Abstract
The creation of accessible DNA in the context of chromatin is a key step in many DNA functions. To reveal how ATP-dependent chromatin remodeling activities impact DNA repair, we constructed mammalian genetic models for the INO80 chromatin remodeling complex and investigated the impact of loss of INO80 function on the repair of UV-induced photo lesions. We showed that deletion of two core components of the INO80 complex, INO80 and ARP5, significantly hampered cellular removal of UV-induced photo lesions but had no significant impact on the transcription of nucleotide excision repair (NER) factors. Loss of INO80 abolished the assembly of NER factors, suggesting that prior chromatin relaxation is important for the NER incision process. Ino80 and Arp5 are enriched to UV-damaged DNA in an NER-incision-independent fashion, suggesting that recruitment of the remodeling activity likely takes place during the initial stage of damage recognition. These results demonstrate a critical role of INO80 in creating DNA accessibility for the NER pathway and provide direct evidence that repair of UV lesions and perhaps most bulky adduct lesions requires chromatin reconfiguration.
Keywords: DNA damage, DNA repair, chromatin, remodeling
The highly condensed nature of the chromatin assembly restricts the interaction of DNA with most nuclear factors. To create accessible DNA for various nuclear events, chromatin dynamics is regulated by the coordinated actions of two types of cellular mechanisms, posttranslational modifications of the core histones and ATP-dependent chromatin remodeling. Whereas covalent histone modifications, instigated mainly from regulation of yeast gene transcription, have been extensively studied, less is known about the chromatin remodeling process and how chromatin remodeling impacts various nuclear events.
ATP-dependent chromatin remodeling is catalyzed by a distinct class of enzymes that comprises four families of structurally related ATP-dependent protein complexes (1). Biological activities of these complexes, defined by a variety of in vitro assays, include disruption of histone–DNA contact within nucleosomes, increased accessibility of nucleosomal DNA to transcription factors or restriction endonucleases, and cis and trans movement of histone octamers (2, 3). The INO80 chromatin remodeling complex was identified from the ino80-1 mutant defective in inositol/choline response (4–6). It contains the Ino80 ATPase, which belongs to the SNF/SWI2 superfamily (7). The Ino80 ATPase associates with 14 proteins to form a 1-MDa complex exhibiting 3′-5′ helicase activity (6, 8). The INO80 complex also contains three actin-related proteins (ARPs), of which ARP5 and ARP8 are specific to the INO80 complex. Deletion of either INO80-specific ARP compromises the ATPase activity of the remaining complex and gives rise to DNA-damage-sensitive phenotypes indistinguishable to the INO80 null mutant (9). Purification of human INO80 revealed a complex with virtually identical core components and a role in transcription (10, 11), indicating that the INO80 complex is highly conserved within eukaryotes. This is further supported by the exceedingly high levels of sequence similarities between the human and budding yeast INO80 protein complex (6).
Several lines of evidence implicate a crucial role of the INO80 complex in DNA damage response. Budding yeast ino80 mutants are hypersensitive to a variety of DNA-damaging and replication-interfering agents. Compared with the budding yeast cell-cycle checkpoint mutant rad9, the severity of the ino80 mutant phenotypes, when exposed to ionizing radiation or UV irradiation, was equal or greater than that of the rad9 mutants (5), suggesting a critical role of INO80 in DNA double-strand break (DSB) repair and nucleotide excision repair (NER). Indeed, Ino80 was found to be recruited to the sites of DSBs and is important for the processing and interhomolog recombinational repair of DSBs (12–15). However, it is unclear whether the INO80 complex is required for the nucleotide excision repair of UV lesions.
NER is the primary mechanism for the removal of bulky adducts, including UV-induced photo lesions. Biochemical studies showed that nucleosome assembly on in vitro NER substrate was severely inhibitory to the dual incision in either cell-extract-based or reconstituted assays (16–18). This inhibition can be mitigated by the presence of the yeast SWI/SNF complex in the reaction (19, 20), which suggests that chromatin reconfiguration is likely a necessary step preceding NER. However, which ATP-dependent remodeling complex or complexes provide the principal in vivo remodeling activity in aiding NER is unknown. In this report, we constructed mammalian genetic models to investigate the impact of loss of INO80 function on nucleotide excision repair. Our results show that the INO80 complex plays an important role in facilitating NER by providing access to lesion-processing factors, suggesting a functional connection between INO80-dependent chromatin remodeling and nucleotide excision repair.
Results
Generation of Conditional Alleles for INO80 and ARP5 Loci.
