Phase transitions are phenomena of enduring fascination for scientists studying condensed matter (e.g., the ubiquitous solids and liquids). Much of our ability to rationalize phenomena in the natural world and to design or make new materials depends on our ability to characterize the properties of different phases that substances can exist in and predict conditions under which transitions occur between them. A unique phase transition that may occur between two liquid states of a pure substance (1–4) has been the focus of considerable attention recently. Beye et al. (5), in work reported in PNAS, study such a liquid–liquid transition in silicon, using femtosecond (fs) pump-probe spectroscopy that allows them to follow the structural evolution of a silicon crystal over tens of picoseconds (ps) after it has been optically excited by a laser. Apart from the impressive developments in technique that make these ultrafast measurements possible, measurements on such short time scales turn out to be quite essential to observe the transition of interest, and therein lies the significance of the reported results.
The possibility of a liquid–liquid transition has been studied for silicon, germanium, water, silica, carbon, hydrogen, etc.—substances that form a significant component of our natural world and of importance for technology. Understanding a phenomenon common to these substances has broad implications, including for biomolecular systems (6). Most of the substances mentioned above possess energetically stabilized open structures (e.g., fourfold coordinated tetrahedral bonding geometry in water and silicon), which characterize the liquid (and solid) at low temperature and pressure, whereas at high pressures and temperatures the liquid is characterized by a denser packing of atoms or molecules. The proposed liquid phases are thus termed low-density liquid (LDL) and high-density liquid (HDL).
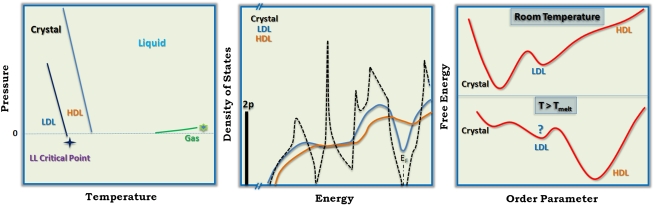
From calorimetric data, Spaepen and Turnbull (7) and Bagley and Chen (8) long ago proposed an unusual transition between amorphous solid and liquid silicon, 200–300 °K below the melting temperature of 1,685 °K. After more recent investigations, this transition has been reinterpreted as a liquid–liquid transition and demonstrated in computer simulations (3, 9–11) but at a lower temperature around 1,100 °K. Clear experimental verification is hard (12) because the transition is expected to occur under conditions in which the crystal is the stable phase. The liquid is therefore metastable (Fig. 1A) and transforms to the stable crystal phase on nanosecond time scales. Such short life times make reliable measurements near the expected transition hard, although indirect evidence has been obtained using, for example, short-duration laser or heavy ion irradiation (13, 14). [Similarly, the estimated transition for water, at 220 °K, lies in the “no man's land” that is inaccessible due to crystallization (15, 16), and attempts to circumvent this limit, for example using confined
Fig. 1.
(A) Schematic of the expected phase diagram of silicon. Liquid–liquid transition occurs below the melting temperature, in the metastable supercooled liquid, and the critical end point is expected to occur at negative pressures. (B) Electronic DOS of the crystal, LDL, and HDL phases is shown schematically. The valence band edge is indicated by the Fermi energy EF for the crystal. (C) Schematic representation of the free energies of the different phases. At room temperature, the crystal phase has the lowest free energy. The LDL is expected to be a local minimum of the free energy, whereas the HDL is not. At temperatures above the melting temperature Tmelt, the crystal will constitute a local free energy minimum, and HDL, the stable phase, the global free energy minimum. Interpretation of the experiments by Beye et al. (5) requires the LDL to be a locally stable phase (represented here by a local free energy minimum).
The work of Beye et al. undoubtedly opens an exciting window to experimental verification of liquid–liquid transitions.
water (17), raise other concerns—about the role of confinement (18)—that need to be resolved.]
The experiments by Beye et al. are the latest attempt to beat the barrier imposed by the short life times of the liquid phases. They do so by using ultrafast spectroscopy that cleverly uses the differences in the electronic structure of the crystal, LDL, and HDL phases of silicon as a probe. The central quantity here is the electronic “density of states” (DOS), a histogram of the number of states available for occupation as a function of energy. The gap between the valence band (the highest band of states corresponding to electrons bound to individual atom) and the conduction band (corresponding to delocalized electrons) determines the electrical properties of a substance. The DOS are characteristically different for the silicon crystal, LDL, and HDL phases. Crystalline silicon is a semiconductor, with a gap between the valence and conduction bands. On the other hand, as recently shown through computer simulation studies (9–11), the band gap gets partially filled in the case of LDL, resulting in moderate conductivity, whereas HDL has no band gap and is a metal (Fig. 1B).
