Abstract
Emerging evidence from neuroimaging and neuropsychology suggests that human speech comprehension engages two types of neurocognitive processes: a distributed bilateral system underpinning general perceptual and cognitive processing, viewed as neurobiologically primary, and a more specialized left hemisphere system supporting key grammatical language functions, likely to be specific to humans. To test these hypotheses directly we covaried increases in the nonlinguistic complexity of spoken words [presence or absence of an embedded stem, e.g., claim (clay)] with variations in their linguistic complexity (presence of inflectional affixes, e.g., play+ed). Nonlinguistic complexity, generated by the on-line competition between the full word and its onset-embedded stem, was found to activate both right and left fronto-temporal brain regions, including bilateral BA45 and -47. Linguistic complexity activated left-lateralized inferior frontal areas only, primarily in BA45. This contrast reflects a differentiation between the functional roles of a bilateral system, which supports the basic mapping from sound to lexical meaning, and a language-specific left-lateralized system that supports core decompositional and combinatorial processes invoked by linguistically complex inputs. These differences can be related to the neurobiological foundations of human language and underline the importance of bihemispheric systems in supporting the dynamic processing and interpretation of spoken inputs.
Keywords: brain, language, morphology, inflection
Considered as a neuroscientific phenomenon, human language comprehension—and human language function in general—is both remarkably specific and remarkably distributed in its neural instantiation. One hundred fifty years of neuropsychological research demonstrate the irreplaceable role of left hemisphere perisylvian networks (linking left inferior frontal and posterior temporal brain areas) in supporting key combinatorial language functions in the domain of inflectional morphology and syntax. At the same time, evidence from lesion studies and neuroimaging of the intact brain shows that dynamic access to lexical meaning, and the ability to rapidly construct semantic and pragmatic interpretations of incoming speech, can remain strikingly intact even when the left perisylvian language areas are destroyed, implying a distributed bihemispheric substrate. We believe that these contrasts reflect a fundamental distinction in the types of processing operations underlying normal language comprehension and in the neural networks that support these functions. In this event-related fMRI study of lexical processing, we test the hypothesis that general purpose processing demands engage both right and left perisylvian systems, whereas linguistically specific processing demands selectively engage left hemisphere systems.
A critical element of this account is its emphasis on the bihemispheric foundations of human speech communication. Functional imaging shows that bilateral activation of temporal lobe structures in and around primary auditory processing areas is not limited to low-level acoustic and phonetic analyses, but also implicates lexical processes. There is complementary evidence from neuropsychological and neuroimaging studies for the involvement of bihemispheric temporal regions in processes related to accessing lexical meaning (1). Compared with low-level acoustic baselines, spoken words activate superior and middle temporal gyri bilaterally, regions related to meaning-based processes in language comprehension. Although researchers disagree on the aspects of lexical processing supported by left and right hemispheres (e.g., ref. 2), neuropsychological data strongly suggest that both hemispheres have access to representations of lexical meaning. Patients with extensive damage to left frontal and superior temporal regions but spared right hemisphere (RH) homologs can still recognize simple spoken words and produce semantic priming effects and reaction times within the normal range, demonstrating rapid and efficient access to lexical identity and meaning from auditory inputs (3).
At the same time, superimposed on this highly effective basic bilateral system is a left-dominant perisylvian core language system, linking left inferior frontal cortex (LIFC) with temporal and parietal cortices. Damage to these regions can cause permanent disruption of language functions although damage to parallel RH regions typically does not. Particularly salient here is disruption to the combinatorial aspects of language. Patients with left hemisphere (LH) damage, especially in LIFC, have problems with syntax and inflectional morphology, both in production and in comprehension (4). Evidence from neuroimaging confirms the salience of LH contributions to language, primarily located in inferior frontal, temporal, and inferior parietal cortex (5). In terms of grammatical function, research focused around regular inflectional morphology reveals a key role for this LH fronto-temporal network in processes related to morpho-phonology and morpho-syntax. The selective deficits for regular inflectional morphology, shown by many LH nonfluent patients, point to a decompositional network linking LIFC with superior and middle temporal cortex (6). This network handles the processing of regularly inflected words (such as joined or treats), which are argued not to be stored as whole forms and instead require morpho-phonological parsing to be segmented into stems and inflectional affixes.
These observations, combining detailed psycholinguistic and neural theories, raise basic questions about the properties of the processing systems that underpin human language function. To understand what is special about the core LH fronto-temporal system, we need a better understanding both of the properties of RH systems and of the aspects of language processing that are common to both hemispheres. These considerations lead to specific questions about the processing and representational properties of the basic bilateral system hypothesized to support the access of simple spoken words. We predict that both hemispheres will be involved to a similar degree when the linguistic input consists of morphologically simple, high-frequency, high-imageability words. These types of words, in a language like English, will engage general purpose perceptual processing mechanisms that are not specifically linguistic in nature. Such mechanisms are engaged by the unfolding nature of the speech signal, where word onsets map onto multiple lexical representations. This triggers competition between these perceptual alternatives and requires additional processing to select the correct candidate. Competition and selection between multiple alternatives are common to a range of cognitive functions, from visual perception to working memory, and are associated with increased bilateral frontal activation (7).
To selectively engage these mechanisms we used words that are not linguistically complex and do not require decompositional analysis, but are nonetheless perceptually complex. These are morphologically simple words like lawn and ramp, which can be directly mapped onto underlying full-form representations, but that also have an onset-embedded stem (law/lawn, ram/ramp). The presence of this second potential candidate for lexical access creates competition between the two simultaneously activated stems. Successful choice of the correct lexical representation will therefore involve additional decision and selection processes. Previous language research indicated that selection processes require the involvement of inferior frontal regions (8), but with few exceptions (e.g., ref. 9) focused on LH effects. On the approach taken here, selection processes operate on lexical candidates whose analysis is conducted bilaterally, triggering the involvement of bilateral IFC.
When the input changes to include linguistically more complex material, we predicted a shift in fronto-temporal involvement across the hemispheres. This shift affects both the degree of left (L) temporal involvement in superior temporal and middle temporal gyri (STG/MTG) and the degree of LH fronto-temporal interaction. Here we focused on regular inflectional morphemes, such as the English past tense {-d}, which lead a spoken lexical input to interact differentially with the machinery of lexical access and linguistic interpretation (10). When a phonetic input such as /preɪd/ is encountered, corresponding to the word prayed, the listener must both access the lexical information associated with the stem {pray} and extract the processing implications of the presence of the grammatical morpheme {-d}. It is the capacity to support this segmentation into stem- and affix-based processes that is disrupted in patients with LH perisylvian damage (6). A key concept here is the notion of the inflectional rhyme pattern (IRP) as a dynamic modulator of fronto-temporal integration that indicates whether the current speech input carries inflectional information necessary for its on-line structural interpretation (11).
The IRP is a phonological pattern in English that signals that the ending of a complex word may be an inflectional affix and therefore should not be treated as part of the stem. It is defined in terms of specific phonological markers—whether the final consonant is coronal [i.e., (d, t, s, z)] and whether it agrees in voice with the preceding segment—which are shared by all regular {-d} and {-s} inflections in English. It may be present both in real inflected forms (bowled, packs), in pseudoinflected forms where a simple word has an apparent inflectional ending (mold, flax), and in nonword forms (nold, dax). Recent fMRI results suggest that the presence of the IRP has an across-the-board triggering effect on LIFC activation, correlated with L temporal lobe and L anterior cingulate activation (11). Consistent with this, patients with LIFC damage show marked disruptions in performance when confronted with IRP stimuli, again independent of lexical status (12). Behavioral studies with unimpaired young adults (13) show increased processing complexity associated with the presence of the IRP for real words and nonwords and for both major types of regular inflection in English (the past tense -d and plural -s).
These results indicate a dynamic interaction between stem-access processes mediated bilaterally by temporal lobe structures and predominantly left frontal systems critical for morpho-phonological and syntactic parsing of the input stream (6, 12). To probe these interactions we covaried the two sources of processing complexity described above: whether a word ends in the IRP, invoking specifically linguistic complexity, and whether it has an embedded stem, modulating general processing complexity. Simple words like dream, exhibiting neither type of complexity, contrast both with words like claim, which have an embedded stem (clay) but no IRP, and with words like trend, which end in a potential suffix, but have no embedded stem. Intermediate cases like played and trade exhibit both forms of complexity—each ends in a potential inflectional affix, and each has a potential embedded stem (play and tray). Variations in the degree of stem competition should modulate activation bilaterally, with greater competition increasing the likelihood of IFC involvement. In contrast, presence or absence of the IRP should primarily modulate LH activation, especially where fronto-temporal links are concerned.
Because this approach predicts that LH networks, especially LIFC, should be sensitive to both of these types of processing complexity, it raises the question of whether the activations elicited will be spatio-temporally distinct or overlapping. Issues of functional specialization in LIFC are widely debated and allow for several possible outcomes: If linguistic computations (tapped into by the IRP conditions) are domain and region specific, they may generate activity in more dorsal LIFC (BA44/45), whereas domain-general computations, elicited by stem competition, should engage more orbito-frontal areas, including BA47 (14, 15). Alternatively, on a network-based account (16), the same LIFC areas may support both linguistic and nonlinguistic processing functions, but do so in the context of different distributed processing networks.
