Abstract
Epithelial-specific activation of the PI3-kinase pathway is the most common genetic alteration in type I endometrial cancer. In the majority of these tumors, PTEN expression is lost in the epithelium but maintained in tumor stroma. Currently reported PTEN knockout mouse models initiate type I endometrial cancer concomitant with loss of PTEN in both uterine epithelium and stroma. Consequently, the biologic outcome of selectively activating the PI3-kinase pathway in the endometrial epithelium remains unknown. To address this question, we established a malleable in vivo endometrial regeneration system from dissociated murine uterine epithelium and stroma. Regenerated endometrial glands responded to pharmacologic variations in hormonal milieu similar to the native endometrium. Cell-autonomous activation of the PI3-kinase pathway via biallelic loss of PTEN or activation of AKT in adult uterine epithelia in this model was sufficient to initiate endometrial carcinoma. AKT-initiated tumors were serially transplantable, demonstrating permanent genetic changes in uterine epithelia. Immunohistochemistry confirmed loss of PTEN or activation of AKT in regenerated hyperplastic glands that were surrounded by wild-type stroma. We demonstrate that cell-autonomous activation of the PI3-kinase pathway is sufficient for the initiation of endometrial carcinoma in naive adult uterine epithelia. This in vivo model provides an ideal platform for testing the response of endometrial carcinoma to targeted therapy against this common genetic alteration.
Keywords: tissue regeneration model, progesterone receptor, uterine cancer
Endometrial cancer, the most common gynecologic cancer in the United States (1), is a hormonally regulated tumor arising from epithelial cells lining the uterine cavity. Effective targeted therapy is unavailable, partly because of lack of knowledge regarding basic biologic pathways and cellular compartments that can initiate this malignancy. Women diagnosed with endometrial cancer are treated in a similar manner irrespective of the heterogeneity of this disease, with surgery or combinations of radiation and chemotherapy. Although endometrial cancer can be cured in early stages, side effects associated with the current therapy can have lifelong debilitating effects on some patients. The majority of women diagnosed with late-stage or metastatic disease die, despite undergoing radical treatments.
Endometrial cancer can be divided into two distinct subtypes (2). Type I tumors occur in younger patients exposed to high levels of estrogen unopposed by progesterone. They are typically localized with better response to hormonal therapy. Type II tumors occur in older patients independent of hormonal status. These tumors are more invasive, have a greater propensity for metastatic spread, and are refractory to hormonal treatment. Activation of distinct biologic pathways has been reported in type I vs. type II cancers. Type I tumors are associated with activation of the PI3-kinase pathway, activating mutations of KRAS, mutant β-catenin, and microsatellite instability (reviewed in ref. 3). Type II tumors are predominantly associated with mutations in p53, amplification of Her2/neu, and inactivation of p16 (reviewed in ref. 3).
One established model for endometrial cancer is the PTEN+/− mouse, characterized by formation of multiple tumors, including type I endometrial carcinoma (4). In this model, one allele of PTEN is deleted in all tissues (4). In an alternative model, mice with progesterone receptor (PR) promoter-driven Cre were crossed with a PTENloxP/loxP strain, resulting in endometrial hyperplasia in prepubertal mice progressing to type I endometrial carcinoma in adult animals (5). Because PR is expressed in uterine epithelium and stroma, it is unclear whether the observed tumors in this model result from genetic alterations in the uterine epithelium, stroma, or both. Human endometrial carcinoma arises predominantly from adult uterine epithelia, unlike transgenic models, where induction of oncogenic signals occur in developing tissue. In type I endometrial cancers, based on immunohistochemical (IHC) studies, loss of PTEN is predominantly detected in the uterine epithelia and not stromal cells (6). Despite the prevalence of PI3-kinase signaling mutations in type I endometrial cancer, the consequence of epithelial-specific activation of this pathway in adult endometrial epithelium remains unknown.