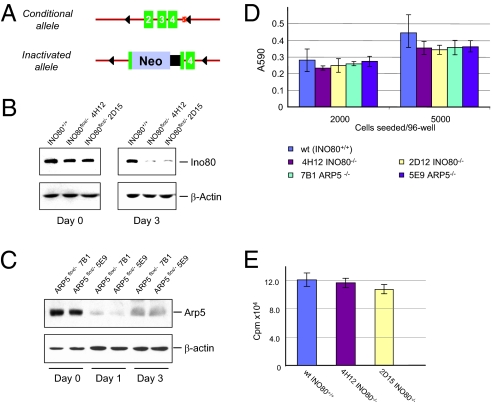
To investigate the function of the INO80 complex in DNA repair response, we carried out replacement gene targeting in HCT116 colon epithelial cells and constructed conditional alleles for INO80 and ARP5, both unique and essential components of the INO80 complex. The INO80 conditional mutants (INO80Flox/−) have one allele of INO80 inactivated by an in-frame insertion of the neomycin-coding/polyadenylation sequences. The second allele has two CreLoxP sites flanking exons 2–4 (Fig. 1A). Similarly, conditional mutants of ARP5 (ARP5Flox/−) were obtained by replacement targeting that resulted in an inactivated ARP5 allele by Neo insertion and a conditional exon 3 in the second ARP5 allele (targeting details of both loci can be found in Figs. S1 and S2). Expression of the Ino80 protein from two independent conditional mutants, 4H12 and 2D12, exhibited ≈50% reduction compared with WT HCT116 parental cells (INO80+/+) (Fig. 1B Left), indicating that presence of the LoxP sites in the conditional allele has minimal effect on the transcription and splicing of the INO80 gene. Upon adenoviral-mediated Cre (AdCre) incision, the INO80 protein was effectively depleted 3 d after AdCre infection (Fig. 1B), as indicated by the drastic decline of Ino80 in both conditional mutants 4H12 and 2D12. As a control, parental HCT116 cells, infected with the AdCre virus in parallel, showed no detectable change in their Ino80 protein levels. Similarly, two independently derived ARP5 conditional mutants, 7B1 and 5E9, also underwent rapid depletion of the Arp5 protein upon AdCre treatment (Fig. 1C). Thus, the AdCre-mediated conditional inactivation of INO80 and ARP5 could serve as loss-of-function models for the Ino80 complex.
Fig. 1.
Conditional inactivation of the INO80 locus. (A) Genotype of the INO80Flox/− conditional mutant with one allele carrying floxed exons 2–4 and the other allele with an in-frame insertion of the Neo-coding sequence. AdCre infection results in the inactivation of the conditional allele. (B) Depletion of the Ino80 protein from the INO80Flox/− conditional mutant. Immunoblotting of Ino80 was performed with an affinity-purified polyclonal antibody and cell extracts were prepared 3 d after AdCre infection. β-Actin was used as a loading control. 4H12 and 2D15 are two independently derived conditional mutants. (C) Depletion of the Arp5 protein from the ARP5Flox/− conditional mutant. Immunoblotting was performed with an affinity-purified polyclonal antibody and cell extracts were prepared 3 d after AdCre infection. β-Actin was used as a loading control. 7B1 and 5E9 are two independently derived conditional mutants. (D) MTT assays on INO80 and ARP5 conditional mutants 5 d after AdCre treatment. Error bars represent SD from three independent experiments. (E) [3H]Thymidine incorporation of INO80+/+ and INO80−/− cells 4 d after AdCre infection. Error bars represent SD from three independent samples.
INO80 is an essential gene for sustained cell proliferation, as assessed by clonogenic analysis of cells expressing anti-INO80 shRNA. However, AdCre-treated INO80Flox/− and ARP5Flox/− cells maintain viability 5 d after AdCre infection, as judged by trypan blue staining (Fig. S3). To ascertain that cells devoid of Ino80 or Arp5 maintain their viability and proliferation status for a sufficient amount of time, we performed the 3(4,5-dimethylthio-zol-2-yl)2,5-diphenyltetrazolium bromide (MTT) assay on INO80−/− and ARP5−/− cells post-AdCre treatment. As shown (Fig. 1D), the MTT activities measured from INO80+/+, INO80−/−, and ARP5−/− cells showed no significant differences 5 d after each had been infected with AdCre. Moreover, tritium thymidine incorporation, tested 4 d after AdCre infection, exhibited similar levels of DNA synthesis between INO80+/+ and INO80−/− cells (Fig. 1E). These results indicate that, despite loss of INO80 or Arp5, cells remain metabolically active and undergo DNA synthesis 4–5 d after depletion of either protein, allowing us to assess the immediate functional impact of loss of Ino80 activity.
Loss of Ino80 Causes Defective Repair of UV-Induced Photo Lesions.
Ino80 has been shown to be important in homologous recombinational repair of DSBs (12, 13, 21), which requires processing of DNA kilobases in length surrounding the DSB ends. In contrast, an excision repair mechanism such as NER involves 28–33 bases, and it is unclear whether chromatin remodeling would significantly impact the repair of UV and bulky adduct lesions. To address this question in vivo, we analyzed repair of UV-induced photo lesions in cells devoid of INO80 or ARP5. We subjected INO80+/+ and INO80Flox/− cells to 8 J/m2 short-wave UV exposure (254 nm) 3 d after AdCre infection. The UV-treated cells were harvested at different time points, and genomic DNA was prepared to measure the repair of photo lesions with antibodies specific to cyclobutane pyrimidine dimers (CPD) and 6-4 photoproducts (6-4PP).
