Abstract
While seeking a new host cell, obligate intracellular parasites, such as the protozoan Toxoplasma gondii, must be able to endure the stress of an extracellular environment. The mechanisms Toxoplasma use to remain viable while deprived of a host cell are not understood. We have previously shown that phosphorylation of Toxoplasma eukaryotic initiation factor-2α (TgIF2α) is a conserved response to stress. Here we report the characterization of Toxoplasma harboring a point mutation (S71A) in TgIF2α that prevents phosphorylation. Results show that TgIF2α phosphorylation is critical for parasite viability because the TgIF2α-S71A mutants are ill-equipped to cope with life outside the host cell. The TgIF2α-S71A mutants also showed a significant delay in producing acute toxoplasmosis in vivo. We conclude that the phosphorylation of TgIF2α plays a crucial role during the lytic cycle by ameliorating the stress of the extracellular environment while the parasite searches for a new host cell.
Keywords: Apicomplexa, toxoplasmosis, stress, translation control, eIF2
Parasites that have adapted to live and replicate within another cell benefit from abundant resources, protection from host immunity, and shelter from therapeutic agents. As the demands of the parasites exceed what the host cell can supply, the parasites must find a new host cell, a journey that can leave them vulnerable to nutrient deprivation and environmental stresses. Protozoa in the phylum Apicomplexa are parasites that require a eukaryotic host cell to replicate. Toxoplasma gondii is one such obligate intracellular parasite, capable of using virtually all warm-blooded vertebrates as host organisms (1). Acute Toxoplasma infection can cause spontaneous abortion or congenital birth defects, as well as severe disease in immunocompromised patients.
The lytic cycle of Toxoplasma tachyzoites consists of discrete stages: adherence to a host cell, invasion, replication, exit from host cell (egress), and movement to a new host cell (2). Tachyzoites remain viable for only a limited time outside of the host cell; the ability of freshly egressed parasites to infect a new host cell monolayer drops significantly between 6 and 12 h of exposure to the extracellular environment (3). The mechanisms the parasite may invoke to cope with the extracellular environment while it searches for a new host cell are not known.
A well-characterized stress response pathway conserved in eukaryotic cells involves translational control by virtue of the phosphorylation of the α subunit of eukaryotic initiation factor-2 (eIF2α) (4, 5). eIF2-GTP escorts Met-tRNAi to the translational machinery for eventual placement into the P-site of ribosomes (5); however, when phosphorylated at a regulatory serine (serine-51), eIF2 becomes an inhibitor of its guanine nucleotide exchange factor, eIF2B. Consequently, global translation initiation is dampened, decreasing the synthesis of the current proteome so the cell can conserve energy and reprogram gene transcription to remedy the stress (6). Four eIF2 kinases have been identified in mammals that phosphorylate eIF2α in response to stress (4, 6): haem-regulated inhibitor (HRI) (EIF2KA1), which responds to heme deficiency and oxidation stress; double-stranded RNA-dependent protein kinase (PKR) (EIF2KA2), which is involved in antiviral defenses; pancreatic eIF2α kinase/PKR (RNA-dependent protein kinase)-like ER kinase (PEK/PERK) (EIF2KA3), which is activated by endoplasmic reticulum (ER) stress; and general control non-derepessible-2 (GCN2) (EIF2KA4), which responds to nutrient deprivation. We identified and characterized an eIF2α ortholog in Toxoplasma (TgIF2α), as well as four TgIF2α kinases (TgIF2K-A through -D) (7, 8). Whereas TgIF2K-C and -D are most closely related to GCN2, TgIF2K-A is localized to the parasite ER and likely mediates the activation of the unfolded protein response analogous to PEK/PERK (8). TgIF2K-B is a unique eIF2 kinase that is not compartmentalized and likely responds to cytoplasmic stresses. