Abstract
Leptin monotherapy reverses the deadly consequences and improves several of the metabolic imbalances caused by insulin-deficient type 1 diabetes (T1D) in rodents. However, the mechanism(s) underlying these effects is totally unknown. Here, we report that intracerebroventricular (icv) infusion of leptin reverses lethality and greatly improves hyperglycemia, hyperglucagonemia, hyperketonemia, and polyuria caused by insulin deficiency in mice. Notably, icv leptin administration leads to increased body weight while suppressing food intake, thus correcting the catabolic consequences of T1D. Also, icv leptin delivery improves expression of the metabolically relevant hypothalamic neuropeptides proopiomelanocortin, neuropeptide Y, and agouti-related peptide in T1D mice. Furthermore, this treatment normalizes phosphoenolpyruvate carboxykinase 1 contents without affecting glycogen levels in the liver. Pancreatic β-cell regeneration does not underlie these beneficial effects of leptin, because circulating insulin levels were undetectable at basal levels and following a glucose overload. Also, pancreatic preproinsulin mRNA was completely absent in these icv leptin-treated T1D mice. Furthermore, the antidiabetic effects of icv leptin administration rapidly vanished (i.e., within 48 h) after leptin treatment was interrupted. Collectively, these results unveil a key role for the brain in mediating the antidiabetic actions of leptin in the context of T1D.
Keywords: brain, leptin monotherapy, glucose homeostasis, glucagon suppression
According to the Juvenile Diabetes Research Foundation, type 1 diabetes (T1D) afflicts 1–3 million people in the United States alone. Regrettably, for reasons yet to be understood, the incidence of T1D has been increasing at an alarming annual rate of ~3%, thus indicating that the number of patients with T1D is predicted to rise significantly in the future (1). T1D occurs as a consequence of pancreatic β-cell destruction leading to insulin deficiency, a defect that causes hyperglycemia, hyperglucagonemia, cachexia, ketoacidosis, and other abnormalities (2, 3). T1D is a deadly condition if not treated. Current life-saving interventions include daily insulin administration; insulin therapy reduces hyperglycemia, glycosylated hemoglobin, and cachexia and prevents or delays some T1D-associated morbidities (3, 4). However, even with insulin therapy, T1D secondary complications include debilitating and long-lasting conditions, such as heart disease, neuropathy, and hypertension (5–7). Moreover, probably because of insulin's lipogenic and cholesterologenic actions, long-term insulin treatment is suspected to underlie the increased ectopic lipid deposition (i.e., in nonadipose tissues) (8) and incidence of coronary artery disease (>90% after the age of 55 y) (9, 10) seen in patients with T1D. Furthermore, in part attributable to insulin's potent, fast-acting, glycemia-lowering effects, intensive insulin therapy significantly increases the risk for hypoglycemia, an event that is disabling and can even be fatal (3, 11–14). Therefore, despite the profound diabetes-improving and life-saving effects of insulin-based therapies, they do not restore metabolic homeostasis and may even lead to serious side effects. Thus, better anti-T1D approaches are urgently needed.
Leptin (a hormone secreted by adipocytes) profoundly affects metabolism (15). By chiefly acting on neurons within the CNS, leptin suppresses food intake and increases energy expenditure, and hence restrains excessive body weight gain (16). In addition, leptin directly governs glucose homeostasis, mainly by activating cognate leptin receptors (LEPRs) in neurons within the hypothalamic arcuate nucleus (ARH) (17–20). Interestingly, leptin and insulin share similar intracellular signaling pathways in hypothalamic neurons, such as the PI3K signaling cascade (21, 22). Furthermore, leptin enhances insulin sensitivity in insulin-resistant rodents and humans (19, 20, 23, 24). Whether leptin's actions on brain neurons can robustly improve hyperglycemia (and other metabolic imbalances) in the context of insulin deficiency has not been tested. Recently, however, exciting results have indicated that overt hyperglycemia and death caused by insulin signaling deficiency can be reversed by leptin therapy alone (25, 26), thus suggesting that insulin is dispensable for the glycemia-lowering actions of leptin. Nevertheless, the mechanism(s) by which leptin monotherapy improves T1D is unknown.
Because of the established importance of the CNS in mediating the glycemia-lowering actions of leptin in the context of either a high (17–20) or low (27–29) circulating insulin level, we hypothesized that leptin's antidiabetic effects could also be mediated by brain neurons in the context of T1D. To test this hypothesis directly, we assessed the metabolic outcomes of CNS-restricted leptin administration in a mouse model of T1D.
Results
Absence of Insulin in Mice That Received Two Injections of Streptozotocin.
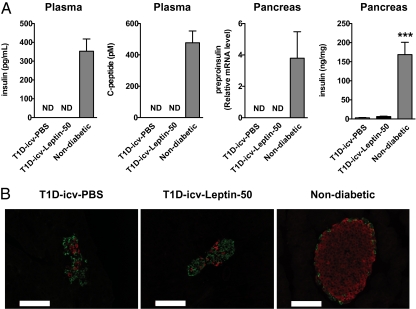
To test directly whether leptin therapy improves T1D via CNS-dependent mechanisms, leptin was delivered by the intracerebroventricular (icv) route in mice rendered insulin-deficient by two i.p. injections of streptozotocin [STZ, a compound known to destroy pancreatic β-cells (30, 31)]. STZ was injected in mice at 8 wk of age and then again at 9 wk of age at a dose of 150 mg/kg of body weight. At 10 wk of age, mice underwent stereotaxic surgery for chronic leptin (50 ng/h) or placebo delivery into the cerebral lateral ventricles (T1D-icv-Leptin-50 and T1D-icv-PBS groups, respectively). Circulating insulin and C-peptide and pancreatic preproinsulin mRNA levels were undetectable (assay detection thresholds: 5 pg/mL, 25 pM, and cycle threshold of 35, respectively) in T1D-icv-PBS and T1D-icv-Leptin-50 mice; conversely, these parameters were all readily measurable in nondiabetic age-matched controls (Fig. 1A). Pancreatic insulin protein contents had also dropped to 2.5% of the normal value in both T1D-icv-PBS and T1D-icv-Leptin-50 mice (Fig. 1A). Consistently, immunohistochemical analyses indicated a near-total loss of insulin-containing cells in pancreas of T1D-icv-PBS and T1D-icv-Leptin-50 mice compared with controls (Fig. 1B). Because T1D-icv-PBS mice also displayed the typical clinical and molecular dysfunctions caused by total absence of insulin (see below), these results indicate that STZ-treated mice are insulin-deficient, and thus a bona fide T1D model.
Fig. 1.
Pancreatic insulin profiles in STZ-treated mice. (A) Plasma insulin and C-peptide as well as pancreatic preproinsulin mRNA and insulin levels in T1D-icv-PBS and T1D-icv-Leptin-50 mice 10 d following stereotaxic surgery and in age-matched nondiabetic control mice. (B) Representative distribution of cells expressing insulin (red) and glucagon (green) in the pancreas of mice shown in A. (Scale bar = 100 μm.) Error bars represent SEM. Statistical analyses were done using one-way ANOVA (Tukey's posttest) (n = 5–7 in each group). ***P < 0.001 vs. T1D-icv-PBS mice. ND, below the threshold of detection.
CNS-Restricted Activation of LEPR Signaling in T1D-icv-Leptin Mice.
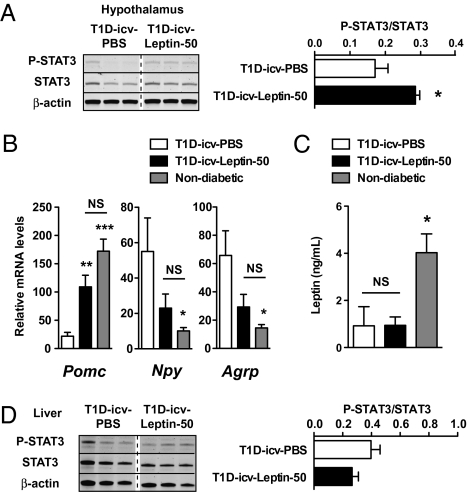
It is unknown whether leptin can successfully activate CNS LEPR signaling in the context of insulin deficiency. A well-established intracellular change that follows activation of LEPR signaling is the phosphorylation of STAT3 (32). Thus, to assess whether icv leptin administration triggers central LEPR signaling in insulin-deficient mice, hypothalamic phosphorylated STAT3 levels were measured. As shown in Fig. 2A, the phosphorylation status of hypothalamic STAT3 was significantly elevated in T1D-icv-Leptin-50 mice compared with the T1D-icv-PBS group. Furthermore, the mRNA content of hypothalamic neuropeptides, the expression of which is known to be directly regulated by leptin [e.g., proopiomelanocortin (POMC), neuropeptide Y (NPY), agouti-related peptide (AgRP) (33–35)], was overtly altered in T1D-icv-PBS mice (Fig. 2B) consistent with their severe hypoleptinemia (Fig. 2C). Of note, the expression of these neuropeptides was greatly improved by icv administration of leptin (Fig. 2B). Importantly, leptinemia and the phosphorylation status of hepatic STAT3 were not different between T1D-icv-Leptin-50 and T1D-icv-PBS mice (Fig. 2 C and D), indicating that leptin delivered by the icv route did not leak into the bloodstream in physiologically significant amounts. Altogether, these results demonstrate that LEPR signaling is restrictedly activated in the brain of T1D-icv-Leptin-50 mice.
Fig. 2.
Delivery of icv leptin is restricted to the brain in STZ-induced insulin-deficient mice. Phosphorylated STAT3 (P-STAT3), STAT3, and β-actin (used as a loading control) protein levels (A) and Pomc, Npy, and Agrp mRNA contents (B) were assessed in the hypothalamus of T1D-icv-PBS and T1D-icv-Leptin-50 mice shown in Fig.1A. (C) Plasma leptin levels were measured in mice shown in Fig. 1A. (D) P-STAT3, STAT3, and β-actin (used as a loading control) protein levels were assessed in the liver of mice shown in Fig 1A. Error bars represent SEM. Statistical analyses were done using a two-tailed unpaired Student's t test or one-way ANOVA (Tukey's posttest) when two or three groups were compared, respectively (n = 5–7 in each group). *P < 0.05; **P < 0.01; ***P < 0.001 vs. T1D-icv-PBS mice. NS, not statistically different.
CNS Leptin Delivery Improves Survival and Diabetes in T1D Mice.
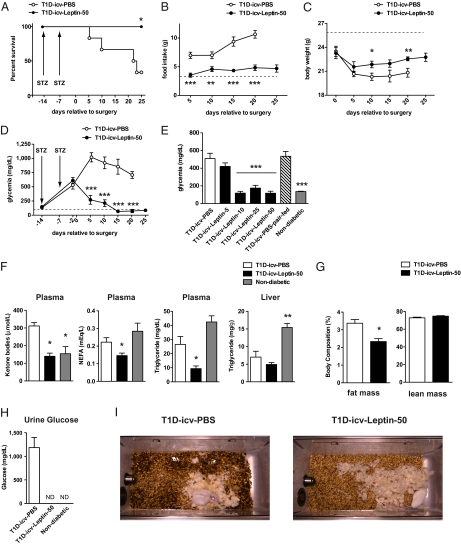
If untreated, insulin deficiency causes premature death. Indeed, very few of the T1D-icv-PBS mice survived for longer than 39 d after receiving the first STZ injection (Fig. 3A). Conversely, the early lethality was totally prevented by icv administration of leptin, because all the T1D-icv-Leptin-50 mice survived for the full extent of the treatment (Fig. 3A). Severe hyperphagia, reduced body weight, hyperglycemia, hyperketonemia, glucosuria, and polyuria are typical clinical manifestations of T1D, and, indeed, these aberrancies were all displayed by T1D-icv-PBS mice (Fig. 3 B–D and F–I). Remarkably, icv leptin administration greatly improved all these defects, because T1D-icv-Leptin-50 mice displayed near-normal food intake, body weight, glycemia, and ketonemia; an undetectable amount of glucose in the urine; and reduced polyuria (Fig. 3 B–D and F–I). Of note, the remarkable glycemia-lowering effects of icv leptin were also observed in other cohorts of STZ-treated mice that received leptin