As shown in Fig. 2 A and B, genomic DNA from each duplicated sample was slot-blotted and probed by anti-CPD and anti-6-4PP antibodies (22), respectively, followed by an HRP-conjugated labeled secondary antibody. Whereas WT cells exhibit progressive reduction of CPD and 6-4PP, consistent with their established kinetics (23), loss of INO80 significantly attenuated the removal of both CPD and 6-4PP. This result suggests that INO80 function is critical for the efficient removal of UV lesions. To directly visualize the UV damage repair defects in INO80−/− and ARP5−/− cells, we performed immunofluorescent staining of CPD and 6-4PP. Consistently, we found that the repair of both types of lesions was significantly abrogated by the loss of either Ino80 or Arp5 (Figs. S4 and S5). Collectively, these results demonstrate that the INO80 complex plays an important role in facilitating the removal of UV-induced photo lesions.
Fig. 2.
Defective repair of UV photo lesions in cells lacking INO80 and transcriptional impact of INO80 depletion. (A) Slot blotting of CPD (Left) in genomic DNA isolated from INO80 WT and INO80 mutant cells (4H12 and 2D15) irradiated with 8 J/m2 of UV (254 nm) and harvested at 0, 6, and 24 h. Quantifications of the relative CPD levels were arrived at by normalizing the chemiluminescent signal against the total DNA signal obtained by Southern hybridization with a total genomic DNA probe (Right). (B) Slot blotting of 6-4PP (Left) in genomic DNA isolated from INO80 WT and INO80 mutant cells (4H12 and 2D15) irradiated with 8 J/m2 of UV (254 nm) and harvested at 0, 1, 3, and 6 h. Quantifications of the relative 6-4PP levels were similarly performed as in A. (C) Immunoblotting of NER proteins in INO80+/+ and INO80−/− cells. (D) In vitro NER activity of INO80 and ARP5 mutants. Nuclear extracts were prepared from wild-type (INO80+/+), heterozygous (P1G8, INO80Flox/+), and two conditional INO80Flox/− cells (4H12 and 2D15) treated (+) and untreated (−) with AdCre and used in the NER synthesis assay. (E) Repair synthesis assay with nuclear extracts prepared from WT (ARP5+/+) and two conditional ARP5Flox/− mutants (7B1 and 5E9) treated (+) or untreated (−) with AdCre. (Upper) DNA substrates from each sample were recovered after the repair synthesis assay and resolved on an agarose gel. (Lower) Autoradiography generated by PhosphorImager (Molecular Dynamics). Relative NER activity of each sample (Bottom) was arrived at by normalizing the [32P]dCTP incorporation in damaged plasmids against each internal control.
Components of the NER Pathway Are Not Affected by the Loss of Ino80.
The INO80 complex creates accessible DNA for DSB repair as well as transcription (10). Thus, the impaired repair of UV lesions from the INO80 and ARP5 mutants could arise from compromised transcription of NER factors, lack of access to DNA lesions, or a combination of both. To distinguish these possibilities, we compared the protein levels of XPA, hhRAD23B, XPD, and ERCC1 in INO80+/+ and INO80−/− cells. As shown (Fig. 2C), the abundance of these proteins was not altered significantly by the loss of INO80.
To functionally assess whether INO80 depletion affects core NER enzymatic activity, we prepared nuclear extracts from INO80+/+, INO80Flox/+, and INO80Flox/− cells 4 d after AdCre infection to inactivate the conditional allele. These extracts were then used in a repair synthesis assay (24) to measure their NER activities with a damaged 5-kb plasmid (UV+) and an undamaged 2.9-kb plasmid (UV−) for control of background incorporation and substrate recovery. As shown in Fig. 2D, NER-dependent repair incorporation into the UV-damaged plasmid, when normalized to the internal undamaged control, was nearly identical among INO80+/+, INO80+/−, and INO80−/− cells. Moreover, AdCre treatment per se did not alter the in vitro NER activity of cells with different INO80 genotypes. These results indicate that loss of one or both INO80 alleles does not significantly impact the transcription of NER components. Consistent with this result, we found that NER synthesis activity was unaltered by the loss of ARP5 (Fig. 2E). Thus, the impaired removal of CPD and 6-4PP observed in INO80−/− or ARP5−/− cells is likely a result of a defect in the chromatin remodeling necessary for the NER mechanism.
Loss of Ino80 Leads to Defective Recruitment of XPC and XPA to the Sites of DNA Lesions.