To study genetic changes that may have a causative role in initiating endometrial cancer, we have developed a regeneration and transformation model from dissociated populations of adult uterine epithelium and cultured neonatal stroma. Previous studies demonstrated that recombined uterine tissue fragments implanted subrenally could regenerate into endometrial-like glands (7, 8). The inability to genetically manipulate tissue fragments limits the utility of this model system. In a separate study, human endometrial glands were regenerated from dissociated adult endometrium, but endometrial glands in these grafts were sparse and appeared atrophic, perhaps because of the limited inductive capacity of adult stroma (9). With the inductive capacity of neonatal stroma, regenerated endometrial glands in our model were secretory, functional, and responded to alterations in hormonal milieu. Herein, we report on the outcome of epithelial-specific PI3-kinase pathway activation using a recently established in vivo endometrial regeneration system. Our findings clearly demonstrate that epithelial specific loss of PTEN or activation of AKT alone is sufficient for initiation of endometrial cancer in adult uterine epithelia. To our knowledge, this in vivo mouse model is unique in accurately recapitulating the epithelial-specific pattern of PTEN loss seen in human endometrial cancer specimens.
Results
Endometrial-Like Glands Regenerated from Dissociated Adult Epithelium and Neonatal Stroma.
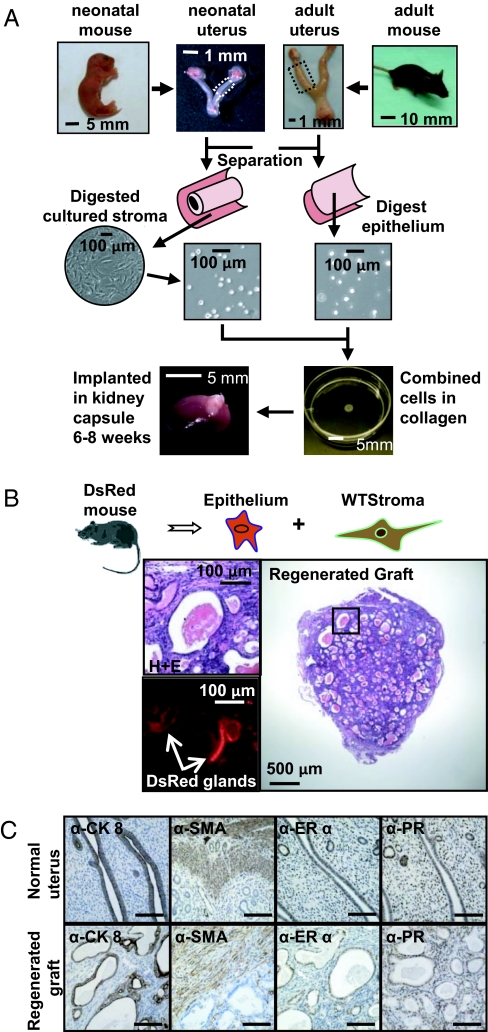
We developed an in vivo murine endometrial regeneration model from dissociated uterine stromal and epithelial cells. Endometrial glands from reproductive-age female mice were harvested as previously described (7, 8) and dissociated (Fig. 1A). Neonatal uterine stroma was isolated (7, 8), dissociated, and cultured short term (Fig. 1A). Efficient separation of epithelium and stroma was demonstrated by IHC (Fig. S1 A and B) and quantitative PCR (Fig. S1C). To differentially color-mark the epithelium, stroma were harvested from WT mice and epithelium was harvested from mice expressing the fluorescent protein DsRed. Equal numbers of dissociated WT uterine stroma and DsRed epithelium were recombined, placed in a collagen plug, and implanted under the kidney capsule of a SCID mouse for 8 wk (Fig. 1 A and B). Predominance of implants, containing a minimum of 1 × 105 epithelia and 1 × 105 stroma, yielded grafts composed of endometrial-like glands surrounded by normal stroma (Fig. 1B). Fluorescent analysis revealed DsRed marker in regenerated glands only, demonstrating their origin from adult epithelial cells (Fig. 1B). To compare the regenerated tissue to native murine uterus, protein expression patterns of known mouse uterine markers were examined by IHC. Similar to mouse uterus, regenerated grafts exhibited cytokeratin 8 (CK8) expression only in epithelial glands and smooth muscle actin (SMA) was present in the outer wall of regenerated grafts (Fig. 1C). Estrogen receptor (ER)α predominated in the endometrial epithelium, and PR was distributed equally in both stromal and epithelial compartments (Fig. 1C).
Fig. 1.
