Abstract
Melanopsin-expressing, intrinsically photosensitive retinal ganglion cells (ipRGCs) form a light-sensitive system separate from rods and cones. Direct light stimulation of ipRGCs can regulate many nonimage-forming visual functions such as photoentrainment of circadian rhythms and pupil responses, and can intensify migraine headache in adults. In mice, ipRGCs are light responsive as early as the day of birth. In contrast, their eyelids do not open until 12–13 d after birth (P12–13), and light signaling from rods and cones does not begin until approximately P10. No physiological or behavioral function is established for ipRGCs in neonates before the onset of rod and cone signaling. Here we report that mouse pups as young as P6 will completely turn away from a light. Light-induced responses of ipRGCs could be readily recorded in retinas of pups younger than P9, and we found no evidence for rod- and cone-mediated visual signaling to the RGCs of these younger mice. These results confirm that negative phototaxis is evident before the onset of rod- and cone-mediated visual signaling, and well before the onset of image-forming vision. Negative phototaxis was absent in mice lacking melanopsin. We conclude that light activation of melanopsin ipRGCs is necessary and sufficient for negative phototaxis. These results strongly suggest that light activation of ipRGCs may regulate physiological functions such as sleep/wake cycles in preterm and neonatal infants.
Keywords: intrinsically photosensitive retinal ganglion cells, phototaxis, retina, visual behavior
Melanopsin, a photopigment sensitive to blue light, is expressed in a subset of retinal ganglion cells (RGCs) in the mammalian eye. Light can activate these neurons in the absence of any visual signaling from rods and cones (1, 2). In adult rodents and primates, including humans, light stimulation of “intrinsically photosensitive” RGCs (ipRGCs) can drive pupillary constriction, suppression of nocturnal locomotor activity (negative masking), suppression of circadian melatonin release, and photo entrainment of circadian locomotor rhythms (3–5). In addition, light activation of ipRGCs exacerbates migraine headache intensity (6) and is implicated in regulation of alertness and cognitive functions (7).
During development, melanopsin expression begins before birth in rodents and humans (8). Physiologically, in mouse retina, melanopsin-expressing ipRGCs can be activated by light as early as the day of birth (9, 10). Concomitantly, by postnatal day 1 (P1), ipRGCs express vesicular glutamate transporter type 2, necessary for the synaptic release of glutamate from ipRGCs (11), and project their axons to the suprachiasmatic nucleus (SCN) of the hypothalamus (12). Furthermore, the retina-SCN tract in rats can be activated by light immediately after birth (9, 10, 13).
In marked contrast to the early onset of ipRGC light sensitivity and signaling, rod- and cone-mediated visual signaling in retina is not known to be functional until at least P10 in rodents (14–16). The significance of having a functional, ipRGC-driven visual system in young neonates before the onset of rod and cone vision is not known.
Although airborne hearing and image-forming vision in rodents do not begin functioning until well after birth (ear canals and eyelids open at ~P10 and P13, respectively), mouse and rat pups exhibit many reflexive sensory behaviors well before the age of P10. Examples are rooting in response to bilateral stimulation of the snout (after P2), righting in response to being on back (after P2), and negative geotropism in which a pup will orient upward on an inclined plane (after P5) (17). Negative phototaxis, a turning away from a bright light, is another reflexive sensory behavior seen in rat pups as early as P6 (18, 19). This behavior requires stimulation of the eyes, but the photodetectors responsible for the negative phototaxis have yet to be identified. Here, we report that neonatal mice exhibit negative phototaxis similar to that in rats. Using mice with a genetic deletion of melanopsin, we tested the hypothesis that melanopsin-expressing retinal neurons mediate this phototaxis.