Beye et al. expose a sample of crystalline silicon to a strong 3.1-eV optical laser pulse of short duration (120 fs) that excites electrons from the valence band into the conduction band. Very quickly, in a few femtoseconds, electron–electron interactions lead to thermalization among the electrons, and one obtains a profile of occupied states that corresponds to high temperatures, in the range of 6,000–7,000 °K. On these time scales, the crystal structure remains unaffected, and consequently, the electronic DOS is unchanged, only occupied with a much higher effective temperature. Interactions between electrons and phonons (lattice vibrations) eventually (on a picosecond time scale) lead to a transfer of energy to the lattice, which melts. As the lattice melts and the atomic structure changes, so does the electronic DOS. If the melting crystal transforms first to LDL before its conversion to HDL, signatures of such a two-step change ought to be seen in the electronic structure, which in turn determines the distribution of occupied states. The key is then to detect the distribution of occupied states, as the transformation proceeds.
Beye et al. achieve this by using a 30-fs-wide, 117-eV probe X-ray pulse, whose arrival after the pump pulse can be controlled with subpicosecond resolution. The energy of the probe photons is comparable to the energy difference (roughly 100 eV) between that of the strongly bound 2p core electrons and the energy at the valence–conduction band gap. The probe pulse therefore excites the 2p electrons into the conduction band (Fig. 1B). The core vacancies so created are almost instantly filled by a valence electron, and the energy difference between the two states is emitted as an X-ray photon, which is detected. The count of such detected photons, resolved according to their energy, then provides an estimate of the electronic DOS.
Monitoring the evolution of the DOS over tens of picoseconds, Beye et al. find that the change occurs as a two-step process. At the end of the first step, in the time window of 2–4 ps, the band gap is partially filled, and at the end of the second step, the band gap is fully filled. Comparison with the calculated DOS for LDL and HDL leads to the interpretation that the silicon crystal transforms to the LDL during the first step and from LDL to HDL during the second.
These measurements are possibly the most direct observations of the existence of two liquid phases in silicon. However, the interpretation of the results is not beyond question. Although the electronic DOS observed at intermediate times is similar to that of LDL, it is possible that what is observed is a transient intermediate structure, with an LDL-like DOS, and no relation otherwise to the proposed phase. The fact that the authors observe a two-step change in the electronic structure argues against this possibility, because the presence of a plateau indicates the existence of a local free energy minimum, which may be associated with a metastable phase. Could, however, the two-step behavior be due to an initial break up of crystalline order, followed by a slower density change, as the authors themselves suggest as part of what is going on? The LDL must exist as a metastable state above the crystal melting temperature of 1,685 °K, to be observed upon melting the crystal. This is not ruled out, but the somewhat large superheating range required for LDL has also not been demonstrated thus far.
Notwithstanding such open questions, the work of Beye et al. undoubtedly opens an exciting window to experimental verification of liquid–liquid transitions, not only in the case of silicon but also, as they note, in other substances, such as water. A study of the electronic structure of the relevant amorphous solids (15, 19) would serve as a useful reference in further investigations. It would also be exciting to perform similar experiments starting with amorphous silicon (20), which could also probe the glass transition in LDL (14), and with sufficient control on the temperature range to which the sample is heated, also resolve questions concerning the transition temperature for the liquid–liquid transition (12). The probe exploited by Beye et al. thus promises significant advances in the experimental investigation of liquid–liquid transitions.
Acknowledgments
I thank Vishwas V. Vasisht for help in preparing the illustrations.
Footnotes
The author declares no conflict of interest.
See companion article on page 16772 in issue 39 of volume 107.