at a dose of 50, 25, or 10 ng/h but not in the mice that received leptin at a dose of 5 ng/h (Fig. 3E). These data indicate that the minimal effective anti-T1D dose of icv leptin is in the range of 5–10 ng/h. Because Friedman and colleagues (36) have shown that a similar concentration of icv-delivered leptin leads to decreased food intake and body weight in nondiabetic normal mice, it seems that the minimal effective anti-T1D concentration of icv-administered leptin is not in the physiological range. Noteworthy, the beneficial effects of icv leptin treatment on hyperglycemia were not secondary to changes in food intake. This is demonstrated by the fact that T1D-icv-PBS mice that were pair-fed to the amount of food that T1D-icv-Leptin-50 mice ate still displayed overt hyperglycemia (Fig. 3E). These results are in agreement with the findings of Wang et al. (26), who reported that restricted feeding does not ameliorate T1D. In addition, in line with leptin's lipid-lowering actions, icv administration of leptin reduced circulating nonesterified fatty acid and triglyceride levels; yet, liver triglyceride contents were not further diminished in T1D-icv-Leptin-50 mice (Fig. 3F). Body fat percentage was also reduced in T1D-icv-Leptin-50 mice compared with T1D-icv-PBS mice (Fig. 3G). Of note, these advantageous lipid-suppressing actions of icv administration of leptin are in opposition to the disadvantageous lipid-rising actions of insulin (26) and, as such, are highly desirable effects of leptin therapy in T1D. Collectively, our results demonstrate that CNS leptin administration reverses lethality and significantly ameliorates metabolic imbalances caused by insulin deficiency in mice.
Fig. 3.
CNS leptin administration reverses lethality and improves diabetes in insulin-deficient mice. (A) Kaplan–Meier survival analyses were performed on T1D-icv-PBS and T1D-icv-Leptin-50 mice; the latter group had increased survival compared with the former group as determined by the Gehan–Breslow–Wilcoxon test. Mice that died during stereotaxic surgery are not included. Food intake (B), body weight (C), and blood glucose level (D) in T1D-icv-PBS and T1D-icv-Leptin-50 mice. Dashed lines represent average values measured in age-matched nondiabetic control mice. (E) Dose–response of icv leptin administration and pair-feeding effects on glycemia in T1D mice. Parameters were determined 10 d following stereotaxic surgery and in age-matched nondiabetic controls. Plasma ketone bodies, nonesterified fatty acid (NEFA), triglyceride levels and liver triglyceride contents (F), body composition (G), and glucose contents (H) in urine of T1D-icv-PBS mice, T1D-icv-Leptin-50 mice, and age-matched, nondiabetic control mice. The parameters shown in F–H were determined 10 d following stereotaxic surgery. (I) Representative photographs of cages in which T1D-icv-PBS or T1D-icv-Leptin-50 mice were housed (the bedding was unchanged for 72 h). Intensity and area of dark spots in the bedding indicate urine excretion. Error bars represent SEM (n = 3–9). Statistical analyses were done using a two-tailed unpaired Student's t test or one-way ANOVA (Tukey's posttest) when two or three groups were compared, respectively. *P < 0.05; **P < 0.01; ***P < 0.001 vs. T1D-icv-PBS mice. ND, below the threshold of detection.
Effects of CNS Leptin Delivery on Peripheral Tissues in T1D Mice.