Results
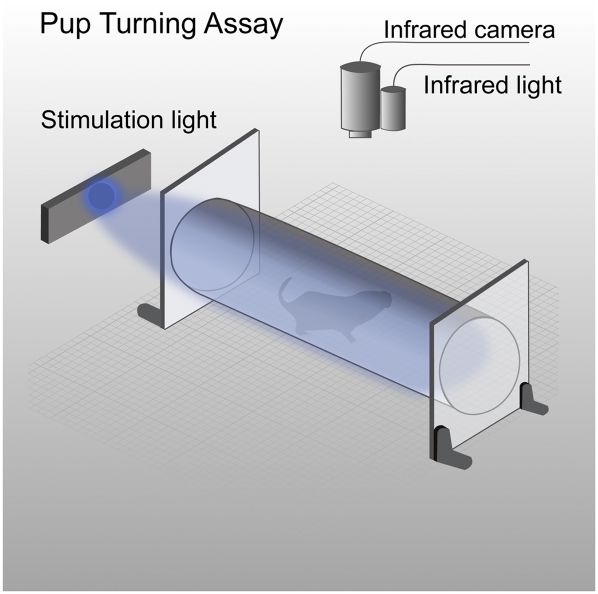
To test and quantify phototaxis in neonatal mice, we recorded the movement of individual pups inside a cylindrical transparent tube with an infrared video camera (Fig. 1). A pup's movement and orientation during periods of darkness were compared with those following light stimulation. The light was directed initially at their face along the long axis of the testing chamber. Following the onset of stimulation, we observed that pups would begin waving their heads back and forth, often termed “pivoting” (17). The pups would then reverse their orientation by turning their whole body completely away from the light, commonly called “negative phototaxis” (Fig. S1 and Movie S1). Because this complete body turn provided an unambiguous outcome, we adopted a 180° turn as the primary metric of a mouse pup's response to light. Each pup's orientation was monitored for 5 min in darkness and 5 min during light stimulation. For the initial characterization of phototactic behavior, we compared (i) the latency to the first complete body turn away from the light to the latency to the first turn from the original orientation in darkness, and (ii) the total time over each 5-min dark or light period that the pup kept its body pointed in its original orientation.
Fig. 1.
Phototaxis assay for neonatal mouse pups. Schematic diagram of test chamber, recording instrument and light stimulator (described in detail in SI Materials and Methods).
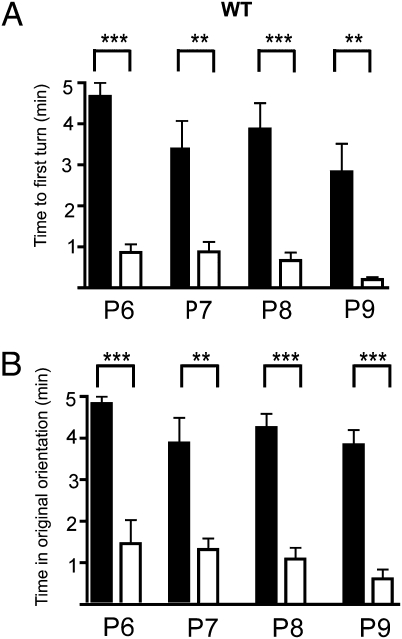
Pups in darkness spent most of their 5-min test period faced in their original orientation and had a relatively long average latency to their first turn. Average latencies to first turn in darkness ranged from 4.66 ± 0.33 (SEM) min at P6 to 2.83 ± 0.68 min at P9. Following light onset, the pups turned away from the light in less than 1 min at all tested ages (P6–P9). The average latency to first turn when stimulated was 76% shorter at P6 and 93% shorter at P9 than in darkness (Fig. 2A). In darkness the P6 animals spent an average 4.83 ± 0.17 min in their original orientation. At older ages the pups were slightly more active in darkness; but even at P9, the animals spent an average of 3.84 ± 0.35 min faced in the original orientation. In response to light, mouse pups spent 66% (P6) to 84% (P9) less total time faced in the original position than in darkness (Fig. 2B). The dark/light time differences were significant (P < 0.001 for all ages). These findings document a robust negative phototaxis in mice as young as P6. Because the mice remained turned away from the light most of the time during stimulation, this argues that the pups could actually detect from which direction the light originated and that light did not simply stimulate locomotor activity in a generalized way.
Fig. 2.
