion is difficult to answer in an in vitro isolated acinar cell system, because physiological exocrine secretion in vivo is likely regulated by several factors including neural pathways and other cell types. Furthermore, rat pancreatic acini (used in the present study) are well known to have functional CCK1 and 2 receptors and to be highly responsive to CCK. Future studies to delineate this issue could include (i) the use of isolated acini from CCK receptor KO animals and normal human PSCs or (ii) an in vivo model (with intact pancreatic anatomy and neurophysiology) using conditional KO animals in which CCK1 and 2 receptors are blocked specifically on pancreatic acini.
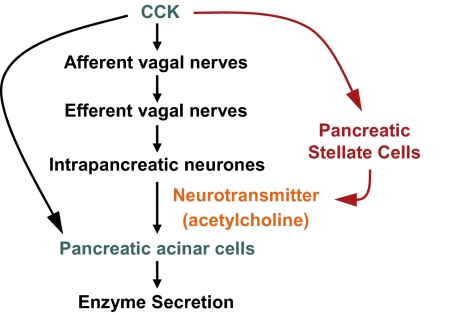
Fig. 5.
A schematic diagram depicting the pathways mediating CCK-induced digestive enzyme secretion by acinar cells. CCK stimulates acinar enzyme secretion indirectly via activation of the vagus nerve and directly via CCK receptors on acinar cells (black arrows). The present study has demonstrated that PSCs respond to CCK (via CCK1/CCK2 receptors) by increased secretion of ACh, which in turn, stimulates amylase secretion by acinar cells. Thus, PSCs may represent an intrapancreatic pathway (depicted in red) regulating CCK-induced exocrine pancreatic secretion.
Experimental Procedures
Materials.
The sources of all reagents and chemicals used in the present study are detailed in SI Experimental Procedures.
Isolation and Culture of PSCs.
Studies were approved by the animal and human research ethics committees of the University of New South Wales, Sydney, Australia. Pancreas tissue samples obtained from human subjects had informed consent. Rat and human PSCs were isolated by density gradient centrifugation using a method developed by our group, which is detailed in SI Experimental Procedures (11). This method takes advantage of the fact that PSCs store vitamin A in the cytoplasm in the form of lipid droplets, which gives the cells a specific density. Purity of freshly isolated PSCs was assessed by dual staining of cells for the presence of PSC-selective features, namely lipid droplets in the cytoplasm and GFAP. Upon culture on plastic, activated PSCs were identified by immunostaining for α-SMA as described previously (11). All cells examined had abundant lipid droplets, ruling out the possibility of neural cell contamination in the freshly isolated PSC preparations.
Assessment of ChAT, VAChT, Synaptophysin, and CCK Receptor Expression by PSCs.
Expression of the aforementioned proteins was assessed by Western blotting and/or immunofluorescence (methodology detailed in SI Experimental Procedures).
Assessment of Synaptic-Like Vesicles by EM.
EM was used to detect the presence of synaptic-like vesicles in PSCs grown on coverslips (methodology detailed in SI Experimental Procedures).
Measurement of ACh by PSCs Using LC-MS/MS.
Collection and cleanup of PSC secretions.
We used culture-activated human PSCs as well as quiescent and activated rat PSCs from the same cell preparation to examine the secretion of ACh by PSCs. It has been previously established that PSCs demonstrate an activated phenotype when cultured on uncoated plastic in medium containing 10% FBS for 48 h (11). Therefore, for rat PSC experiments, cells in their quiescent state (within 24 h after isolation) were compared with cells in their activated state (after first passage, approximately 1 wk in culture). Subconfluent cells were then washed twice with PBS solution. Cells were equilibrated for 1 h in a Hepes buffer (116 mM NaCl, 1.8 mM CaCl2, 1 mM NaH2PO4, 0.81 mM MgCl2, 5.4 mM KCl, 25 mM Hepes, 25 mM glucose, pH 7.4). Following this, cells were incubated with 5 mL of Hepes buffer containing choline chloride (10 μM) in the presence and absence of the ACh esterase inhibitor neostigmine (50 μM) for 15 min. PSC secretions were collected and TFA (final concentration, 0.4%) was added to prevent any ACh degradation. Secretions were passed through 3-kDa cutoff membrane filtration spin cartridges (Amicon) to remove large proteins and then freeze-dried. The lyophilized secretions were resuspended in 125 μL of 0.4% TFA spun briefly to remove excess undissolved salt and the supernatant filtered through a PVDF syringe filter to remove additional particulates. Ten microliters of this concentrated secretion was then loaded directly onto the column (details provided in a subsequent section) and duplicate LC-MS/MS runs were performed as outlined later. The recovery of ACh following the sample cleanup procedure, as estimated from the pre and postcleanup standard curves, was 86.5%.