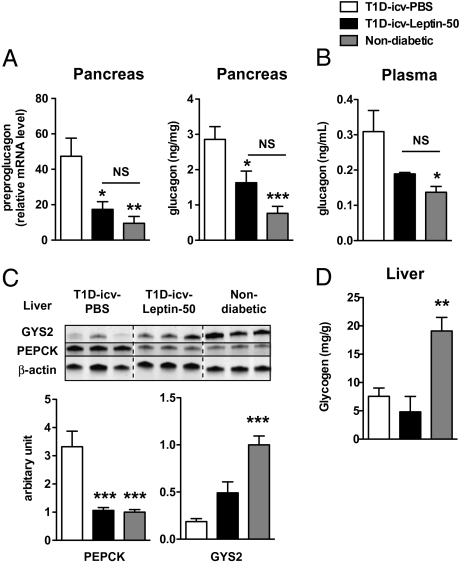
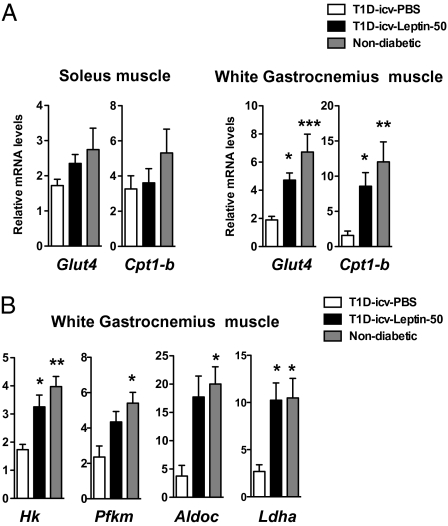
Lack of insulin increases production of the glycemia-increasing hormone glucagon, an effect thought to underlie uncontrolled hyperglycemia in T1D rodents (26) and humans (37). Pancreatic preproglucagon mRNA and glucagon levels as well as plasma glucagon levels were elevated in T1D-icv-PBS mice (Fig. 4 A and B). Strikingly, pancreatic and circulating glucagon levels were almost normalized by icv administration of leptin (Fig. 4 A and B), suggesting that leptin suppresses glucagon via CNS LEPR-descending pathways in the context of insulin deficiency. Alternatively, these glucagon improvements may be secondary to reduced hyperglycemia, because it has been suggested that without insulin, glucose stimulates glucagon secretion from α-cells (38–41). Consistent with reduced glucagon, the hepatic content of phosphoenolpyruvate carboxykinase 1 (an enzyme that mediates the first catalytic reaction in the gluconeogenic pathway, the amounts of which are increased by glucagon) was also elevated in T1D-icv-PBS mice but was normal in T1D-icv-Leptin-50 mice (Fig. 4C). Wang et al. (26) have shown that systemic leptin treatment rescues hepatic glycogen levels in T1D rodents. Our data suggest that this is an effect probably mediated by peripheral LEPRs, because icv administration of leptin did not rescue the reduced levels of hepatic glycogen synthetase 2 (a rate-limiting enzyme in the glycogen synthesis pathway) and glycogen levels seen in T1D mice (Fig. 4 C and D). In the presence of insulin, leptin has been suggested to enhance glucose uptake and fatty acid oxidation in slow-twitch (e.g., soleus) but not fast-twitch (e.g., white gastrocnemius) skeletal muscles fibers (42). Surprisingly, we found that icv leptin treatment increased mRNA levels of the glucose transporter 4 and carnitine palmitoyltransferase 1-b (a protein that mediates the transfer of cytosolic long-chain fatty acid into the mitochondria) in white gastrocnemius muscle but not in soleus muscle (Fig. 5A). In addition, we found that the mRNA levels of key enzymes of the glycolytic pathway (hexokinase, phosphofructokinase, and aldolase) and lactate dehydrogenase were significantly reduced in white gastrocnemius muscle of T1D-icv-PBS mice, defects that underlie, at least in part, the impaired skeletal muscle glucose utilization in T1D. Of note, the expression of all these genes was normalized by icv administration of leptin (Fig. 5B). Altogether, these results indicate that administration of leptin in the CNS reverses hyperglucagonemia and significantly improves metabolic dysfunction in the liver and skeletal muscle of T1D mice.
Fig. 4.
CNS leptin administration improves hyperglucagonemia. Pancreatic levels of preproglucagon mRNA and glucagon levels (A); plasma glucagon contents (B); hepatic phosphoenolpyruvate carboxykinase 1 (PEPCK), glycogen synthetase 2 (GYS2), and β-actin (used as a loading control) protein levels (C); and hepatic glycogen contents (D) in T1D-icv-PBS and T1D-icv-Leptin-50 mice 10 d following stereotaxic surgery and in age-matched nondiabetic control mice. Error bars represent SEM (n = 4–7). Statistical analyses were done using one-way ANOVA (Tukey's posttest). *P < 0.05; **P < 0.01; ***P < 0.001 vs. T1D-icv-PBS mice. NS, not statistically different.
Fig. 5.
CNS leptin administration improves parameters related to glucose and fat metabolism in skeletal muscle. Glut4 and Cpt1-b mRNA levels in soleus muscle and white gastrocnemius muscle (A) and mRNA levels of glycolytic pathway enzymes in WG muscle (B) in T1D-icv-PBS and T1D-icv-Leptin-50 mice 10 d following stereotaxic surgery and in age-matched nondiabetic control mice. Error bars represent SEM (n = 4–7). Statistical analyses were done using one-way ANOVA (Tukey's posttest). *P < 0.05; **P < 0.01; ***P < 0.001 vs. T1D-icv-PBS mice.
CNS Leptin Delivery Does Not Induce Significant β-Cell Regeneration in T1D Mice.
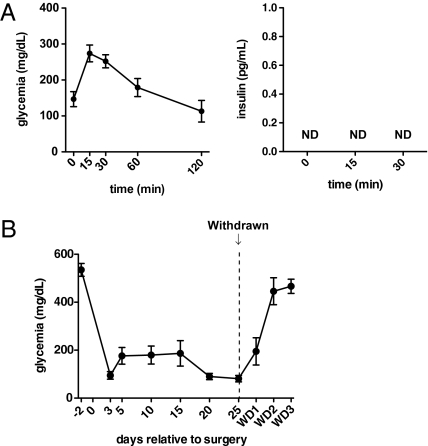
Under extreme circumstances, such as after the loss of more than 70% of pancreatic β-cells, regeneration of β-cells can occur from homologous and/or heterologous origins in mice (43). Thus, we investigated whether icv administration of leptin promotes β-cell regeneration, an effect that, in principle, could explain the remarkable metabolic improvements observed in T1D-icv-Leptin-50 mice. Our data argue against this idea, because (i) pancreatic insulin content and β-cell number were not different between T1D-icv-Leptin-50 and T1D-icv-PBS mice (Fig. 1 A and B) and (ii) serum insulin levels were undetectable before (Fig. 1A) and even after a glucose overload in T1D-icv-Leptin-50 mice (Fig. 6A). Moreover, within 48 h after the icv leptin treatment was interrupted, glycemia increased and reached the extremely high levels seen before leptin administration began in T1D-icv-Leptin-50 mice (Fig. 6B). These data suggest that in T1D, icv leptin administration does not cause long-lasting changes (e.g., increased β-cell number) able to maintain near-normal glycemia after treatment is halted.
Fig. 6.
Effects of icv leptin delivery in insulin-deficient mice quickly vanish after leptin withdrawal (WD). (A) Oral glucose tolerance test (2 g/kg of body weight) in T1D-icv-Leptin-50 mice 10 d after surgery. Glycemia levels (Left) and insulin levels (Right) in the blood. ND, below the threshold of detection. (B) Glycemia before and after icv leptin administration was interrupted in T1D-icv-Leptin-50 mice. WD1, WD2, and WD3 in the x axis indicate 1, 2, and 3 d after leptin administration was interrupted, respectively. Error bars represent SEM (n = 7).