Negative phototaxis is evident as early as postnatal day 6 (P6) in WT mice. (A) Average latencies to first complete turn for P6-, P7-, P8-, and P9-aged pups. (B) Average time that pups faced in their original orientation (detailed descriptions of protocols, measurements, and statistical analyses in SI Materials and Methods) Dark and light bars show data from dark and light-stimulation, respectively. Statistical differences (t test) between light–dark latencies were significant: P < 0.0001 at P6; P < 0.003 at P7; P < 0.0001 at P8 and P < 0.005 at P9. The statistical differences in original position duration in the same animals were also significant at all ages: P < 0.0002 (P6; n = 6), P < 0.001 (P7; n = 9), P < 0.0001 (P8; n = 15), and P < 0.0001 (P9; n = 5). ***P < 0.001; **P < 0.01. Error bars represent SEM.
The responses to light were observed at irradiance levels consistent with activation of ipRGCs and were not due to heat emitted by the light-emitting diode (LED). We found threshold irradiance for negative phototaxis was ~1.5 × 1013 photons/s/cm2 at the eyelids (λ = 468 nm). Conservatively estimating 1% light transmission through mouse pup eyelid at 470 nm (~1%, 2%, 10% for Siamese, black, and white cat eyelids, respectively, and 7% for macaque eyelids) (20), this irradiance is comparable to the light intensities required to induce pupil constrictions and photic shifts in the phase of circadian rhythms in adult mice lacking rod and cone function (1010–1011 photons/s/cm2; 3, 21). No changes in the thresholds for negative phototaxis were discerned when infrared emissions were blocked with a heat filter. Direct measurement of the spectral output of the LED at infrared wavelengths (>700 nm) indicated the flux density was at least four orders of magnitude below the flux density at the peak wavelength (468 nm; manufacturer's specification and direct measurement; Photo Research, PR670 spectraradiometer). Moreover, Crozier and Pincus (22) noted that neonatal rat pups are attracted to heat sources and repelled by light. We conclude that the negative phototaxis is due to visible light emitted by the LED and that the irradiance level is in the range to activate ipRGCs.
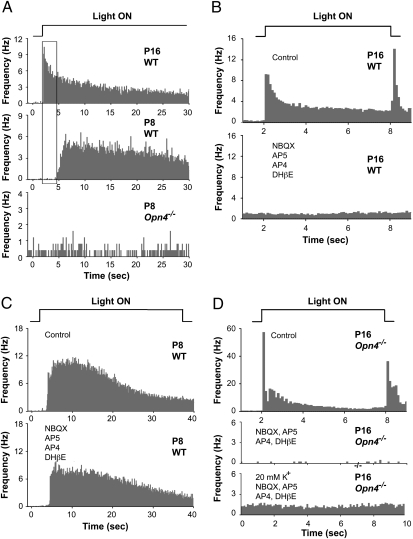
In adult rats, ipRGCs receive synaptic inputs from bipolar and amacrine cells (23). These inputs provide pathways for visual signals from rods and cones to activate ipRGCs (24) and also to elicit melanopsin-independent nonimage-forming behaviors (25–27). Although there is no evidence for visual signaling from rods and cones to inner retina before P10 in the mouse (14–16), it is not known whether rods and cones could be exciting ipRGCs via an unconventional pathway from rods and cones directly to ipRGCs (28). To test this possibility and to confirm earlier reports, we recorded from large samples of ipRGCs and nonipRGCs in neonatal mice using a multielectrode array (16). By comparing the temporal coding of light responses and by blocking putative rod and cone synaptic inputs pharmacologically, we conclude that no conventional rod- and cone-driven cone excitation of any type of RGC exists in neonatal WT mice younger than P10. Figure 3A shows light-evoked, rod/cone-driven spiking from RGCs in the retina of a P16 WT mouse. This spiking occurs within 100–200 ms of light onset, typical in WT mice after P12 (29). In contrast, no equivalent short latency light-evoked spiking was ever recorded in more than 20 retinas from WT or Opn4−/− neonates younger than P9 (Fig. 3A, middle and bottom traces). However, slower-onset, more sustained ipRGC responses were readily recorded in neonates (Fig. 3A, middle trace, and Fig. 3C).
Fig. 3.