LC-MS/MS.
For highly specific and sensitive measurement of ACh, we used C18 reverse-phase LC coupled to tandem MS. Serially diluted ACh was used to construct a standard curve. All standards and samples received a uniform aliquot of isotope-labeled ACh internal standard to correct for variations in extraction efficiency. ACh was separated by liquid chromatography using an Accela pump (Thermo Scientific). Detailed methodology is provided in SI Experimental Procedures. All spectra were processed and area-integrated using XCalibur software (version 2.07). Peak area ratios of ions 87.2/94.2 were used for quantification, together with an ACh calibration curve consisting of six duplicate standards in the concentration range from 0 to 400 fmol/10 μL (on column).
Effect of CCK on ACh Secretion by PSCs.
To determine whether CCK stimulates ACh secretion by PSCs, human PSCs (1 million cells in a 100-mm Petri dish) were treated in 5 mL of Hepes buffer with or without physiological concentrations (1 or 20 pM) of CCK-8 for 15 min in the presence of neostigmine (50 μM). ACh secreted by PSCs was measured by LC-MS/MS (as described earlier). We also examined the effect of CCK receptor antagonists on CCK-induced ACh release by PSCs. Human PSCs were treated with two doses of CCK8 (1 and 20 pM) in the presence and absence of the CCK1 receptor antagonist (lorglumide 0.1 μM) or the CCK2 receptor antagonist (L-365,260 0.1 μM in 0.01% DMSO) for 15 min.
Isolation of Rat Pancreatic Acini.
Rat acini were isolated as described by Haber et al. (30) and detailed in SI Experimental Procedures.
Coculture of PSCs with Acini.
Human PSCs were cultured for 24 h before direct coculture with rat acini. Before coculture, the PSCs were washed twice with PBS solution and allowed to equilibrate for 1 h in Hepes buffer (116 mM NaCl, 1.8 mM CaCl2, 1 mM NaH2PO4, 0.81 mM MgCl2, 5.4 mM KCl, 25 mM Hepes, 25 mM glucose, pH 7.4). Coculture experiments were performed in a six-well plate containing 150,000 human PSCs. The total volume of the coculture was 1 ml. Acini were preincubated with or without atropine (1 or 10 μM) for 5 min. Acini were then washed in Hepes buffer and resuspended in Hepes buffer containing choline (10 μM) and neostigmine (50 μM) and placed into a six-well plate with the PSCs for 30 min coculture at 37 °C with gentle agitation. At the end of the incubation period, the acini and their secretions were removed from the plate and amylase was estimated using a colorimetric assay (Amylase–SL assay reagent; Diagnostic Chemicals). Amylase release was then calculated as the percentage of total cell content. For comparison between groups, we calculated the difference as percentage of control (i.e., acini without PSC coculture). Based on cell pellet size and protein content, we estimated that we had 2 million acinar cells per well for the coculture with PSCs. Thus, with 150,000 PSCs per 2 million acinar cells, PSCs constituted approximately 6.9% of the total cells. This proportion is similar to the known ratio of stellate cells to acinar cells in the healthy pancreas (31).
Effect of CCK on PSC Functions.