Finally, to be able to isolate the lexical processing network from lower-level auditory processing, we compared word processing against a baseline that unambiguously separates complex auditory processing from specifically acoustic–phonetic analyses. Correct choice of baseline is critical for a convincing demonstration of RH lexical processing, where inappropriate baselines lead either to false negatives or to false positives. Our baseline for speech sounds was “musical rain” (MuR) as described in ref. 17. MuR was chosen over acoustic baselines such as spectrally rotated, noise-vocoded or reversed speech because it provides a principled basis for claiming that it closely tracks the acoustic properties of speech, while at the same time not being interpretable as speech. The temporal envelope of each MuR segment was extracted from the corresponding speech segment, and the two were matched in terms of the long-term spectro-temporal distribution of energy and root mean square (RMS) level. Nevertheless, despite the similarities in the distribution of energy over frequency and time, the absence of continuous formants in the signal means that MuR is not interpretable as being the output of a human vocal tract and is not heard as speechlike. MuR produces a similar level of neural activation to that of vowels in the auditory pathway up to and including primary auditory cortex in Heschl's gyrus and planum temporale (17). In secondary auditory regions, however, such as anterior superior temporal cortex, it produces much less activation than the corresponding speech.
In the context of this strict auditory baseline, we can evaluate directly the hemispheric distribution of contrasting lexical processing systems, using a set of stimuli that covary stem competition and suffix decomposition demands (Table 1). Words in the regular past tense condition (e.g., prayed) were all morphologically complex, consisting of stems and suffixes. These words should trigger a decomposition process on the basis of the presence of the suffix. Although they have a phonologically embedded stem, this may not trigger strong lexical competition because prayed is not thought to be separately lexically represented (6). The second condition consisted of monomorphemic words with a real word embedded at the onset and an IRP ending, e.g., trade. Words from this condition can be processed as tray+ed, thus triggering both stem competition and suffix decomposition. The third condition consisted of monomorphemic words with an IRP ending but without an onset embedded word, e.g., blend. Here, the presence of the IRP ending can generate a decomposition process, but there is no basis for stem competition because the remaining set of phonemes (e.g., blen) does not form a real word. Words in the fourth condition were monomorphemic, with an embedded stem but no IRP ending, e.g., claim. These words should trigger stem competition because of the embedded stem (e.g., clay). The fifth condition consisted of monomorphemic words with neither embedded stems nor IRPs, e.g., dream. These are simple words that do not exhibit either linguistic or perceptual processing complexity, placing least demands on both bilateral and L perisylvian processing systems.
Table 1.
Experimental conditions and behavioral results in milliseconds
| Experimental condition | Example | Embedded stem | Suffix (IRP) | RT (SD)* |
| 1. Regular past tense | Prayed | ? (pray) | Y | 970 (90) |
| 2. Pseudoregulars | Trade | Y (tray) | Y | 942 (65) |
| 3. No stem, with IRP | Blend | N | Y | 955 (68) |
| 4. Stem only | Claim | Y (clay) | N | 967 (92) |
| 5. Simple | Dream | N | N | 924 (71) |
N, no; Y, yes.
*Behavioral results, reaction time.
Results
Behavioral Data.
Participants performed a gap detection task (18). All errors (1.5%) and time-outs [reaction time (RT) >3,000 ms, 0.1%] were removed, and data were inverse transformed. Mean reaction times and SD for the test conditions (“no” responses in the gap detection task) are shown in Table 1.
A univariate ANCOVA was performed on the inverse-transformed RTs, with condition as a fixed factor and speech file duration as a covariate. The results showed a significant difference in RTs between conditions [F1(4, 44) = 11.6, P < 0.01; F2(4, 194) = 2.6, P < 0.05]. Effects of IRP presence and embeddedness were tested by grouping conditions according to presence of IRP and embedded stems and comparing them against the RTs for simple words. Simple words (e.g., dream) were responded to significantly faster than words with IRPs (prayed, trade, blend) [F1(3, 33) = 10.5, P < 0.01; F2(1, 159) = 6.9, P < 0.01], or embedded stems (trade, claim) [F1(2, 22) = 18.2, P < 0.01; F2(1, 119) = 5.7, P < 0.05]. There was no significant difference between RTs for the words with IRPs and embedded stems [F1 (3, 33) = 5.2, P < 0.01; F2(3, 159) = 0.83, P > 0.1]. These results indicate a general increase in lexical processing load for the complex items, consistent with earlier studies using gap detection (18).
Imaging Data.