Multielectrode array (MEA) recordings of retinal ganglion cell spiking demonstrate that rod and cone-mediated light responses do not drive ipRGCs or nonipRGCs in mice younger than P10. (A) Histograms of light-evoked spikes from RGCs in response to a 40 s step of light. Top trace: P16 WT mouse; middle trace: P8 WT mouse, and bottom trace: P8 Opn4−/− mouse. (Note: although spontaneous waves of spiking were observed in P8 retinas, none of the spike activity shown here was induced by light.) Only the P16 recording (top record) shows short latency responses attributable to rod and cone signals. Top, middle, and bottom traces are averages of 16, 26, and 5 RGCs that responded to the onset of light, respectively. (B) Histograms of light-evoked spikes recorded from P16 WT mouse. Light stimuli were 6 s in duration, and the timescale is expanded compared with A. Top trace: Responses in control saline. Bottom trace: Recordings from same retina in synaptic blocker mixture of NBQX (20 μM, AMPA/KA glutamate receptor antagonist), DL-AP5 (100 μM, NMDA receptor antagonist), DL-AP4 (20 μM, mGluR6 agonist that blocks light responses in ON bipolar cells) and DHβE (2 μM, an agonist for nicotinic ACh receptors). All light-evoked spiking was eliminated in this mixture. Top and bottom traces are averages of 102 and 85 RGCs, respectively, recorded in the same retina. (C) Histograms of longer latency light-evoked spike responses from ipRGCs in P8 mouse. Top trace: control saline. Bottom trace: Synaptic blocker mixture. Same synaptic blockers used in B did not eliminate the light-evoked ipRGC responses. Top and bottom traces are averages of 66 and 62 RGCs, respectively, recorded in the same retina. Preservation of the long latency sustained light responses attributable to ipRGCs was observed in four of four retinas treated with the drug mixture (P8 and P9). (D) Histograms of light-evoked spikes in P16 Opn4−/− retina. Top trace: Short latency rod- and cone-mediated ON and OFF responses recorded in control saline. Middle trace: recordings in synaptic blocker mixture used in B and C. Bottom trace: spiking activity recorded in response to 20 mM K+ in the presence of the synaptic blocker mixture. Top, middle and bottom traces are averages of 56, 12, and 45 RGCs, respectively, recorded in the same retina. These records demonstrate that rod- and cone-mediated light responses are present in older Opn4−/− mice, and that all this activity is blocked with synaptic blockers, yet the RGCs themselves are still capable of spiking when depolarized with potassium.
To test whether the ipRGC responses required synaptic inputs, we blocked synaptic transmission to RGCs. Figure 3B shows the mixture of NBQX, AP5, AP4, and DHβE (middle trace) (11, 30) effectively blocks the short latency photoreceptor-driven responses in RGCs recorded from a P16 WT mouse (top trace). This same mixture of synaptic blockers failed to block the light-driven ipRGC responses in a P8 WT mouse (Fig. 3C). We interpret these results as evidence that the visual signals that generate either the well known short latency ON and OFF responses or the recently discovered long-latency ON responses (31) in adult mice do not produce similar responses in ipRGCs or non-ipRGCs in WT mice ≤P9.
Given that adult melanopsin-null mice exhibit rod- and cone-driven pupil constrictions (Fig. S2), masking and photoentrainment (25), it would be assumed that photoreceptor visual signals are transmitted through the retina in these older mice. Consistent with postulated rod/cone visual signaling in older Opn4−/− mice, we recorded short-latency, photoreceptor-driven responses in retinas of juvenile Opn4−/− mice (Fig. 3D, top trace). These responses were eliminated in the synaptic blocker mixture, verifying that these were visual responses from rod and/or cones and not an intrinsic response of RGCs (Fig. 3D, middle trace). Increased spiking in high potassium verified that RGC spiking was conserved in the blocker mixture (Fig. 3D, bottom trace). These results substantiate that rod and cone signaling to second- and third-order retinal neurons, including ipRGCs, does not begin until at least P10 (14–16), and that rod and cone signaling is preserved in adult Opn4−/− mice.