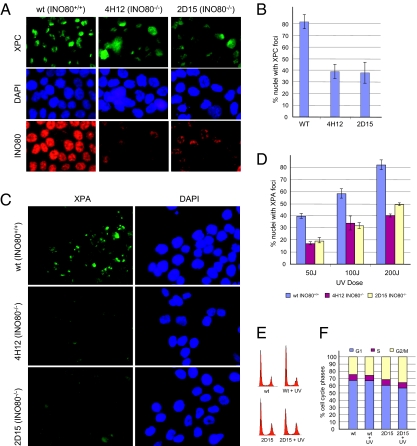
A key initiating step of NER is damage recognition by the XPC-hhRAD23 complex (25, 26). We reasoned that the binding of XPC-hhRAD23B to UV lesions could be limited by the diminished access to damaged DNA in INO80-deficient cells, causing the defect in CPD and 6-4PP repair. To test this premise, we examined whether the recruitment of XPC to the sites of UV damage was affected by INO80. We microirradiated INO80+/+ and INO80−/− cells with a 5-μm filter and fixed the cells 30 min later for the immunostaining of XPC. As shown in Fig. 3A, microirradiated INO80+/+ cells displayed discrete XPC nuclear foci corresponding to the filter pore size. However, mutant cells lacking INO80 (4H12 and 2D15) showed a visible decrease in XPC foci both in terms of the intensity and the number of foci-positive nuclei (Fig. 3B). This result suggests INO80 function is important for the damage binding step of NER, presumably by creating access for the recognition of UV lesions.
Fig. 3.
XPC and XPA foci formation in INO80-deficient cells. (A) Formation of UV-induced XPC foci in INO80 mutant cells 30 min after UV exposure (2,000 J/m2). (B) Quantification of XPC foci-positive cells in WT and INO80−/− cells. (C) Formation of UV-induced XPA foci in INO80 mutant cells. (D) Quantification of XPA foci-positive cells in WT and INO80−/− cells. UV-induced foci were generated by localized irradiation with 5-μm (XPC) and 2-μm (XPA) Isopore filters (Millipore). (E) WT (INO80+/+) and 2D15 (INO80−/−) cells were mock-treated or treated (+ UV) with 50 J/m2. Cell-cycle profiles were measured 1 h after treatment by flow cytometry. Representative data from two experiments are shown. (F) Quantification of E.
A decrease in damage recognition is expected to affect the recruitment of the XPA protein, which is responsible for the subsequent recruitment and assembly of NER incision activity (27, 28). Accordingly, we measured foci formation of XPA in INO80+/+ and INO80−/− cells microirradiated through a 2-μm filter. As shown in Fig. 3 C and D, cells devoid of INO80 exhibited markedly reduced XPA foci, when compared with INO80-proficient cells. Whereas INO80+/+ cells displayed dose-dependent XPA foci formation, INO80−/− cells exposed to the same doses of UV consistently showed reduced foci number per nuclei and decreased foci intensity. To exclude the possibility that the deficient foci formation in INO80−/− cells was a result of potential cell-cycle differences between WT and INO80 mutants, we analyzed mock-treated or UV-irradiated (1 h) cells and found no significant alteration in cell-cycle distribution between INO80+/+ and INO80−/− cells (Fig. 3 E and F). Together, these results suggest that INO80 function is important for damage binding of NER lesions and for the subsequent assembly of NER incision activity, which is likely the mechanistic basis for the attenuated UV lesion removal in INO80- and ARP5-deficient cells.
Ino80 and Arp5 Are Recruited to UV-Damaged DNA.
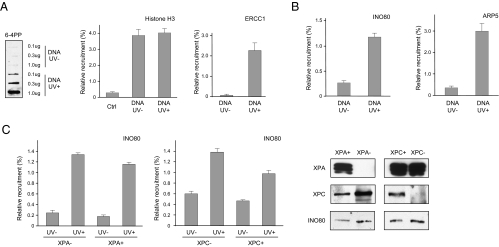
To test whether the Ino80 complex is directly present at the sites of UV lesions, we performed a modified episomal chromatin immunoprecipitation (eChIP) analysis (29) to determine whether Ino80 is recruited to UV-irradiated plasmid DNA transfected into repair-proficient cells. We transfected 293T cells with either UV-irritated or mock-treated plasmid DNA (Fig. 4A Left) and incubated the cells for 0.5 h before formaldehyde fixation and subsequent ChIP analysis with PCR primers specific to the plasmid DNA but not the genomic DNA. Using an antibody against histone H3, we detected the presence of histone H3 on both UV-damaged and mock-treated plasmid DNA (Fig. 4A Middle), indicating the chromatinized nature of the transfected DNA, as previously reported (30, 31). We also detected a strong recruitment of ERCC1, a subunit of the 5′ NER incision endonuclease, suggesting that the UV lesions on the damaged plasmid are actively engaged by NER (Fig. 4A Right).
Fig. 4.