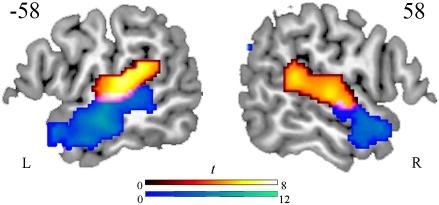
On the basis of previous evidence (1, 15) and our predictions, we focused on bilateral fronto-temporo–parietal regions as the volume of interest for the analyses. Using WFU Pickatlas, a mask was constructed, consisting of bilateral temporal lobes (superior, middle and inferior temporal gyri, and angular gyrus), inferior frontal gyri (pars orbitalis, pars opercularis, pars triangularis, and precentral gyrus), insula, and the anterior cingulate. We first established the network that supports complex acoustic processing by subtracting null events from MuR. This contrast showed bilateral activity in primary auditory cortices and surrounding superior temporal regions (BA42/22, peaks at −66 −30 14 and 68 −34 14), consistent with previous results (5, 17) (Fig. 1 and SI Appendix, Table S1). Laterality analyses showed generally bilateral activation, with only medial temporal activation stronger in LH compared with RH. To conduct these laterality analyses, EPI images were normalized onto a symmetrical T1 template, and the standard first-level analysis was performed. The resulting maps were flipped along the y axis and compared with the original maps in a series of t tests (19).
Fig. 1.
Significant activation for complex acoustic processing (orange) and linguistic processing (blue) rendered onto the surface of a canonical brain. Threshold at P < 0.001 voxel level and P < 0.05 cluster level corrected for multiple comparisons is shown.
To extract the activity specifically related to speech-driven lexical processing we contrasted words with the MuR baseline. This comparison showed that lexical processes activated regions that are anterior and ventro-lateral to the activity observed for lower-lever auditory processing (Fig. 1). These lexical activations were in bilateral middle temporal gyri (BA21) extending into fusiform and hippocampal regions (BA20/37), angular gyrus (BA39), and anterior cingulate (BA32), as well as L inferior frontal regions on the lower statistical threshold of 0.01. Despite the significant RH activity, these activations had a strong LH bias: The laterality analyses showed that lexical activity (words minus MuR) was significantly stronger in LIFC (BA44/45/47) and left angular gyrus (BA39), whereas no region showed significantly more RH lexical activation (SI Appendix, Table S2).
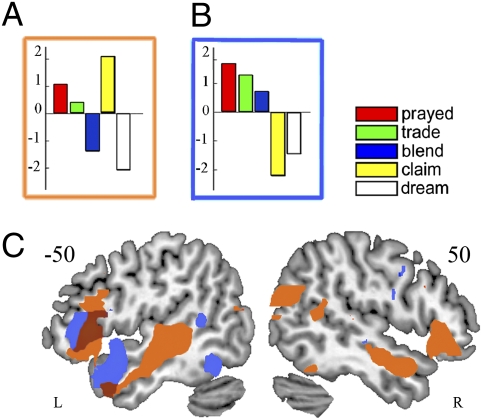
Turning to our key questions concerning patterns of fronto-temporal activation as a function of different types of lexical processing complexity, and their hemispheric distribution, we first conducted exploratory analyses using the multivariate linear approach implemented in the MLM toolbox (20). These analyses classified conditions on the basis of the patterns of activation they evoke in the fronto-temporal volume of interest (relative to the MuR baseline) and calculated orthogonal eigencomponents that show greatest difference between conditions. Two eigencomponents speak directly to the critical contrasts in this study (details in SI Appendix). The first of these (Fig. 2A) dissociates conditions with and without an embedded stem, with the divergence between claim, trade, and prayed (each with actual or potential embedded stems) versus blend and dream (no embedded stems). Overlaid on the brain (Fig. 2C) this eigencomponent shows a bilateral fronto-temporal distribution with IFC activation focused in BA47 (pars orbitalis) and extending dorsally into BA45 (pars triangularis). In separate analyses on RIFC and LIFC regions of interest (ROIs), the same dominant pattern emerges, accounting for 77% of the variance in RIFC and 64% of the variance in LIFC. The second eigencomponent of interest (Fig. 2B) dissociates conditions with the IRP ending (prayed, trade, blend) from the conditions without it (claim, dream). Overlaid on the brain, this eigencomponent was primarily left lateralized (Fig. 2C), with activity concentrated in BA45/44 but not BA47. In analyses carried out on separate LIFC and RIFC ROIs, this pattern is not seen at all for the RIFC, but is clearly present in the LIFC. It is noteworthy that the trade condition, which combines stem competition with the presence of an IRP, contributes differentially to each of the corresponding multivariate components (Fig. 2 A and B).
Fig. 2.
Selected MLM eigencomponents and their overlay. Bar graphs are in the unit of the beta images. (A) Component dissociating embedded from nonembedded words; (B) component dissociating IRP from non-IRP words; (C) overlay on a canonical brain of the embeddedness (orange) and IRP (blue) components, shown at t > 1, with the overlap between them in purple.