Dissociated populations of uterine epithelium and stroma form endometrial-like glands in the in vivo regeneration model. (A) Schematic of the endometrial regeneration system. Dissociated endometrial epithelia harvested from adult female mice were combined with cultured uterine stroma obtained from neonatal mice. The collagen plug containing both cellular fractions was implanted subrenally into a SCID mouse. (B) Regenerated tissue contained endometrial-like glands. These glands arose from adult donor epithelium (DsRed transgenic) demonstrated by the expression of fluorescence in Cy3/RFP channel. (C) The marker expression profile of regenerated grafts (Lower) closely resembled the native murine uterus (Upper). The regenerated endometrial glands expressed CK8 in the endometrial epithelium and SMA in the outer wall of the graft. ERα was predominantly expressed in the epithelium and PR was detected both in the epithelium and the stroma. (Scale bars, 100 μm.)
Dissociated Endometrial Glands Were Highly Enriched for Epithelial Cells.
To verify that isolated adult endometrial cells used in the regeneration assay were enriched for epithelia, we established an in vitro colony-forming assay. Isolated and dissociated endometrial cells were plated in matrigel coated six-well plates and the number and type of colonies were scored after 8 d based on morphology and expression of epithelial vs. stromal markers. Trop2 is a cell surface marker expressed in uterine epithelium (10) (Fig. 2A). CD90 (Thy-1) is expressed in human endometrial stroma (11) and upon immunostaining, was detected in murine stromal cells adjacent to endometrial glands (Fig. 2A). Isolated murine endometrial cells were dissociated, costained for Trop2 and CD90, fractionated using FACS (Fig. 2B), and placed in the in vitro colony assay. Lineage depletion was performed to remove contaminating hematopoietic and endothelial cells (Fig S2A). The predominance of isolated endometrial cells was Trop2+/CD90− (91%). These cells expressed high levels of CK8 and E-Cadherin message assessed by quantitative PCR (qPCR) and generated epithelial colonies as determined by morphology and expression of CK8 (Fig. 2 B and C and Fig. S2B). Trop2−/CD90+ (7%) cells predominantly yielded stromal colonies expressing SMA and vimentin (Vim) (Fig. 2 B and C and Fig. S2B). All Trop2+/CD90− cells were CD49f+ and CD24+ by FACS and their epithelial-specific expression pattern was confirmed by immunostaining (Fig. S2C).
Fig. 2.
Isolated populations of endometrium are highly enriched for uterine epithelial cells. (A) Cell surface markers that define endometrial epithelium vs. stroma. Trop2 is a marker that is solely expressed in the endometrial epithelium. CD90 marks stromal cells adjacent to the uterine epithelium. (B) Isolated adult endometria used in the regeneration assay were predominantly Trop2+/CD90− (blue oval, 91% of total isolated cells). Trop2+/CD90− cells were epithelial as they gave rise to epithelial colonies in vitro. Trop2−/CD90+ cells (green oval) were stromal and formed stromal colonies in the 2D in vitro assay. Error bars represent 1 SD. (C) The epithelial nature of the Trop2+/CD90− colonies was confirmed with the expression of CK8. In contrast, Trop2−/CD90+ colonies were CK8− and Vim+ or SMA+. (Scale bars, 100 μm.)
These results demonstrate that our isolated populations of adult endometrium used in the regeneration assay were highly enriched for endometrial epithelia, and these cells express Trop2, CD49f, and CD24.
Regenerated Endometrial-Like Glands Were Functional and Responded to Alterations in the Hormonal Milieu.
In response to fluctuating levels of estrogen and progesterone, mouse and human endometria undergo cyclic apoptosis/shedding and regrowth during the estrous and menstrual cycle, respectively (12, 13). Endometrial estrogen- and progesterone-mediated effects are thought to occur in a paracrine manner from adjacent stroma (14, 15). Alterations in these steroids induce changes in the endometrium essential for implantation of the embryo (16), and result in secretion of leukemia inhibitory factor (LIF), essential for blastocyst implantation (17, 18).