Discussion
We found that icv administration of leptin is sufficient to restore near-normal metabolic homeostasis without the use of therapeutic insulin in insulin-deficient mice. These findings significantly differ from those of previously published work, in which enhanced CNS LEPR signaling was shown to improve glucose homeostasis in hypoinsulinemic rodents (29). In fact, mainly because (i) leptin and insulin partly share their intracellular signaling pathways (e.g., the PI3K signaling cascade) (21, 22) and (ii) leptin has been shown to enhance insulin sensitivity in rodents and humans (19, 20, 23, 24), the effects of central leptin delivery on glucose balance in the context of hypoinsulinemia were likely attributable to synergistic actions between administered leptin and residual insulin. As such, the general consensus has been that circulating insulin, even at very low levels, is required for the antidiabetic actions of leptin (44). Here, we provide data establishing the brain as a critical site for mediating leptin's metabolic-improving actions in the context of T1D, in which insulin is absent. In fact, many of the major improvements caused by s.c.-administered leptin (e.g., reduced hyperglycemia and hyperglucagonemia, increased body weight and survival) (26) were also observed in our T1D mice, in which leptin was administrated centrally. Additionally, we found that this treatment improves expression of genes of the gluconeogenic and glycolytic pathways in liver and fast-twitch skeletal muscle fibers, respectively, suggesting that icv leptin may also lead to suppressed hepatic glucose production and enhanced skeletal muscle glucose uptake. These effects could, in principle, underlie the improved glucose homeostasis observed in icv leptin-treated T1D mice. However, future studies using radiolabeled tracers are required to assess the effects of icv administration of leptin directly on hepatic glucose production and skeletal muscle/adipose tissue glucose uptake in the context of T1D.
Because leptin was chronically delivered into the cerebral ventricles, it is possible that icv-administered leptin may have leaked into the systemic circulation in physiologically significant amounts, an effect that could have confounded our analyses. However, our data argue against this possibility, because (i) to prevent lethality and ameliorate diabetes, s.c.-administered leptin had to cause leptinemia to increase up to ~40 ng/mL (26), an amount that is ~40 time higher than that seen in T1D-icv-Leptin-50 mice (Fig. 2C); (ii) the phosphorylation status of STAT3 was significantly elevated in the liver of T1D mice receiving sc-administered leptin (indicating enhanced hepatic LEPR signaling) (26) but not in our T1D-icv-Leptin-50 mice compared with placebo-treated T1D controls (Fig. 2D); and (iii) leptinemia was not different between T1D-icv-PBS mice and T1D-icv-Leptin-50 mice (Fig. 2C). Our data also suggest that icv leptin effects are not the consequence of β-cell regeneration, because (i) pancreatic insulin levels in T1D-icv-PBS and T1D-icv-Leptin-50 mice were similarly extremely low (Fig. 1A), (ii) plasma insulin was not detectable even after administration of oral glucose (Fig. 6A), and (iii) the glycemia-lowering actions of leptin vanished within 48 h after the treatment was halted (Fig. 6B).
An important step forward in our understanding of the CNS LEPR descending pathways critical for mediating the effects of leptin in T1D will be the identification of the LEPR-expressing neurons governing these pathways. Because our icv leptin delivery was not specific to any brain structures, the identity of the neurons mediating leptin's anti-T1D effects is still unknown. However, we suggest that two distinct neuronal groups located in the ventromedial hypothalamic (VMH) and ARH nuclei may be key components of neurocircuitries that mediate the glycemia-lowering actions of leptin in T1D. For example, ARH neurons (specifically, POMC neurons) are known to convey leptin signaling into coordinated glucose homeostasis (17, 18). In addition, VMH neurons are important, as demonstrated by the fact that microinjections of leptin into the VMH of lean mice increase glucose uptake in the skeletal muscle, heart, and brown adipose tissue (45, 46). Also, VMH glutamatergic tone has been shown to be required for normal glucagon secretion (47). Future work in which leptin monotherapy effects are assayed in insulin-deficient mice expressing LEPRs in a neuron type-specific fashion, such as only in POMC and/or VMH neurons, is therefore warranted. Results from these studies will likely uncover previously undescribed molecular components that could become the targets of better, and perhaps even insulin-free anti-T1D strategies.
Methods
Mice and Stereotaxic Surgery.
Friend virus B/N male mice were housed with food (standard show diet) and water available ad libitum in a light-controlled (12-h light/dark cycle with lights-on from 6:00 AM until 6:00 PM and lights-off from 6:00 PM until 6:00 AM) and temperature-controlled (23 °C) environment. Care of mice was within the Institutional Animal Care and Use Committee (IACUC) guidelines, and all the procedures were approved by the University of Texas Southwestern Medical Center IACUC. Additional details regarding methods can be found in SI Methods.