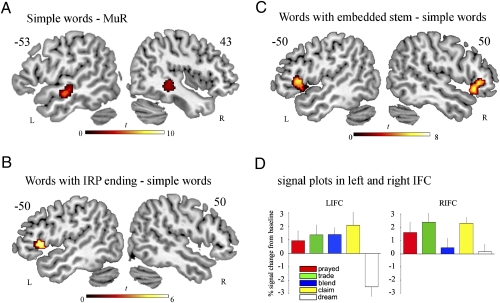
These exploratory analyses are consistent with the hypothesis that dissociable processing systems, differing in their hemispheric distribution, underlie different aspects of language processing function. Next, two sets of conventional GLM analyses were performed to pin down the effects of different types of complex internal structure on lexical processing. We first delineated the network that supports the processing of simple words. A comparison of simple words (dream) against MuR showed activation in bilateral middle temporal gyri (peak at −60 −16 −6 on the left and more medially at 38 −40 −8 on the right, SI Appendix, Table S3a, and Fig. 3A). Compared with simple words, words with IRP endings (prayed, trade, blend) produced a single cluster of significant activation in LIFC BA45 (peak at −48 18 4, SI Appendix, Table S3b, and Fig. 3A). Viewed at a lower statistical threshold (SI Appendix, Table S3b and Fig. S3a), activation can be seen to extend into the L temporal pole and the L STG. In contrast, words with embedded stems (trade, claim) compared with simple words produced significant activation in bilateral IFC, BA 45/47 (peaks at −44 22 2 and 54 30 0, SI Appendix, Table S3c and Fig. 3C). At a lower threshold, activity extends to bilateral STG/MTG regions (SI Appendix, Table S3c and Fig. S3b). The two sets of activation overlap in LBA45, where the signal intensity plot (Fig. 3D) does not show any dissociation between complexity due to the presence of embedded stems or IRPs. RIFC, on the other hand, clearly dissociates between the two types of complex words: It shows increased activation for words with embedded stems, but not for words with IRPs but no embedded stem.
Fig. 3.
Significant activation for (A) simple words minus the MuR baseline, (B) words with IRP endings minus simple words, and (C) words with embedded stems minus simple words. Threshold at P < 0.001 voxel level and P < 0.05 cluster level corrected for multiple comparisons is shown. (D) Signal plots in 5-mm-radius spheres around the peaks of the L and R frontal clusters from C.
In a separate parametric analysis, we tested directly whether bilateral frontal regions show modulation in activity as a function of the degree of lexical competition between the carrier word and its embedded stem. Lexical competition between the two words was defined as a ratio of their frequencies and entered as a parametric modulator with linear expansion for each item. Consistent with the results obtained from comparisons between conditions with and without stem competition, there was significant modulation of activity in both L- and RIFC, focused in BA47 and the insula in both hemispheres (peaks at −40 14 −2 and 46 6 −4, SI Appendix, Table S4).
Discussion
This experiment tested a set of hypotheses about the basic architecture of the human speech comprehension system, focusing on processing activities within a theoretically defined set of fronto-temporal brain regions. We proposed, in particular, that speech comprehension is supported by two interdependent but separable systems with distinct functional roles: a bihemispheric fronto-temporal system, supporting sound-to-meaning mapping and general perceptual processing demands (as well as supporting processes of pragmatic inference), and a more specialized LH perisylvian system, required to support the core morpho-syntactic functions unique to human language.
The results confirm these hypotheses. First, we provide unambiguous evidence for the bilateral foundations of the network that underpins speech comprehension. Compared with a well-matched acoustic baseline, spoken words produced bilateral activation in middle temporal lobes, angular gyrus, and anterior cingulate. However, consistent with the well-established LH dominance for aspects of language function, we saw stronger LH lexical activation in inferior frontal and angular gyri (BA 44/45/47, BA39).
Second, our results showed a functional dissociation between the components of this complex fronto-temporal network. Compared with the MuR baseline, simple words (e.g., dream) showed an essentially bilateral pattern of middle temporal activation. When general processing complexity increased due to the presence of embedded stems (e.g., activation in the claim condition compared with simple words), the activation pattern shifted anteriorly, to inferior frontal regions. Crucially, this frontal activation was fully bilateral, engaging L and R inferior frontal gyri (BA45/47). The bilateral foundation of this subsystem was further confirmed in a parametric analysis that showed significant modulation of the activity in bilateral frontal regions by competition due to the presence of embedded stems. However, when a different type of processing demand—specifically linguistic complexity—was introduced, the resulting activation shifted to a strongly left-lateralized pattern of IFC activity (BA45). These peaks of activity in prefrontal sites extend into more posterior temporal regions at a lower threshold, consistent with the view that activity in LIFC and RIFC reflects membership of broader fronto-temporal networks, as indicated in earlier research (e.g., refs. 7, 12, 16, 21). Parallel results emerged from the illustrative multivariate analyses (Fig. 2): A component that dissociates words that do and do not have an embedded stem showed a robust bilateral fronto-temporal distribution, whereas an orthogonal component that dissociated words with and without a potential past tense ending showed a primarily left-lateralized activation pattern.