To assess the effect of CCK on PSC signaling, we assessed PSC proliferation and α-SMA expression (a marker of PSC activation). MAPK activation was assessed in response to CCK exposure. Human PSCs were incubated with CCK8 at a physiological dose (20 pM) and supraphysiological doses (100 pM and 500 pM) for 24 h at 37 °C. Cell proliferation was then assessed using the Dojindo Cell Counting Kit-8 per the manufacturer's instructions. For proliferation experiments, we used 2,500 PSCs per well of a 96-well plate in 100 μL of media containing 10% FBS. α-SMA expression was assessed by Western blotting, as previously described (32) and detailed in SI Experimental Procedures. MAPK activation was measured by assessment of phosphorylated ERK1/2 and p38 kinase using Western blotting of cell lysate proteins, as described previously (32) and detailed in SI Experimental Procedures.
Supplementary Material
Acknowledgments
This work was funded by Discovery Project Grant DP0772223 from the Australian Research Council and a fellowship from the Gastroenterological Society of Australia (to P.A.P., 2007--2009). P.A.P. is currently supported by a Cancer Institute NSW fellowship (2009--2012).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000359107/-/DCSupplemental.
References
- 1.Bockman DE. Anatomy of the pancreas. In: Go VLW, DiMagno EP, Gardner JD, editors. The Pancreas: Biology, Pathobiology and Disease. New York: Raven; 1993. pp. 1–8. [Google Scholar]
- 2.Williams JA, Yule DI. Stimulus-secretion coupling in pancreatic acinar cells. In: Johnson LR, et al., editors. Physiology of the Gastrointestinal Tract. 4th Ed. New York: Elsevier; 2006. [Google Scholar]
- 3.Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957–969. doi: 10.1053/j.gastro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Jensen RT, Wank SA, Rowley WH, Sato S, Gardner JD. Interaction of CCK with pancreatic acinar cells. Trends Pharmacol Sci. 1989;10:418–423. doi: 10.1016/0165-6147(89)90192-2. [DOI] [PubMed] [Google Scholar]
- 5.Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TV. The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem. 2007;7:1154–1165. doi: 10.2174/156802607780960483. [DOI] [PubMed] [Google Scholar]
- 6.Wank SA, Pisegna JR, de Weerth A. Cholecystokinin receptor family. Molecular cloning, structure, and functional expression in rat, guinea pig, and human. Ann N Y Acad Sci. 1994;713:49–66. doi: 10.1111/j.1749-6632.1994.tb44052.x. [DOI] [PubMed] [Google Scholar]
- 7.Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology. 2001;121:1380–1390. doi: 10.1053/gast.2001.29557. [DOI] [PubMed] [Google Scholar]
- 8.Morisset J, Lainé J, Biernat M, Julien S. What are the pancreatic target cells for gastrin and its CCKB receptor? Is this a couple for cancerous cells? Med Sci Monit. 2004;10:RA242–RA246. [PubMed] [Google Scholar]
- 9.Weinberg DS, et al. Cholecystokinin A and B receptors are differentially expressed in normal pancreas and pancreatic adenocarcinoma. J Clin Invest. 1997;100:597–603. doi: 10.1172/JCI119570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy JA, et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. 2008;135:632–641. doi: 10.1053/j.gastro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Apte MV, et al. Periacinar stellate shaped cells in rat pancreas: Identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips PA, et al. Rat pancreatic stellate cells secrete matrix metalloproteinases: Implications for extracellular matrix turnover. Gut. 2003;52:275–282. doi: 10.1136/gut.52.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner J, et al. Alcoholic pancreatitis in rats: Injury from nonoxidative metabolites of ethanol. Am J Physiol Gastrointest Liver Physiol. 2002;283:G65–G73. doi: 10.1152/ajpgi.00419.2001. [DOI] [PubMed] [Google Scholar]