Supplementary Material
Acknowledgments
We thank the following individuals at the University of Texas Southwestern Medical Center: Kristen Wertz and Charlotte Lee for technical assistance; Dr. Joyce Repa (for quantitative real-time PCR primers); Dr. Makoto Fukuda (for helping with Western blotting assays); and Drs. Roger Unger, Joel Elmquist, and Jeffrey Zigman (for advice). This work was supported by start-up funding from the Department of Internal Medicine/Division of Hypothalamic Research, University of Texas Southwestern Medical Center (to R.C.), an American Heart Association postdoctoral fellowship (to G.R.) and Scientist Development grant (to R.C.), and National Institutes of Health Grants DK080836 (to R.C.) and DK068069-01A2 to Dr. Jeffrey Zigman.
Footnotes
This article is a PNAS Direct Submission.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008025107/-/DCSupplemental.
References
- 1.Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev. 2010;9:A355–A365. doi: 10.1016/j.autrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 3.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus—Progress made, more to be done. J Clin Endocrinol Metab. 2006;91:3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- 6.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 7.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu HY, et al. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM) J Biol Chem. 2009;284:27090–27100. doi: 10.1074/jbc.M109.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen J, et al. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes. 2002;51:2637–2641. doi: 10.2337/diabetes.51.8.2637. [DOI] [PubMed] [Google Scholar]
- 10.Orchard TJ, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 11.Cryer PE. Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J Clin Invest. 2006;116:1470–1473. doi: 10.1172/JCI28735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cryer PE. Hypoglycemia: Still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14:750–756. doi: 10.4158/EP.14.6.750. [DOI] [PubMed] [Google Scholar]
- 14.Cryer PE. Preventing hypoglycaemia: What is the appropriate glucose alert value? Diabetologia. 2009;52:35–37. doi: 10.1007/s00125-008-1205-7. [DOI] [PubMed] [Google Scholar]
- 15.O'Rahilly S, Farooqi IS, Yeo GS, Challis BG. Minireview: Human obesity-lessons from monogenic disorders. Endocrinology. 2003;144:3757–3764. doi: 10.1210/en.2003-0373. [DOI] [PubMed] [Google Scholar]
- 16.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 17.Coppari R, et al. The hypothalamic arcuate nucleus: A key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Huo L, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton GJ, et al. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 20.German J, et al. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009;150:4502–4511. doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill JW, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda M, et al. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 24.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 25.Koch L, et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang MY, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2002;282:E1084–E1091. doi: 10.1152/ajpendo.00489.2001. [DOI] [PubMed] [Google Scholar]
- 28.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 29.Kojima S, et al. Central leptin gene therapy, a substitute for insulin therapy to ameliorate hyperglycemia and hyperphagia, and promote survival in insulin-deficient diabetic mice. Peptides. 2009;30:962–966. doi: 10.1016/j.peptides.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Mansford KR, Opie L. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet. 1968;1:670–671. doi: 10.1016/s0140-6736(68)92103-x. [DOI] [PubMed] [Google Scholar]
- 31.Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 32.Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MW, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 36.Halaas JL, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobbs R, et al. Glucagon: Role in the hyperglycemia of diabetes mellitus. Science. 1975;187:544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- 38.Salehi A, Vieira E, Gylfe E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes. 2006;55:2318–2323. doi: 10.2337/db06-0080. [DOI] [PubMed] [Google Scholar]
- 39.Braaten JT, Faloona GR, Unger RH. The effect of insulin on the alpha-cell response to hyperglycemia in long-standing alloxan diabetes. J Clin Invest. 1974;53:1017–1021. doi: 10.1172/JCI107638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamori D, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen HL, et al. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology. 2005;146:4861–4870. doi: 10.1210/en.2005-0800. [DOI] [PubMed] [Google Scholar]
- 42.Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 43.Thorel F, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalra SP. Central leptin gene therapy ameliorates diabetes type 1 and 2 through two independent hypothalamic relays; A benefit beyond weight and appetite regulation. Peptides. 2009;30:1957–1963. doi: 10.1016/j.peptides.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haque MS, et al. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–1712. doi: 10.2337/diabetes.48.9.1706. [DOI] [PubMed] [Google Scholar]
- 46.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 47.Tong Q, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.