This set of results enables us to pull together a range of existing but fragmented evidence about the functional architecture of the human speech comprehension system. Complementary results from neuropsychological and neuroimaging research suggest that dynamic speech comprehension processes rely on a distinctly bilateral neural network (1, 3, 16). In healthy volunteers activation for words against a low-level acoustic baseline is typically observed in superior and middle temporal regions (2, 5), with the exact localization determined by complexity of the baseline stimulus and task demands (22). Temporal lobe structures have long been implicated in lexical-semantic processes (23), even though authors disagree on the precise role of different temporal lobe regions in the process of sound-to-meaning mapping. The bilateral foundation of the speech comprehension system is further supported by neuropsychological data showing that patient performance on linguistically simple spoken words remains preserved even when the LH perisylvian areas are destroyed (3, 16). In addition to the involvement of bilateral MTG, we also observed activation in bilateral angular gyri, thought to be involved in the mapping between spoken forms and their meanings (11), medial temporal lobe structures, required for the encoding and maintenance of verbal material (24), and anterior cingulate, implicated in modulating fronto-temporal integration in lexical context (21).
Although researchers generally agree on the role of temporal regions in speech comprehension, there is much less agreement on the functional role of inferior frontal regions and how they interact during spoken language comprehension. The focus has typically been on LIFC, which has been implicated in language-specific functions such as phonological analysis, syntactic parsing, and morphological segmentation, as well as wider, non-language-specific processes such as memory retrieval or selection between competing alternatives (8, 14). A variety of different mechanisms have been proposed for these different functions. The most salient contrast is between functional modularity accounts, where specific areas within the LIFC perform unique language-specific functions, and network-based accounts, where the processing capacities of a given region are recruited to support both domain-specific and domain-general functions, as determined by the properties of the different distributed networks to which these regions are linked and are differentially activated by specific inputs.
Our results, although consistent with earlier claims that different regions of LIFC support different aspects of language-related function, are clearly inconsistent with the claim that this is based on functional modularity. We saw some spatial dissociation between linguistic IRP-related activations and general processing activations, in the sense that both IRP and embeddedness contrasts activated BA45, but only the embeddedness contrast extended to the more ventral BA47. This distribution of effects is broadly consistent with proposals that anterior ventral regions (BA47/45) support processing of lexical semantics, whereas more posterior dorsal areas (BA45/44) support syntactic processing (14)—although it is important to note that the contrasts in this experiment did not directly involve the phrasal and sentential aspects of syntactic and semantic combination. However, as is made clear in SI Appendix, Fig. S4, the IRP-related activations in L BA45 seem to fully overlap with those related to embeddedness. This overlap of linguistic and nonlinguistic operations suggests that the functional role and the degree of left-lateralization of fronto-temporal interaction patterns will vary depending on the nature of the speech input. Lexical processing demands that are not specifically linguistic can clearly activate inferior frontal areas bilaterally.
Competition and selection in speech comprehension reflects the sequential nature of the speech signal. As acoustic information unfolds over time, it triggers the simultaneous activation of multiple competing lexical candidates (25). Successful comprehension requires selection of the correct lexical candidate, and although we focused on lexical selection here, it is unlikely that the underlying processes are language specific. Instead, they reflect an increase in processing demands due to competing perceptual alternatives, general across a wide range of cognitive functions and commonly associated with increased activation in bilateral inferior frontal regions (7). These frontal areas may exert top–down control over posterior regions that store representations and mediate access to these representations from spoken inputs, by biasing the network toward the correct interpretation and supporting information retrieval in ambiguous contexts. Within the language domain, recent studies show that semantically ambiguous words and pairs of near-synonyms, which can map onto multiple competing representations in semantic space, trigger a bilateral increase in activation in BA45/47 (8, 26). This pattern of results is consistent with the effects we observed here: Compared with simple words, words with embedded stems activate bilateral frontal regions (BA45/47), and this pattern was dissociable from the left-lateralized activation due to the presence of linguistic complexity.
In sum, the results of the current study and existing evidence about the neuro-functional properties of the speech comprehension network indicate a functional dissociation between bilateral and left-lateralized fronto-temporal subsystems. A neurobiological context for this duality of organization is suggested by recent research into the neural systems underlying complex auditory object processing (including conspecific vocal calls) in nonhuman primates (27). Basic similarities have emerged between the functional architecture of the macaque and the human comprehension systems. Following bilateral input to primary auditory cortex, processing streams extend ventrally and laterally in a hierarchical manner, projecting to frontal, parietal, and posterior temporal regions. Species-specific calls in the macaque activate L and R fronto-temporal regions (homologs of Broca's and Wernicke's areas), possibly supporting the interpretation of vocal calls in their situational and social contexts. These observations point to a bihemispheric substrate for the processing and interpretation of complex auditory signals that remains fundamental to human speech comprehension as well.