To assess the optimal condition for regeneration of endometrial glands, mice harboring grafts were pharmacologically exposed to defined hormonal conditions. To exclude the influence of endogenous female hormones, recipient mice were oophorectomized before graft implantation. The regeneration of endometrial glands was poor in the absence of any hormonal supplementation (Fig. S3A). Exposure to unopposed estrogen for 8 wk resulted in regeneration of crowded endometrial glands, histologically consistent with simple endometrial hyperplasia, but normal secretory glands regenerated with the administration of an additional progesterone pulse (Fig. 3A and Fig. S3B). The combination of steady-state estrogen with progesterone pulse was deemed the optimal condition for in vivo regeneration of the endometrium. Withdrawal of progesterone led to breakdown of the endometrium glands similar to the cycling uterus (Fig. 3A and Fig. S3B).
Fig. 3.
Regenerated endometrial-like glands were functional and responded to hormonal variations. (A) The response of regenerated tissue to alterations in hormonal milieu was similar to normal uterine endometrium. Regenerated glands became hyperplastic in response to unopposed estradiol (E2), secretory with the administration of progesterone pulse (P4), and underwent breakdown after the withdrawal of P4. (B) The regeneration of endometrial glands depends on stromal and epithelial interactions. Combinations of epithelium and stroma led to the formation of normal secretory glands. Implantation of epithelium alone resulted in the formation of atrophic glands, but glands were absent in the stromal grafts. (C) Similar to the native uterus, LIF was detected in regenerated grafts composed of epithelium and stroma. Importantly, expression of LIF was absent in atrophic glands regenerated from epithelium alone. (D) Prolonged exposure to unopposed estrogen resulted in the formation of progressive endometrial hyperplasia. Simple endometrial hyperplasia developed within 8 wk. At 12 wk, complex hyperplasia was seen, which progressed to atypical complex hyperplasia at 16 wk. (Scale bars, 100 μm.)
To assess if regeneration of endometrial glands was dependent on stromal inductive effects, dissociated populations of epithelium and stroma were implanted alone or in varying ratios in the regeneration assay (Fig. 3B and Fig. S4). Implantation of stromal cells resulted in overgrowth of solely stromal tissue, but regeneration of epithelial cells alone produced atrophic endometrial glands lined by flattened cells (Fig. 3B). Combinations of epithelium and stroma generated differentiated secretory endometrial glands, and optimal regeneration was achieved when equal or greater numbers of stromal cells were used (Fig. 3B and Fig. S4). LIF expression was only detected in regenerated secretory endometrial glands and was notably absent in atrophic glandular tissue (Fig. 3C). These results suggest that regeneration of functional endometrial glands depends on stromal and epithelial interactions.
Chronic exposure to unopposed estrogen is a well-known risk factor for endometrial hyperplasia and cancer (19, 20). In our model, increasing durations of exposure to high-dose estrogen unopposed by progesterone resulted in progressive endometrial pathology, demonstrated by increased gland to stromal ratio, and irregularly contoured glands with cytologic atypia (Fig. 3D and Figs. S3 C and D and S5).
These results collectively demonstrate that regenerated endometrial glands not only resemble the endometrium morphologically, but also function in a similar manner to native endometrial tissue.
Cell-Autonomous PI3-Kinase Pathway Activation in Adult Naive Uterine Epithelia Resulted in Type I Endometrial Adenocarcinoma.
Common genetic changes in type I endometrial cancer include PI3-kinase pathway activation via loss of PTEN function (21) or mutations in AKT (22). In human cancer specimens, PTEN is predominantly lost in the epithelium and maintained in the stroma (6). The ability to work with dissociated uterine cells makes our model an ideal platform for testing consequences of cell-autonomous genetic changes in naive adult epithelia.
Activating mutations in the pleckstrin homology domain of AKT oncogene have been detected in endometrial cancers (22). Dissociated populations of WT endometrial epithelium were infected with control or myristoylated AKT-expressing lentivirus (23). These cells were combined with WT stroma and placed in the in vivo regeneration model. Cell-autonomous AKT activation resulted in formation of larger grafts that contained areas of complex endometrial hyperplasia and well-differentiated adenocarcinoma, as determined histologically and by pankeratin staining (Fig. 4A).
Fig. 4.