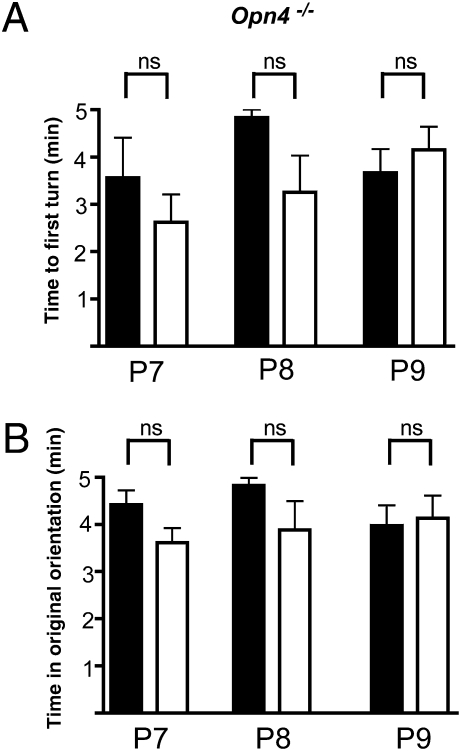
To determine experimentally whether melanopsin ipRGCs mediate negative phototaxis, we tested visual behavior in melanopsin null mice (Opn4−/−) (31). Unlike in WT pups, no statistically significant dark/light differences were observed in turning behavior of Opn4−/− pups. We found no difference in the latency to the first turn between Opn4−/− mice in darkness compared with in light (Fig. 4A). The Opn4−/− pups spent most of the 5-min test period faced in their original orientation whether in darkness or while being illuminated. These findings are consistent with the hypothesis that negative phototaxis in neonatal mice requires melanopsin.
Fig. 4.
Negative phototaxis is absent in neonatal melanopsin null (Opn4−/−) mice. Experiments are identical to those described for Fig. 2. All times are reported in minutes ± SEM. Latencies to first turn in dark averaged 3.56 ± 0.85, 4.83 ± 0.16, and 3.66 ± 0.49, at P7, P8, and P9, respectively. In light, latencies were 2.62 ± 0.58, 3.26 ± 0.77, and 4.15 ± 0.48 at the same ages. Durations in original orientations in the dark averaged 4.82 ± 0.16 min, 3.56 ± 0.85, and 3.97 ± 1.56, respectively at P7, P8, and P9, respectively. In light, average durations were 3.88 ± 0.58, 2.62 ± 0.16, and 4.13 ± 0.48 at the same ages. No light–dark time differences of significance were detected in the latencies to the first complete body turn or for the duration of times spent in the original orientation. P7, n = 7; P8, n = 7; P9, n = 13. Error bars represent SEM.
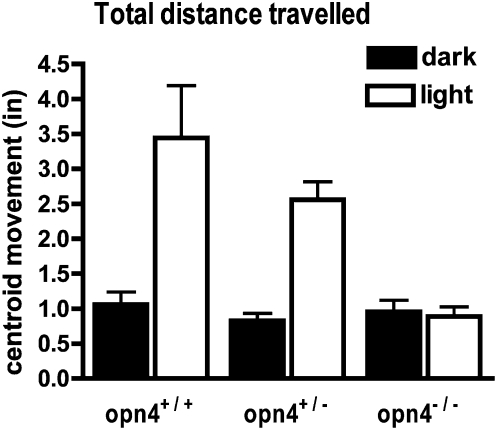
A mouse's ability to learn and perform visual tests or motor tasks can depend significantly on its strain (32, 33). To rule out the possibility that the absence of negative phototaxis could be reflecting a strain difference between the WT (C57BL/6J) mice and the Opn4−/− (mixed 129SvJxC57Bl/6J background) mice, we tested phototactic behavior in littermates bred from Opn4+/− mice. For these comparisons we measured the movement of the pups that occurred before complete turn-around (Movie S1). This alternative measure allowed us to determine behavioral responses over shorter testing epochs. For quantification, we measured and compared the total distance traversed by the calculated center-of-mass (centroid) of a high-contrast 2D image of the mouse pup during a 1-min period of darkness followed by a 1-min period in light (34) (SI Text and Movie S2). Figure 5 shows the average total centroid movement in Opn4+/+, Opn4+/−, and Opn4−/−mice. During the first minute of light stimulation, Opn4+/+ pups moved >3-fold farther than they did in the preceding minute of darkness. Opn4+/− pups moved >2.5-fold farther in light. Light stimulation had no effect on Opn4−/− mice. In both light and dark the total distance of movement of the Opn4−/− mice was comparable to Opn4+/− and Opn4+/+ mice in darkness. Thus, strain differences cannot account for the lack of photic behavior in the Opn4−/− mice compared with WT.