The critical difference from these primate systems is the human-specific LH specialization that supports the core morpho-syntactic properties of language. These grammatical capacities are uniquely dependent on the left fronto-temporal system, and RH fronto-temporal regions cannot take over these functions. Recent research comparing white matter connections between frontal and temporal regions in humans, macaques, and chimpanzees (28) shows that major evolutionary changes have taken place in this LH fronto-temporal network. Comparing humans with chimpanzees, and chimpanzees with macaques, there is a striking, hemispherically asymmetrical increase in the LH arcuate fasciculus that links posterior temporal and inferior frontal areas critical for human language. This emerging LH perisylvian system, however, should be seen as continuing to function in the broader context of the bihemispheric systems for processing and interpreting speech inputs that have emerged so clearly in the current study.
Methods
Participants.
Twelve right-handed (seven males) native speakers of British English participated in the study. They had normal or corrected-to-normal vision and had been screened for neurological or developmental disorders. All gave informed consent and were paid for their participation. The study was approved by the Peterborough and Fenland Ethical Committee.
Experimental Design.
Stimuli.
There were five test conditions with 40 words each (Table 1). All words were matched across conditions on word and lemma frequency, familiarity, imageability sound file length, and cohort-related variables (all P > 0.1, based on CELEX and MRC Psycholinguistic databases; see SI Appendix for details). The 200 test words were mixed with 200 filler words, 200 acoustic baseline trials, and 160 silence trials. Filler words were a mix of simple words (two-thirds) and words with embedded stems or suffixes (one-third), to balance out the structure of the test items. The acoustic baseline trials were constructed to share the complex auditory properties of speech but not trigger phonetic interpretation. They were produced by applying a temporal envelope, extracted for each of the speech tokens, to MuR to produce envelope-modulated MuR tokens. The procedure for generating musical rain itself is described in ref. 17 and summarized in SI Appendix. The technique produces nonspeech auditory stimuli in which the long-term spectro-temporal distribution of energy is closely matched to that of the corresponding speech stimuli (SI Appendix, Fig. S1).
Procedure.
The design of the experiment required a task that engages lexical processing but allows us to keep the task requirements constant across speech and nonspeech stimuli. To this end we used gap detection, a nonlinguistic task known to engage lexical processing on line (18). Short silent gaps (400 ms) were inserted in 25% of trials (100 filler words and 50 MuR trials) and participants were required to decide as quickly and accurately as possible whether words and MuR sounds contained a silent gap. For “no” responses participants pressed the button under their index finger and for “yes” responses the button under their middle finger. Only gap-absent trials were subsequently analyzed.
The words were recorded in a sound-proof room by a female native speaker of British English onto a DAT recorder, digitized at a sampling rate of 22 kHz with 16-bit conversion, and stored as separate files using CoolEdit. CoolEdit was also used for gap insertion. Items were presented using in-house software and participants heard the stimuli binaurally over Etymotic R-30 plastic tube phones. There were a total of 760 trials, pseudorandomized with respect to their type (test, filler, baseline, null) and presence or absence of gaps and presented in four blocks of 190 items (10.7 min) each. There were 5 items at the beginning of each block to allow the signal to reach equilibrium. The experiment started with a short practice session outside the scanner, where participants were given feedback on their performance. Participants were instructed to keep their eyes closed during the scanning.
Scanning was performed on a 3T Trio Siemens Scanner at the Medical Research Council Cognition and Brain Sciences Unit (MRC-CBU), University of Cambridge, using a fast sparse imaging protocol to minimize the interference of scanner noise (gradient-echo EPI sequence, repetition time [TR] = 3.4 s, acquisition time [TA] = 2 s, echo delay time [TE] = 30 ms, flip angle 78, matrix size 64 × 64, field of view [FOV] = 192 × 192 mm, 32 oblique slices 3 mm thick, 0.75 mm gap). MPRAGE T1-weighted scans were acquired for anatomical localization. Stimuli were onset-jittered relative to scan offset by 100–300 ms to allow more effective sampling. Imaging data were preprocessed and analyzed in SPM5. Preprocessing was performed using Automated Analyses Software (MRC-CBU) and involved image realignment to correct for movement, segmentation, and spatial normalization of functional images to the MNI reference brain and smoothing with a 10-mm isotropic Gaussian kernel. The data for each subject were analyzed using the general linear model, with four blocks and 10 event types (five conditions, fillers, MuRs, gap words, gap MuRs, and null). The neural response for each event type was modeled with the canonical haemodynamic response function (HRF) and its temporal derivative. Motion regressors were included to code for the movement effects. Sound length was entered as a parametric modulator for words and musical rain sounds without embedded gaps. A high-pass filter with a 128-s cutoff was applied to remove low-frequency noise. Contrast images were combined into a group random effects analysis, and results were thresholded at uncorrected voxel level of P < 0.001 and cluster level of P < 0.05 corrected for multiple comparisons.