Consequences of cell autonomous PI3-kinase pathway activation in naive adult endometrial epithelium. (A) Activation of AKT in endometrial epithelium led to the development of hyperplasia and well-differentiated adenocarcinoma. Expression of Myr-AKT with a lentiviral vector resulted in the formation of larger grafts comprised of hyperplastic glands compared with controls. Expression of activated AKT was confirmed by immunostaining. Heterogeneous expression of PR was detected in AKT tumors with many areas exhibiting low levels of epithelial PR. (B) AKT initiated tumors result from cell autonomous genetic changes. Primary AKT tumors were dissociated and retransplanted into a secondary graft with WT stroma. The histology of the secondary regenerant was consistent with endometrial adenocarcinoma. (C) Loss of PTEN resulted in formation of well-differentiated endometrial adenocarcinoma. PTEN was absent only in the epithelial compartment of these tumors, recapitulating the PTEN expression pattern observed in human endometrial adenocarcinoma. (Scale bars, 100 μm.)
Histopathologic criteria used for the diagnosis of type I endometrial carcinoma included uncontrolled proliferation of endometrial glands, resulting in higher gland/stromal ratio compared with control. Tumor glands were irregular, angulated, back-to-back with minimal intervening stroma, and had an invasive growth pattern.
Regenerated abnormal glands in these tumors expressed phosphorylated AKT, confirming AKT activation in hyperplastic areas (Fig. 4A). The predominance of abnormal glands expressed ERα (Fig. 4A) but PR expression was variable, with many hyperplastic regions exhibiting decreased levels of epithelial PR (Fig. 4A). Loss of PR is clinically associated with poorer prognosis and resistance to hormonal therapy (24, 25). These results suggest that cell autonomous AKT activation is sufficient to initiate hyperplasia and well-differentiated carcinoma in naive adult uterine epithelium.
To ensure that observed AKT-mediated effects were cell-autonomous, primary AKT tumors were dissociated, combined with WT stroma, and reimplanted as a secondary graft in the regeneration assay. The resulting secondary tumor was phenotypically similar to the primary tumor based on histology and AKT activation (Fig. 4B and Fig. S6A). These results demonstrate that activation of AKT in adult uterine epithelia resulted in permanent cell-autonomous changes that could be serially propagated.
A more common genetic alteration in type I endometrial cancer is PI3-kinase pathway activation via deletion, inactivating mutation, or promoter hypermethylation of the PTEN tumor-suppressor gene (6, 21, 26, 27). Mutations in PTEN are found in 83% of endometrial cancer specimens (6). IHC analysis revealed undetectable levels of PTEN in 61% of type I endometrial carcinomas, suggesting biallelic loss or inactivation of PTEN in these tumors (6). PTEN loss not only activates AKT but also leads to the activation of additional downstream targets, such as Rac1 and CDC42, involved in regulation of cell growth and cell cycle (reviewed in ref. 28). To test the consequences of epithelial-specific PTEN loss, endometrial glands were harvested from adult PTENloxP/loxP mice where PTEN is floxed by loxP sites (29). The dissociated endometrial epithelia were infected with FUCRW control or FU-Cre-CRW virus, which expresses Cre-recombinase (Fig. S7), resulting in excision of PTEN. Infected epithelial cells were combined with WT stroma and placed in the in vivo regeneration assay. Epithelial-specific PTEN loss in naive adult endometria resulted in the formation of well-differentiated endometrial cancers (Fig. 4C). High levels of ERα were noted in the epithelial tumor cells (Fig. 4C). In contrast to AKT tumors, robust expression of epithelial PR was detected in hyperplastic glands (Fig. 4C). These abnormal glands had a lower proliferation index compared with AKT tumors (Fig. S6C). Loss of PTEN was observed in tumor epithelia surrounded by WT stroma (Fig. 4C and Fig. S6B). The compartment-specific pattern of PTEN loss in these tumors resembles findings in clinical endometrial cancer specimens.
Collectively, these results demonstrate that epithelial-specific PI3-kinase pathway activation via loss of PTEN or activation of AKT can initiate type I endometrial carcinoma or hyperplasia in naive adult endometrial cells.