- 14.Yago MD, et al. Effects of the type of dietary fat on acetylcholine-evoked amylase secretion and calcium mobilization in isolated rat pancreatic acinar cells. J Nutr Biochem. 2006;17:242–249. doi: 10.1016/j.jnutbio.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Yago MD, et al. Effect of sodium nitroprusside and 8-bromo cyclic GMP on nerve-mediated and acetylcholine-evoked secretory responses in the rat pancreas. Br J Pharmacol. 2002;136:49–56. doi: 10.1038/sj.bjp.0704693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chariot J, de la Tour J, Anglade P, Rozé C. Cholinergic mechanisms in the pancreas after extrinsic denervation in the rat. Am J Physiol. 1987;252:G755–G761. doi: 10.1152/ajpgi.1987.252.6.G755. [DOI] [PubMed] [Google Scholar]
- 17.Erickson JD, et al. Functional identification of a vesicular acetylcholine transporter and its expression from a “cholinergic” gene locus. J Biol Chem. 1994;269:21929–21932. [PubMed] [Google Scholar]
- 18.Thomas-Reetz AC, De Camilli P. A role for synaptic vesicles in non-neuronal cells: Clues from pancreatic beta cells and from chromaffin cells. FASEB J. 1994;8:209–216. doi: 10.1096/fasebj.8.2.7907072. [DOI] [PubMed] [Google Scholar]
- 19.Cassiman D, et al. Synaptophysin: A novel marker for human and rat hepatic stellate cells. Am J Pathol. 1999;155:1831–1839. doi: 10.1016/S0002-9440(10)65501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DK, Natarajan N, Prabhakar NR, Kumar GK. Facilitation of dopamine and acetylcholine release by intermittent hypoxia in PC12 cells: Involvement of calcium and reactive oxygen species. J Appl Physiol. 2004;96:1206–1215. doi: 10.1152/japplphysiol.00879.2003. [DOI] [PubMed] [Google Scholar]
- 21.Steffl M, et al. Non-neuronal acetylcholine and choline acetyltransferase in oviductal epithelial cells of cyclic and pregnant pigs. Anat Embryol (Berl) 2006;211:685–690. doi: 10.1007/s00429-006-0132-y. [DOI] [PubMed] [Google Scholar]
- 22.Anderson AA, et al. Morphoregulation by acetylcholinesterase in fibroblasts and astrocytes. J Cell Physiol. 2008;215:82–100. doi: 10.1002/jcp.21288. [DOI] [PubMed] [Google Scholar]
- 23.Seva C, Scemama JL, Bastié MJ, Pradayrol L, Vaysse N. Lorglumide and loxiglumide inhibit gastrin-stimulated DNA synthesis in a rat tumoral acinar pancreatic cell line (AR42J) Cancer Res. 1990;50:5829–5833. [PubMed] [Google Scholar]
- 24.Bachem MG, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Hwang RF, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vonlaufen A, et al. Pancreatic stellate cells: Partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 27.Cattaneo MG, D'atri F, Vicentini LM. Mechanisms of mitogen-activated protein kinase activation by nicotine in small-cell lung carcinoma cells. Biochem J. 1997;328:499–503. doi: 10.1042/bj3280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song P, et al. Synthesis of acetylcholine by lung cancer. Life Sci. 2003;72:2159–2168. doi: 10.1016/s0024-3205(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 29.Schuller HM. Neurotransmission and cancer: implications for prevention and therapy. Anticancer Drugs. 2008;19:655–671. doi: 10.1097/CAD.0b013e3283025b58. [DOI] [PubMed] [Google Scholar]
- 30.Haber PS, et al. Non-oxidative metabolism of ethanol by rat pancreatic acini. Pancreatology. 2004;4:82–89. doi: 10.1159/000077608. [DOI] [PubMed] [Google Scholar]
- 31.Bachem MG, Zhou Z, Zhou S, Siech M. Role of stellate cells in pancreatic fibrogenesis associated with acute and chronic pancreatitis. J Gastroenterol Hepatol. 2006;21(suppl 3):S92–S96. doi: 10.1111/j.1440-1746.2006.04592.x. [DOI] [PubMed] [Google Scholar]
- 32.McCarroll JA, et al. Pancreatic stellate cell activation by ethanol and acetaldehyde: is it mediated by the mitogen-activated protein kinase signaling pathway? Pancreas. 2003;27:150–160. doi: 10.1097/00006676-200308000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.