Fig. 5.
Light stimulates movement in Opn4+/+ and Opn4+/− pups, but not in Opn4−/− littermates. Movement was quantified as the distance the centroid of the pup's image traveled during the 60 s test periods (Materials and Methods). Mice were placed in the chamber for periods of 2–5 min in darkness. Distances during the 60-s period before (dark bars) and after light onset (light bars) are plotted for Opn4+/+ (n = 11), Opn4+/− (n = 20), and Opn4−/− (n = 9) mice. Results from P7–P9 aged animals are grouped together. Statistically significant differences between light and dark were observed in the Opn4+/+ and Opn4+/− mice (P < 0.001). No statistically significant light–dark differences were observed in the Opn4−/− mice (P > 0.05). Posttest analysis (Bonferroni) revealed no statistical differences between distances traversed in darkness of +/+, +/−, or −/− littermates. Error bars represent SEM.
Discussion
In summary, we demonstrate here that neonatal mice between P6 and P9 are negatively phototactic. Several findings provide strong evidence that this phototactic behavior is mediated by melanopsin ipRGCs: (i) light activates ipRGCs at birth (10); (ii) visual signaling from rods and cones to RGCs does not begin until P10; (iii) light generates ipRGC responses in the retinas of mice younger than P9 (35) (Fig. 3); and (iv) negative phototaxis is absent in melanopsin null mice (Fig. 4). These findings demonstrate a previously unrecognized function of ipRGCs and suggest that light activation of ipRGCs could regulate other physiological functions in neonates and preterm infants.
Our conclusion about melanopsin being the light detector rests substantially on the absence of negative phototaxis in the Opn4−/− mice. Alternative explanations for the lack of negative phototaxis in these melanopsin null mice include the possibility that either the retinas in these animals have lost their ability to provide visual signaling via ganglion cells, or that these mice have locomotor deficits. Several findings do not support these interpretations. First, histological examination of the retinas of our Opn4−/− mice showed no deterioration of the inner retina (Fig. S3). Second, we observed pupillary responses and light-driven RGCs spikes, presumably originating in rods and cones, in adult and P16 Opn4−/− mice (Fig. 3 and Fig. S2). Third, there was no difference in the latencies to the first turn in darkness for Opn4−/− and WT mice (Fig. 4). Finally, photo entrainment and negative masking by light, as assessed by locomotor activity, is evident in older Opn4 −/− mice (3).
Could we observe negative phototaxis at ages before P6 or after P9? We found the lack of coordinated head and forepaw movements prevented us from reliably observing negative phototaxis before P6 (36). Certainly at ages older than P9, mice have a preference to be located in darker environments. However, quantifying phototaxis in our turning assay proved problematical with older mice. After P9, pups are much less passive in the chamber and spent much more time exploring in the dark or light.
The specific neural pathways mediating negative phototaxis, a complex and coordinated sensory motor behavior, remain to be identified. Three findings suggest signaling via the tectospinal tract: (i) Retrograde WGA-HRP injections demonstrate innervation of spinal cord by superior colliculus (SC) neurons as early as P5 in rat (37), (ii) lesions of the dorsal midbrain, involving the SC and tectum, abolished negative phototaxis in neonatal rat pups (38); and (iii) melanopsin ipRGCs project to the SC in adult mice (39). Whether ipRGCs project to the SC in P6- to P9-aged mice, and whether coordination of movement from the cerebellum or cortex is involved in negative phototaxis in neonates are unknown.