UV damage-specific recruitment of the INO80 complex detected by ChIP analysis. (A) Plasmid DNA (pOriP) was UV-irradiated (UV+) or mock-treated (UV−) to induced UV lesions as detected by slot blotting with an anti-64-PP antibody (Left) and electroporated into HCT116 cells. ChIP analyses were carried out 0.5 h after transfection with anti-histone H3 (Middle) and ERCC1 (Right) antibodies, as indicated. Percentages of relative enrichment of each protein to UV-damaged DNA or mock-treated DNA were arrived at by normalizing comparative concentrations of each sample with that of its input lysate. (B) Recruitment of INO80 and ARP5 proteins to UV-treated and mock-treated plasmid DNA. (C) UV damage-dependent recruitment of INO80 in XPA/XP2OS (XPA−) (Left) and XPC/XP4PA (XPC−) (Right) mutants and their isogenic, complemented controls (XPA+ and XPC+, respectively). Error bars representing SD were derived from three or more experiments with triplicate quantitative PCR reactions. The P values were derived from paired t tests.
Importantly, both Ino80 and Arp5 exhibited significant enrichment on UV-damaged DNA. As shown (Fig. 4B), eChIP analyses of both Ino80 and Arp5 indicate that both were recruited to UV-treated DNA but not to mock-treated controls, suggesting that the Ino80 complex has a direct role at the sites of UV lesions, most likely to provide the remodeling activity for the NER mechanism. To further test whether the function of Ino80 is required before the NER incision process, we performed eChIP in XPA and XPC mutants and isogenic, complemented cells (Fig. 4C). In both mutants, UV damage-dependent recruitment of Ino80 was not affected by the absence of XPA or XPC. This observation again suggests that the function of the Ino80-dependent remodeling activity acts during the initial stage of lesion removal, consistent with the fact that XPC and XPA foci formation are rendered defective by INO80 or ARP5 loss (Fig. 3).
Association Between DDB1 and the INO80 Complex.
The diminished XPC and XPA foci formation in INO80−/− cells and the XPC-XPA-independent recruitment of Ino80 components indicate that INO80-mediated chromatin relaxation likely takes place before XPC/hhRAD23B binding to the lesion. To address how the INO80 remodeling activity is directed to the site of a lesion, we screened protein interactions between Ino80-Arp5 and NER factors via coimmunoprecipitation. As shown in Fig. 5A, Ino80 and Arp5 can be reciprocally immunoprecipitated, consistent with the fact that they are both core components of the stable Ino80 complex. No interactions with XPC, XPA, and XPD could be detected in cells with or without UV treatment (Fig. S6A). However, DDB1 was found to interact with both Ino80 and Arp5 (Fig. 5A) in HCT116 cell extracts, as DDB1 was immunoprecipitated by Ino80 and Arp5 antibodies but not by control IgG. Reciprocal IP with an affinity-purified DDB1 antibody also detected Ino80 and Arp5 (Fig. 5A, lane 5). This interaction appears to be independent of UV exposure, as it can be detected in UV-treated as well as in mock-treated cell extracts. The interaction between INO80 and DDB1 can also be reproduced in vitro using GST-DDB1 fusion and cell extract (Fig. S6B).
Fig. 5.
Interaction between the Ino80 complex and NER factors. (A) Immunoprecipitation of Ino80 and Arp5 from untreated HCT116 cell extracts. Endogenous proteins precipitated with the indicated antibodies were immunoblotted with antibodies against Ino80, Arp5, and DDB1. (B) Reciprocal IP between DDB1 and Ino80 complex components. Myc-DDB1 (125 kDa) or the negative control Myc-CtIP (125 kDa) was coexpressed with SFB-tagged Ino80 (Upper) or SFB-tagged Arp5 (Lower). (Upper, Middle) Cell lysates were immunoprecipitated with an anti-Myc antibody followed by immunoblotting with anti-Myc and anti-Flag antibodies. (Upper, Right) Cell lysates were subjected to streptavidin-bead (S-beads) pull-down assays to recover SFB-Ino80 and its associated proteins, followed by immunoblotting with an anti-Myc antibody. Similarly, detection of Myc-DDB1–Arp5 interaction was performed (Right). Coimmunoprecipitations and pull-down assays were performed in the presence of ethidium bromide (50 μg/mL).
To further verify the interaction between DDB1 and INO80 components, we coexpressed Myc-tagged DDB1 and SFB (S, Flag, and streptavidin-binding protein)-tagged INO80 in 293T cells. Myc-tagged CtIP, with a molecular weight identical to Myc-DDB1, was also coexpressed with SFB-INO80 as a negative control. When cell lysates were immunoprecipitated with an anti-Myc antibody followed by immunoblotting, SFB-INO80 was readily detectable by an anti-Flag antibody (Fig. 5B Upper, Middle). In contrast, in spite of the extremely high Myc-CtIP expression level, no interaction between SFB-INO80 and Myc-CtIP could be detected. When SFB-INO80 was pulled down by streptavidin beads, a strong signal of Myc-DDB1 was detected (Fig. 5B Upper, Right). Similarly, a reciprocal interaction between Myc-DDB1 and SFB-Arp5 was observed (Fig. 5B Lower) when both proteins were coexpressed. Collectively, these results suggest that the Ino80 complex is associated with the DDB1/DDB2 complex, which is a likely mechanism for the recruitment of chromatin remodeling activity to the sites of UV lesions.