Discussion
We report an endometrial cancer mouse model that accurately recapitulates the epithelial-specific PTEN loss observed in human endometrial cancer specimens. Regenerated tumors resulting from loss of PTEN in this in vivo model exhibit normal expression of PTEN in the stroma with loss of PTEN in the epithelium. Currently published PTEN mouse models result in concomitant loss of PTEN in the epithelium and stroma (4, 5). Although the consequences of stromal PTEN loss in the endometrium are unknown, loss of PTEN in mammary stromal cells has been shown to promote tumor formation in cooperation with cell-autonomous genetic changes (30).
The ability to accurately recapitulate human endometrial cancer makes this model system a unique tool for testing therapeutics in an in vivo setting. For example, progesterone is a well-tolerated noncytotoxic drug with 50 to 70% response rates in primary type I endometrial cancers and 17% response rates in recurrent disease (reviewed in ref. 31). In clinical practice, progesterone therapy is under-used, as there are no reliable methods for stratification of endometrial hyperplasia or cancer into those that may respond to progesterone. Endometrial tumors from PTEN+/− mice were refractory to hormonal treatment (32). However, in tumors of these mice, PTEN was lost in the uterine epithelium and stroma, which does not recapitulate epithelial specific loss of PTEN in human endometrial cancer specimens. Based on retrospective clinical reports, it is unclear if loss of PTEN is a predictor of response to progesterone therapy (33–36). Findings in these clinical studies are widely variable, with some reporting PTEN loss as a positive predictor of response (34, 35) but others reporting loss of PTEN as a negative predictor for response to progesterone treatment (33, 36). These conflicting clinical findings may be because of tumor heterogeneity, resulting from genetic alterations in addition to PTEN loss. The expression of PR in endometrial cancer specimens correlates with a positive response to progesterone therapy (25, 37). In our in vivo model, differential expression of epithelial PR was observed in AKT vs. PTEN-initiated tumors. Lower levels of epithelial PR were detected in AKT tumors compared with PTEN-initiated cancers. To determine predictors of response to hormonal therapy, we plan to use this model to ascertain response to progesterone treatment in the context of defined genetic changes in an in vivo setting.
The cell of origin for endometrial carcinoma is unknown. Stem cells are efficient targets of oncogenic transformation in some epithelial cancers (38–40). Stem cells must exist in the uterine lining as estrogen-deprived endometrium undergoes atrophy but maintains the capacity to regrow with the addition of exogenous hormones. In many hormonally responsive epithelia, stem cells either lack hormone receptor expression (41) or active signaling (38, 40). Murine uterine epithelial label-retaining cells were found to be ERα-negative (12), suggesting these progenitors may have quiescent hormone-receptor transcription machinery. Because type II tumors are resistant to hormonal therapy and are predominantly ER-/PR-negative, they may arise from endometrial stem cells. In contrast, hormonally responsive type I tumors that are ER-/PR-positive, may arise from more differentiated endometrial progenitors. To date, mouse models have not recapitulated type II endometrial cancers. One possible reason for the lack of animal models that recapitulate type II tumors could be the inability to target endometrial stem cells with oncogenic signals using the PR-driven promoter.
The ability to genetically manipulate fractionated endometrial epithelia in our model provides the tools necessary for identifying cells and differential oncogenic signals that may give rise to type I vs. type II endometrial cancers. This experimental approach can help elucidate genetic pathways that can initiate endometrial cancer in target cells. Results from these studies could lead to scientifically based clinical trials that can help individualize therapeutic interventions for women affected by endometrial cancer.
Methods
Experimental Animals.
WT C57BL/6, β-actin DsRed [C57BL/6-Tg(ACTB-DsRed.MST)1Nagy/J], PTENloxPloxP (C;129S4-Ptentm1Hwu/J), and CB17Scid/Scid mouse strains were purchased from The Jackson Laboratory. Mice were maintained in accordance with the University of California at Los Angeles, Division Laboratory of Animal Medicine-approved protocols. All animal experiments were performed under University of California at Los Angeles Animal Research Committee approval.
Plasmids and Virus Production.
Lentiviral plasmids expressing GFP (FUCGW), myristoylated-AKT and GFP (FU-myrAKT-CGW), and RFP (FUCRW) were previously described (23, 42). To construct the cre-expressing vector FU-cre-CRW, cDNA for cre recombinase was cloned into the EcoRI site of the FU-CRW vector. Lentivirus preparation and titering was performed as previously reported (23).
Preparation of Endometrial and Stromal Cells.