Given that negative phototaxis could be classified as a sensory reflex with other reflexes such as negative geotaxis, rooting, and the righting reflex, it is quite plausible that negative phototaxis has important survival value for young pups. Because of limited fat reserves adult mice forage during the day and the night (40). When pups are ~1 wk old, their mothers spend a total of 4–5 h per day away from the nest (41), which under natural conditions would most likely be located in a darker niche. If unattended neonatal pups wandered from the nest into a lighted environment while the mother was absent from the nest, they would be driven back toward the nest by negative phototaxis.
That melanopsin ipRGCs can mediate a complex visual behavior in the absence of rods and cones raises the important possibility that this system might provide a rudimentary visual sense in circumstances when the rods and cones are nonfunctional. This situation could include congenital blindness in childhood diseases such as Lebers congenital amaurosis and adult onset diseases such as retinitis pigmentosa. Even should ipRGCs not support image-forming vision, these photosensitive cells could regulate sleep/wake cycles and other well-known photo entrainable physiological processes. In support of this idea, light has been shown to control biological clock genes in premature primate infants (42), modulate heart rates in neonatal rat pups (43), and entrain pineal N-acetyltransferase rhythms in rat pups as young as P6 (44). In blind adult human patients lacking functional rod and cone function, blue light, by presumably activating melanopsin cells, was able to reset patients’ circadian clocks and increase alertness (7) and exacerbate the intensity of migraine headaches (6). Thus, the melanopsin-expressing ganglion cells may serve many more nonimage-forming visual functions than appreciated at present. In particular, lighting conditions may play an under-recognized role in controlling physiological functions, sleep-wake cycles, alertness, and cognitive functions in preterm and neonatal infants.
Materials and Methods
Details of materials and methods used are presented in SI Materials and Methods. Institutional animal care and use committees at the University of California, San Francisco, and Washington University approved all animal procedures. All of the experiments met the guidelines of the National Institutes of Health, Public Health Service Policy, and the Society for Neuroscience Policy on the Use of Animals in Neuroscience Research.
Phototaxis Assay.
Pup movements in a cylindrical chamber were recorded with an infrared video camera. Each pups’ position was monitored for a 5-min period in darkness and during a 5-min period after a light was directed at the pup's face. Responses to light were quantified by comparing latencies to first turn from original position (in darkness) to first turn after light onset. Other measures of phototaxis were comparisons of total time spent in original position (light versus dark), and the amount of movement 1 min before light onset versus 1 min after light onset.
Light Stimuli.
A bright-blue LED (Jameco 183222, 468 nm λmax, 0.2 mW/cm2) provided photic stimulation.
Supplementary Material
Acknowledgments
We acknowledge valuable discussions with Lawrence Sincich, Kata Rabl, and Anton Delvig and technical help from Christina Chun, Doug Yasumura, Charlotte Oelerich, and Suling Wang. This research was supported by National Institutes of Health Grants EY01869, EY002162, and EY07120 and by Research to Prevent Blindness, March of Dimes, the Plum Foundation, and That Man May See.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008533107/-/DCSupplemental.