Discussion
Using conditional alleles derived from homologous targeting, we established mammalian genetic INO80 and ARP5 models and showed that human cells lacking the essential components of the INO80 complex suffer from a major deficit in removing UV-induced photo lesions. Our analyses indicate that the likely cause of defective UV damage repair is the lack of chromatin remodeling, presumably critical for the access of NER factors. These results reveal a functional link between the INO80-dependent chromatin remodeling and the nucleotide excision repair mechanism.
INO80 and ARP5 yeast mutants display marked hypersensitivity to UV irradiation (5, 21). Such hypersensitivity can arise from disrupted transcription of DNA damage repair proteins and/or defective chromatin remodeling necessary for the proper access of DNA damage to repair factors. Whereas genomewide transcription array analyses from yeast indicated that a very limited set of DNA damage response-unrelated genes are transcriptionally affected by INO80 loss (15), the human INO80 complex has nevertheless been found to interact with the general transcription factor YY-1 (10, 21). Our results from the in vitro NER assay show that extracts prepared from INO80 and ARP5 mutant cells possessed nearly identical levels of repair synthesis activities compared with wild-type cells (Fig. 2). Because the in vitro NER assay utilizes naked DNA substrate and is independent of chromatin remodeling, this result argues that NER incision and repair synthesis factors are minimally affected by INO80 loss at the protein level. The only noticeable reduction (≈50%) by Western blotting was in XPA protein (Fig. 2C). However, an in vivo XPA titration study indicated that XPA levels must be reduced to <10% of that present in a normal cell to render XPA a limiting factor for NER and consequently cellular sensitivity (32). Together, these results allow us to associate the attenuated photo lesion repair to the loss of INO80-mediated remodeling activity.
Our eChIP analyses (Fig. 4) showed that components of the INO80 complex are highly enriched at the site of UV lesions soon after the damaged DNA was introduced into the cells (30 min). The onset of recruitment also coincides with the binding of NER incision factors such as ERCC1. This result provides the molecular evidence that INO80 is localized to the sites of damage. The observation that INO80 recruitment was independent of XPC and XPA argues that the involvement of INO80 mostly likely precedes the lesion incision process to create damage accessibility. Consistent with this notion, we observed that UV exposure led to markedly increased relaxation of the chromatin structure in INO80+/+ cells. However, the extent of UV-dependent chromatin relaxation was significantly diminished in INO80−/− cells, albeit a relatively relaxed global chromatin density (Figs. S7 and S8).
The involvement of INO80 in NER is further corroborated by the observation that loss of INO80 led to significant reductions in XPA and XPC foci formation (Fig. 3). Although the moderate decrease of XPA protein level in INO80 mutant cells (Fig. 2C) might contribute to the reduction of XPA foci signal, it is less likely to account for the overall loss of foci occurrence. Using affinity-purified antibodies, we found that INO80 and ARP5 both exhibit exclusive nuclear localization in undamaged cells (Fig. 3A). However, in UV-microirradiated cells, we were unable to detect any redistribution of the INO80 staining signal upon testing several cell lines with various doses. It is possible that the local concentration of INO80 is sufficient to provide chromatin remodeling required for the efficient repair of UV damage. Alternatively, the strong basal levels of INO80 and ARP5 staining signal may require extensive enrichment to be visualized.
How chromatin remodeling coordinates with the NER process is a key question. It is unlikely that remodeling mechanisms possess intrinsic damage recognition ability. Thus, prior damage recognition by NER components and/or histone marking of the damaged sites would be the most plausible mechanism to guide remodeling activities. In INO80 mutant cells, we observed that formation of XPC and XPA foci both decreased significantly. This indicates that nucleosome relocation/eviction needs to take place before damage binding of the XPC/hhRAD23B complex, thus placing the involvement of INO80 upstream of XPC/hhRAD23B. Our finding that DDB1 interacts with the Ino80 complex provides a mechanistic link between chromatin remodeling activity and the initiating step of NER.
The DDB1/DDB2 complex binds UV damage and participates in global genome repair. However, DDB1/DDB2 is not required in in vitro NER reactions using unchromatinized DNA substrates (33, 34), suggesting an in vivo function for XPE before the direct damage binding by XPC/Rad23B. Recently, DDB1/DDB2 were identified as components of the CUL4-DDB1-DDB2-ROC1 E3 ubiquitin ligase complex that targets core histones such as H2A, H3, and H4 at the site of UV lesions (35, 36). Importantly, ubiquitinated H3 and H4 appear to facilitate the damage recognition of XPC/hhRAD23B by promoting nucleosome release. Thus, UV-induced posttranslational modification of core histones may be an alternative recruitment anchor for remodeling activities.