References
- 1.Poole PH, Sciortino F, Essmann U, Stanley HE. Phase behaviour of metastable water. Nature. 1992;360:324–328. [Google Scholar]
- 2.Saika-Voivod I, Sciortino F, Poole PH. Computer simulations of liquid silica: Equation of state and liquid-liquid phase transition. Phys Rev E. 2000;63:011202. doi: 10.1103/PhysRevE.63.011202. [DOI] [PubMed] [Google Scholar]
- 3.Sastry S, Austen Angell C. Liquid-liquid phase transition in supercooled silicon. Nat Mater. 2003;2:739–743. doi: 10.1038/nmat994. [DOI] [PubMed] [Google Scholar]
- 4.Scandolo S. Liquid-liquid phase transition in compressed hydrogen from first-principles simulations. Proc Natl Acad Sci USA. 2003;100:3051–3053. doi: 10.1073/pnas.0038012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beye M, Sorgenfrei S, Schlotter WF, Wurth W, Föhlisch A. The liquid–liquid phase transition in silicon revealed by snapshots of valence electrons. Proc Natl Acad Sci USA. 2010;107:16772–16776. doi: 10.1073/pnas.1006499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar P, et al. Protein glass transition and liquid-liquid critical point of water. Phys Rev Lett. 2006;97:177802. doi: 10.1103/PhysRevLett.97.177802. [DOI] [PubMed] [Google Scholar]
- 7.Spaepen F, Turnbull D. Kinetics of motion of crystal-melt interfaces. In: Ferris SD, Leamy HJ, Poate JM, editors. Laser–Solid Interactions and Laser Processing AIP Conference Proceedings No. 50. New York: American Institute of Physics; 1978. pp. 73–83. [Google Scholar]
- 8.Bagley BG, Chen HS. A calculation of the thermodynamic first order amorphous semiconductor to metallic liquid transition temperature. In: Ferris SD, Leamy HJ, Poate JM, editors. Laser–Solid Interactions and Laser Processing. AIP Conference Proceedings No. 50. New York: American Institute of Physics; 1978. pp. 97–101. [Google Scholar]
- 9.Ashwin SS, Waghmare UV, Sastry S. Metal-to-semimetal transition in supercooled liquid silicon. Phys Rev Lett. 2004;92:175701. doi: 10.1103/PhysRevLett.92.175701. [DOI] [PubMed] [Google Scholar]
- 10.Jakse N, Pasturel A. Liquid-liquid phase transformation in silicon: Evidence from first-principles molecular dynamics simulations. Phys Rev Lett. 2007;99:205702–205704. doi: 10.1103/PhysRevLett.99.205702. [DOI] [PubMed] [Google Scholar]
- 11.Ganesh P, Widom M. Liquid-liquid transition in supercooled silicon determined by first-principles simulation. Phys Rev Lett. 2009;102:075701. doi: 10.1103/PhysRevLett.102.075701. [DOI] [PubMed] [Google Scholar]
- 12.Kim TH, et al. In situ high-energy x-ray diffraction study of the local structure of supercooled liquid Si. Phys Rev Lett. 2005;95:085501. doi: 10.1103/PhysRevLett.95.085501. [DOI] [PubMed] [Google Scholar]
- 13.Thompson MO, et al. Melting temperature and explosive crystallization of amorphous silicon during pulsed laser irradiation. Phys Rev Lett. 1984;52:2360–2363. [Google Scholar]
- 14.Hedler A, Klaumünzer SL, Wesch W. Amorphous silicon exhibits a glass transition. Nat Mater. 2004;3:804–809. doi: 10.1038/nmat1241. [DOI] [PubMed] [Google Scholar]
- 15.Mishima O, Stanley HE. The relationship between liquid, supercooled and glassy water. Nature. 1998;396:329–335. [Google Scholar]
- 16.Debenedetti PG, Stanley HE. Supercooled and glassy water. Phys Today. 2003;56:40–46. [Google Scholar]
- 17.Mallamace F, et al. Evidence of the existence of the low-density liquid phase in supercooled, confined water. Proc Natl Acad Sci USA. 2007;104:424–428. doi: 10.1073/pnas.0607138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angell CA. Insights into phases of liquid water from study of its unusual glass-forming properties. Science. 2008;319:582–587. doi: 10.1126/science.1131939. [DOI] [PubMed] [Google Scholar]
- 19.Deb SK, Wilding M, Somayazulu M, McMillan PF. Pressure-induced amorphization and an amorphous-amorphous transition in densified porous silicon. Nature. 2001;414:528–530. doi: 10.1038/35107036. [DOI] [PubMed] [Google Scholar]
- 20.Johnson SL, et al. Properties of liquid silicon observed by time resolved x-ray absorption spectroscopy. Phys Rev Lett. 2003;91:157403. doi: 10.1103/PhysRevLett.91.157403. [DOI] [PubMed] [Google Scholar]