Uterine epithelial and stromal separation was performed as previously described (7, 8). Uterine epithelial sheets were digested in 0.8 mg/mL collagenase (Invitrogen; 17018–029) in DMEM/10% FBS by incubation at 37 °C with gentle agitation. Dissociated cell preparations were passed through a 100-μm nylon mesh to remove clumps. Dissociated epithelia were either used directly or virally infected before placement in the regeneration system. Uterine stromal fragments were obtained from 0- to 2-d-old C57BL/6 pups, as described previously (7, 8). Stromal fragments were digested with collagenase, passed through a 100-μm nylon mesh, and cultured short term in BFS media (DMEM, 5% FBS, 5% Nu-serum, and 5 μg/mL insulin).
Epithelial Infection and Regeneration.
Dissociated epithelial cells were lentivirally infected with centrifugation at a multiplicity of infection of 40. Dissociated epithelial and stromal cells were mixed and resuspended in rat-tail collagen (BD Biosciences; 354236) neutralized according to the manufacturer's instructions. Cell and collagen mixtures were dispensed into 15-μL grafts and incubated for 30 min at 37 °C. Grafts were overlaid with BFS media and incubated overnight. The following day, endometrial grafts were implanted under the kidney capsule of oophorectomized CB17Scid/Scid mice. Estrogen pellets (90-d time release, 0.72-mg β-estradiol/pellet; Innovative Research of America) were implanted subcutaneously unless otherwise noted. A progesterone pulse (2.5 mg/d) was administered for the final 8 d of regeneration, unless otherwise indicated. In endometrial hyperplasia experiments, two estrogen pellets were implanted per mouse. All surgical procedures were performed under the Division of Laboratory Animal Medicine regulations for the University of California, Los Angeles.
Immunohistochemistry.
Tissue was formalin-fixed and paraffin-embedded. Primary antibodies used for IHC are listed in Table S1. Bioiotinylated goat anti-rabbit, rabbit anti-rat, streptavidin-FITC, and streptavidin-HRP were from Jackson ImmunoResearch, and biotinylated anti-mouse antibody was purchased from Vector Laboratories.
FACS and in Vitro Colony Assay.
Endometrial cells were suspended in DMEM/10% FBS and stained with antibody for 20 min at 4 °C with shaking. FACS cell sorting was performed using the BD FACS AriaII (BD Biosciences) machine. FACS population analyses were performed on a BD FACSCanto. Antibodies used for FACS sorting are listed in Table S1. For colony-forming assays, triplicate samples were plated at 10,000 cells per well in PrEGM in matrigel-coated six-well plates. Colony formation was scored after 8 d. Epithelial and stromal colony designations were confirmed by immunocytochemical staining.
Quantitative PCR.
RNA was extracted using Qiagen RNeasy Mini kit according to the manufacturer's instructions. Reverse transcription was performed with a SuperScript III first-strand synthesis kit (Invitrogen). Quantitative PCR was performed using iQ SYBR Green Supermix for Real-Time PCR (Bio-Rad) on a Bio-Rad iCycler and iQ5 2.0 Standard Edition Optical System Software. Data were analyzed by using the Pfaffl method. Primer sets used are outlined in Table S2.