Although loss of INO80 results in defective UV damage repair, it is unlikely an exclusive chromatin remodeler involved in NER. For example, a direct interaction between Rad4/Rad23 and components of the SWI/SNF complex was suggested to load the SWI/SNF complex in aiding NER in budding yeast (37). Thus, generation of accessible DNA for lesion removal requires coordination of remodeling complexes with distinct catalytic functions. The role for various remodeling activities is likely to promote the timely repair of lesions, rather than being an essential component for lesion removal. For example, INO80 mutants were able to remove the majority of the 6-4PP, albeit taking four times longer compared with WT cells (Fig. S9). This observation suggests that loss of remodeling activity leads to attenuation of photo lesion repair, but not complete impairment. Together, findings from this work support the model that INO80 functions in an important chromatin remodeling activity for an efficient nucleotide excision repair mechanism. The link between INO80 and NER function may reflect the underlying mechanism for the UV hypersensitivities of INO80 mutant cells and the broadening connections between chromatin remodeling and DNA repair in general.
Materials and Methods
Chromatin Immunoprecipitation.
Damaged (2,000 J/m2) and mock-treated pOriP plasmid DNA was introduced to cells by electroporation (Amaxa). Thirty minutes after transfection, cells were washed and fixed with 1% formaldehyde for 8 min at room temperature. The cell suspensions were then lysed and subjected to sonication (Sonifier; Branson) to shear genomic DNA. Supernatants were collected and precleared for 1 h with 25 μL of a 50% slurry of protein A Sepharose beads (Amersham Biosciences) followed by antibody incubation overnight at 4 °C. After incubation with antibody, the beads were washed with RIPA buffer, high salt buffer, LiCl buffer, and finally Tris-EDTA buffer. DNA was recovered after proteinase K treatment (6 h, 37 °C), reversal of formaldehyde crosslink (65 °C, overnight), and phenol/chloroform extraction and ethanol precipitation.
Supplementary Material
Acknowledgments
Yue Xiong (University of North Carolina, Chapel Hill, NC) and Jiyong Zhao (University of Rochester, Rochester, NY) kindly provided affinity-purified DDB1 and NPAT antibodies, respectively. We thank Jiemin Wong (Institute of Biomedical Sciences, East China Normal University, Shanghai, China) for providing technical help, and Junjie Chen (M. D. Anderson Cancer) for providing the SFB expression system and for helpful discussions. This work was supported by National Cancer Institute Grants CA 127945 and CA97175 Project 3 (to L.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008388107/-/DCSupplemental.
References
- 1.Lusser A, Kadonaga JT. Chromatin remodeling by ATP-dependent molecular machines. Bioessays. 2003;25:1192–1200. doi: 10.1002/bies.10359. [DOI] [PubMed] [Google Scholar]
- 2.Owen-Hughes T, et al. Analysis of nucleosome disruption by ATP-driven chromatin remodeling complexes. Methods Mol Biol. 1999;119:319–331. doi: 10.1385/1-59259-681-9:319. [DOI] [PubMed] [Google Scholar]
- 3.Peterson CL, Côté J. Cellular machineries for chromosomal DNA repair. Genes Dev. 2004;18:602–616. doi: 10.1101/gad.1182704. [DOI] [PubMed] [Google Scholar]
- 4.Ebbert R, Birkmann A, Schüller H-J. The product of the SNF2/SWI2 paralogue INO80 of Saccharomyces cerevisiae required for efficient expression of various yeast structural genes is part of a high-molecular-weight protein complex. Mol Microbiol. 1999;32:741–751. doi: 10.1046/j.1365-2958.1999.01390.x. [DOI] [PubMed] [Google Scholar]
- 5.Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406:541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 6.Shen X, Xiao H, Ranallo R, Wu WH, Wu C. Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates. Science. 2003;299:112–114. doi: 10.1126/science.1078068. [DOI] [PubMed] [Google Scholar]
- 7.Peterson CL. Multiple SWItches to turn on chromatin? Curr Opin Genet Dev. 1996;6:171–175. doi: 10.1016/s0959-437x(96)80047-5. [DOI] [PubMed] [Google Scholar]
- 8.Shen X. Preparation and analysis of the INO80 complex. Methods Enzymol. 2004;377:401–412. doi: 10.1016/S0076-6879(03)77026-8. [DOI] [PubMed] [Google Scholar]
- 9.Shen X, Ranallo R, Choi E, Wu C. Involvement of actin-related proteins in ATP-dependent chromatin remodeling. Mol Cell. 2003;12:147–155. doi: 10.1016/s1097-2765(03)00264-8. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y, et al. YY1 functions with INO80 to activate transcription. Nat Struct Mol Biol. 2007;14:872–874. doi: 10.1038/nsmb1276. [DOI] [PubMed] [Google Scholar]
- 11.Jin J, et al. A mammalian chromatin remodeling complex with similarities to the yeast INO80 complex. J Biol Chem. 2005;280:41207–41212. doi: 10.1074/jbc.M509128200. [DOI] [PubMed] [Google Scholar]