Supplementary Material
Acknowledgments
We thank Carolyn McGettigan for her help with stimulus preparation. This research was supported by Medical Research Council Cognition and Brain Sciences Unit funding (U.1055.04.002.00001.01) (to W.D.M.-W.), a Medical Research Council program grant (to L.K.T.), and a European Research Council Advanced Grant (to W.D.M.-W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The fMRI data have been deposited in XNAT Central, http://central.xnat.org (Project ID: speech).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000531107/-/DCSupplemental.
References
- 1.Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cogn Sci. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- 3.Longworth CE, Marslen-Wilson WD, Randall B, Tyler LK. Getting to the meaning of the regular past tense: Evidence from neuropsychology. J Cogn Neurosci. 2005;17:1087–1097. doi: 10.1162/0898929054475109. [DOI] [PubMed] [Google Scholar]
- 4.Goodglass H, Christiansen JA, Gallagher R. Comparison of morphology and syntax in free narrative and structured tests: Fluent vs. nonfluent aphasics. Cortex. 1993;29:377–407. doi: 10.1016/s0010-9452(13)80250-x. [DOI] [PubMed] [Google Scholar]
- 5.Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26:100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 6.Marslen-Wilson WD, Tyler LK. Morphology, language and the brain: The decompositional substrate for language comprehension. Philos Trans R Soc Lond B Biol Sci. 2007;362:823–836. doi: 10.1098/rstb.2007.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 8.Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 9.Raposo A, Moss HE, Stamatakis EA, Tyler LK. Repetition suppression and semantic enhancement: An investigation of the neural correlates of priming. Neuropsychologia. 2006;44:2284–2295. doi: 10.1016/j.neuropsychologia.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Marslen-Wilson WD, Tyler LK. Rules, representations, and the English past tense. Trends Cogn Sci. 1998;2:428–435. doi: 10.1016/s1364-6613(98)01239-x. [DOI] [PubMed] [Google Scholar]
- 11.Tyler LK, Stamatakis EA, Post B, Randall B, Marslen-Wilson WD. Temporal and frontal systems in speech comprehension: An fMRI study of past tense processing. Neuropsychologia. 2005;43:1963–1974. doi: 10.1016/j.neuropsychologia.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Tyler LK, Randall B, Marslen-Wilson WD. Phonology and neuropsychology of the English past tense. Neuropsychologia. 2002;40:1154–1166. doi: 10.1016/s0028-3932(01)00232-9. [DOI] [PubMed] [Google Scholar]
- 13.Post B, Marslen-Wilson WD, Randall B, Tyler LK. The processing of English regular inflections: Phonological cues to morphological structure. Cognition. 2008;109:1–17. doi: 10.1016/j.cognition.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagoort P. On Broca, brain, and binding: A new framework. Trends Cogn Sci. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Friederici AD, Rüschemeyer S-A, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- 16.Tyler LK, Marslen-Wilson WD. Fronto-temporal brain systems supporting spoken language comprehension. Philos Trans R Soc Lond B Biol Sci. 2008;363:1037–1054. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uppenkamp S, Johnsrude IS, Norris D, Marslen-Wilson WD, Patterson RD. Locating the initial stages of speech-sound processing in human temporal cortex. Neuroimage. 2006;31:1284–1296. doi: 10.1016/j.neuroimage.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Mattys SL, Clark JH. Lexical activity in speech processing: Evidence from pause detection. J Mem Lang. 2002;47:343–359. [Google Scholar]
- 19.Liégeois F, et al. A direct test for lateralization of language activation using fMRI: Comparison with invasive assessments in children with epilepsy. Neuroimage. 2002;17:1861–1867. doi: 10.1006/nimg.2002.1327. [DOI] [PubMed] [Google Scholar]
- 20.Kherif F, et al. Multivariate model specification for fMRI data. Neuroimage. 2002;16:1068–1083. doi: 10.1006/nimg.2002.1094. [DOI] [PubMed] [Google Scholar]
- 21.Stamatakis EA, Marslen-Wilson WD, Tyler LK, Fletcher PC. Cingulate control of fronto-temporal integration reflects linguistic demands: A three-way interaction in functional connectivity. Neuroimage. 2005;28:115–121. doi: 10.1016/j.neuroimage.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Giraud AL, et al. Contributions of sensory input, auditory search and verbal comprehension to cortical activity during speech processing. Cereb Cortex. 2004;14:247–255. doi: 10.1093/cercor/bhg124. [DOI] [PubMed] [Google Scholar]
- 23.Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marslen-Wilson WD, Welsh A. Processing interactions and lexical access during word recognition in continuous speech. Cogn Psychol. 1978;10:29–63. [Google Scholar]
- 26.Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- 27.Gil-da-Costa R, et al. Species-specific calls activate homologs of Broca's and Wernicke's areas in the macaque. Nat Neurosci. 2006;9:1064–1070. doi: 10.1038/nn1741. [DOI] [PubMed] [Google Scholar]
- 28.Rilling JK, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci. 2008;11:426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.