References
- 1.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 2.Van Gelder RN. Non-visual photoreception: sensing light without sight. Curr Biol. 2008;18:R38–R39. doi: 10.1016/j.cub.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 5.Lucas RJ, Freedman MS, Muñoz M, Garcia-Fernández JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 6.Noseda R, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaidi FH, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarttelin EE, et al. Expression of opsin genes early in ocular development of humans and mice. Exp Eye Res. 2003;76:393–396. doi: 10.1016/s0014-4835(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 9.Hannibal J, Fahrenkrug J. Melanopsin containing retinal ganglion cells are light responsive from birth. Neuroreport. 2004;15:2317–2320. doi: 10.1097/00001756-200410250-00003. [DOI] [PubMed] [Google Scholar]
- 10.Sekaran S, et al. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson J, et al. Vesicular glutamate transporter 1 is required for photoreceptor synaptic signaling but not for intrinsic visual functions. J Neurosci. 2007;27:7245–7255. doi: 10.1523/JNEUROSCI.0815-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver DR, Reppert SM. Definition of the developmental transition from dopaminergic to photic regulation of c-fos gene expression in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1995;33:136–148. doi: 10.1016/0169-328x(95)00117-b. [DOI] [PubMed] [Google Scholar]
- 14.Takada Y, et al. A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Invest Ophthalmol Vis Sci. 2004;45:3302–3312. doi: 10.1167/iovs.04-0156. [DOI] [PubMed] [Google Scholar]
- 15.Bakall B, et al. Expression and localization of bestrophin during normal mouse development. Invest Ophthalmol Vis Sci. 2003;44:3622–3628. doi: 10.1167/iovs.03-0030. [DOI] [PubMed] [Google Scholar]
- 16.Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 17.Fox WM. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 1965;13:234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- 18.Crozier WJ, Pincus G. Phototropism in young rats. J Gen Physiol. 1927-27;10:407–417. doi: 10.1085/jgp.10.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandel GL, Bedell HE, Walker R, Wolf BM. Negative phototaxis in pigmented, albinotic and RCS rat pups measured with a new technique. Vision Sci. 1987;1:357–366. [Google Scholar]
- 20.Crawford ML, Marc RE. Light transmission of cat and monkey eyelids. Vision Res. 1976;16:323–324. doi: 10.1016/0042-6989(76)90118-8. [DOI] [PubMed] [Google Scholar]
- 21.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 22.Crozier WJ, Pincus G. Photic stimulation of young rats. J Gen Psychol. 1937;17:105–111. [Google Scholar]
- 23.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 24.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Göz D, et al. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatori M, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Güler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson PT, Williams RR, Cusato K, Reese BE. Rods and cones project to the inner plexiform layer during development. J Comp Neurol. 1999;414:1–12. [PubMed] [Google Scholar]
- 29.Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, et al. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Invest Ophthalmol Vis Sci. 2007;48:1268–1275. doi: 10.1167/iovs.06-0925. [DOI] [PubMed] [Google Scholar]
- 31.Rentería RC, et al. Intrinsic ON responses of the retinal OFF pathway are suppressed by the ON pathway. J Neurosci. 2006;26:11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 33.Dierssen M, et al. Neurobehavioral development of two mouse lines commonly used in transgenic studies. Pharmacol Biochem Behav. 2002;73:19–25. doi: 10.1016/s0091-3057(02)00792-x. [DOI] [PubMed] [Google Scholar]
- 34.Lagali PS, et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 35.Tu DC, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Blanck A, Hård E, Larsson K. Ontogenetic development of orienting behavior in the rat. J Comp Physiol Psychol. 1967;63:327–328. doi: 10.1037/h0024367. [DOI] [PubMed] [Google Scholar]
- 37.Okoyama S. Anatomical plasticity of the tectospinal tract after unilateral lesion of the superior colliculus in the neonatal rat. Exp Brain Res. 1991;85:552–558. doi: 10.1007/BF00231739. [DOI] [PubMed] [Google Scholar]
- 38.Routtenberg A, Strop M, Jerdan J. Response of the infant rat to light prior to eyelid opening: Mediation by the superior colliculus. Dev Psychobiol. 1978;11:469–478. doi: 10.1002/dev.420110510. [DOI] [PubMed] [Google Scholar]
- 39.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: Cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goulding EH, et al. A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci USA. 2008;105:20575–20582. doi: 10.1073/pnas.0809053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King JH. In: Maternal Behavior in Mammals. Rheingold HL, editor. New York: Wiley; 1963. [Google Scholar]
- 42.Hao H, Rivkees SA. The biological clock of very premature primate infants is responsive to light. Proc Natl Acad Sci USA. 1999;96:2426–2429. doi: 10.1073/pnas.96.5.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dailey W, Wigal SB, Amsel A. Effects of photic stimulation on heart rate of infant rats. Int J Psychophysiol. 1986;3:183–204. doi: 10.1016/0167-8760(86)90027-9. [DOI] [PubMed] [Google Scholar]
- 44.Duncan MJ, Banister MJ, Reppert SM. Developmental appearance of light-dark entrainment in the rat. Brain Res. 1986;369:326–330. doi: 10.1016/0006-8993(86)90544-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.