- 12.Morrison AJ, et al. INO80 and γ-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell. 2004;119:767–775. doi: 10.1016/j.cell.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Tsukuda T, Fleming AB, Nickoloff JA, Osley MA. Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature. 2005;438:379–383. doi: 10.1038/nature04148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukuda T, et al. INO80-dependent chromatin remodeling regulates early and late stages of mitotic homologous recombination. DNA Repair (Amst) 2009;8:360–369. doi: 10.1016/j.dnarep.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 15.van Attikum H, Fritsch O, Hohn B, Gasser SM. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell. 2004;119:777–788. doi: 10.1016/j.cell.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Hara R, Mo J, Sancar A. DNA damage in the nucleosome core is refractory to repair by human excision nuclease. Mol Cell Biol. 2000;20:9173–9181. doi: 10.1128/mcb.20.24.9173-9181.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ura K, et al. ATP-dependent chromatin remodeling facilitates nucleotide excision repair of UV-induced DNA lesions in synthetic dinucleosomes. EMBO J. 2001;20:2004–2014. doi: 10.1093/emboj/20.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, et al. Genomic instability and endoreduplication triggered by RAD17 deletion. Genes Dev. 2003;17:965–970. doi: 10.1101/gad.1065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara R, Sancar A. The SWI/SNF chromatin-remodeling factor stimulates repair by human excision nuclease in the mononucleosome core particle. Mol Cell Biol. 2002;22:6779–6787. doi: 10.1128/MCB.22.19.6779-6787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitch ME, Nakajima S, Yasui A, Ford JM. In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. J Biol Chem. 2003;278:46906–46910. doi: 10.1074/jbc.M307254200. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, et al. A YY1-INO80 complex regulates genomic stability through homologous recombination-based repair. Nat Struct Mol Biol. 2007;14:1165–1172. doi: 10.1038/nsmb1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowley DJ, Hanawalt PC. Induction of the SOS response increases the efficiency of global nucleotide excision repair of cyclobutane pyrimidine dimers, but not 6-4 photoproducts, in UV-irradiated Escherichia coli. J Bacteriol. 1998;180:3345–3352. doi: 10.1128/jb.180.13.3345-3352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volker M, et al. Sequential assembly of the nucleotide excision repair factors in vivo. Mol Cell. 2001;8:213–224. doi: 10.1016/s1097-2765(01)00281-7. [DOI] [PubMed] [Google Scholar]
- 24.Wood RD, Robins P, Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988;53:97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- 25.Min J-H, Pavletich NP. Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature. 2007;449:570–575. doi: 10.1038/nature06155. [DOI] [PubMed] [Google Scholar]
- 26.Sugasawa K, et al. A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev. 2001;15:507–521. doi: 10.1101/gad.866301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Z, Henricksen LA, Wold MS, Ingles CJ. RPA involvement in the damage-recognition and incision steps of nucleotide excision repair. Nature. 1995;374:566–569. doi: 10.1038/374566a0. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Lu X, Peterson CA, Legerski RJ. An interaction between the DNA repair factor XPA and replication protein A appears essential for nucleotide excision repair. Mol Cell Biol. 1995;15:5396–5402. doi: 10.1128/mcb.15.10.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen X, et al. Recruitment of Fanconi anemia and breast cancer proteins to DNA damage sites is differentially governed by replication. Mol Cell. 2009;35:716–723. doi: 10.1016/j.molcel.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves R, Gorman CM, Howard B. Minichromosome assembly of non-integrated plasmid DNA transfected into mammalian cells. Nucleic Acids Res. 1985;13:3599–3615. doi: 10.1093/nar/13.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong W, Kulaeva OI, Clark DJ, Lutter LC. Topological analysis of plasmid chromatin from yeast and mammalian cells. J Mol Biol. 2006;361:813–822. doi: 10.1016/j.jmb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Köberle B, Roginskaya V, Wood RD. XPA protein as a limiting factor for nucleotide excision repair and UV sensitivity in human cells. DNA Repair (Amst) 2006;5:641–648. doi: 10.1016/j.dnarep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Aboussekhra A, et al. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80:859–868. doi: 10.1016/0092-8674(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 34.Mu D, et al. Reconstitution of human DNA repair excision nuclease in a highly defined system. J Biol Chem. 1995;270:2415–2418. doi: 10.1074/jbc.270.6.2415. [DOI] [PubMed] [Google Scholar]
- 35.Kapetanaki MG, et al. The DDB1-CUL4ADDB2 ubiquitin ligase is deficient in xeroderma pigmentosum group E and targets histone H2A at UV-damaged DNA sites. Proc Natl Acad Sci USA. 2006;103:2588–2593. doi: 10.1073/pnas.0511160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, et al. Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol Cell. 2006;22:383–394. doi: 10.1016/j.molcel.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Gong F, Fahy D, Smerdon MJ. Rad4-Rad23 interaction with SWI/SNF links ATP-dependent chromatin remodeling with nucleotide excision repair. Nat Struct Mol Biol. 2006;13:902–907. doi: 10.1038/nsmb1152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.