Supplementary Material
Acknowledgments
We thank Ms. Shirley Quan for her contributions in arranging mice needed for experiments and Ms. Barbara Anderson for her help in preparing this manuscript. S.M. is supported by Building Interdisciplinary Research Careers in Women's Health Grant (National Institutes of Health/National Institute of Child Health and Human Development 5 K12 HD001400), a Prostate Cancer Foundation Young Investigator's Award, the Stein Oppenheimer Clinical Translational Seed Grant, the Liz Tilberis Scholars Program from the Ovarian Cancer Research Fund, and a Scholars in Translational Medicine gift. J.H. is supported by American Cancer Society RSG-07-092-01-TBE, Department of Defense Prostate Cancer Research Program PC061456, a Development Research Award from the University of California at Los Angeles Prostate Cancer Specialized Program in Research Excellence [Primary Investigator (PI): R. Reiter], a Challenge Award from the Prostate Cancer Research Foundation (PI: O.N.W.), and a Creativity Award from the Prostate Cancer Research Foundation (PI: M. Rettig). A.S.G. is supported by the Ruth L. Kirschstein National Research Service Award GM07185. T.K. is supported by 1R01CA154358-01 through the National Cancer Institute. O.N.W. is a Howard Hughes Medical Institute Investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012548107/-/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Samarnthai N, Hall K, Yeh IT. Molecular profiling of endometrial malignancies. Obstet Gynecol Int. 2010;2010:162363. doi: 10.1155/2010/162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stambolic V, et al. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- 5.Daikoku T, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutter GL, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 7.Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- 8.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Dev Biol. 2001;240(1):194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- 9.Masuda H, et al. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc Natl Acad Sci USA. 2007;104:1925–1930. doi: 10.1073/pnas.0604310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein AS, et al. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci USA. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23:934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 12.Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 13.McLennan CE, Rydell AH. Extent of endometrial shedding during normal menstruation. Obstet Gynecol. 1965;26:605–621. [PubMed] [Google Scholar]
- 14.Cooke PS, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurita T, et al. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- 16.Finn CA, Martin L. The control of implantation. J Reprod Fertil. 1974;39(1):195–206. doi: 10.1530/jrf.0.0390195. [DOI] [PubMed] [Google Scholar]
- 17.Stewart CL, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 18.Cullinan EB, et al. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci USA. 1996;93:3115–3120. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–1167. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- 20.McDonald TW, et al. Exogenous estrogen and endometrial carcinoma: Case-control and incidence study. Am J Obstet Gynecol. 1977;127:572–580. doi: 10.1016/0002-9378(77)90351-9. [DOI] [PubMed] [Google Scholar]
- 21.Kong D, et al. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat Genet. 1997;17(2):143–144. doi: 10.1038/ng1097-143. [DOI] [PubMed] [Google Scholar]
- 22.Cohen Y, et al. AKT1 pleckstrin homology domain E17K activating mutation in endometrial carcinoma. Gynecol Oncol. 2010;116:88–91. doi: 10.1016/j.ygyno.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Memarzadeh S, et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrlich CE, Young PC, Stehman FB, Sutton GP, Alford WM. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol. 1988;158:796–807. doi: 10.1016/0002-9378(88)90075-0. [DOI] [PubMed] [Google Scholar]
- 25.Thigpen JT, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: A dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–1744. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 26.Peiffer SL, et al. Allelic loss of sequences from the long arm of chromosome 10 and replication errors in endometrial cancers. Cancer Res. 1995;55:1922–1926. [PubMed] [Google Scholar]
- 27.Nagase S, Yamakawa H, Sato S, Yajima A, Horii A. Identification of a 790-kilobase region of common allelic loss in chromosome 10q25-q26 in human endometrial cancer. Cancer Res. 1997;57:1630–1633. [PubMed] [Google Scholar]
- 28.Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev Biol. 2004;273(2):175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Lesche R, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32(2):148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 30.Trimboli AJ, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28(1):81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fyles A, et al. Neither ovariectomy nor progestin treatment prevents endometrial neoplasia in pten+/- mice. Gynecol Oncol. 2008;108:395–401. doi: 10.1016/j.ygyno.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Milam MR, et al. Loss of phosphatase and tensin homologue deleted on chromosome 10 and phosphorylation of mammalian target of rapamycin are associated with progesterone refractory endometrial hyperplasia. Int J Gynecol Cancer. 2008;18(1):146–151. doi: 10.1111/j.1525-1438.2007.00958.x. [DOI] [PubMed] [Google Scholar]
- 34.Ørbo A, Rise CE, Mutter GL. Regression of latent endometrial precancers by progestin infiltrated intrauterine device. Cancer Res. 2006;66:5613–5617. doi: 10.1158/0008-5472.CAN-05-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng W, Baker HE, Mutter GL. Involution of PTEN-null endometrial glands with progestin therapy. Gynecol Oncol. 2004;92:1008–1013. doi: 10.1016/j.ygyno.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Minaguchi T, et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer Lett. 2007;248(1):112–122. doi: 10.1016/j.canlet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Yamazawa K, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22:1953–1958. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 38.Lawson DA, et al. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci USA. 2010;107:2610–2615. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asselin-Labat ML, et al. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- 42.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.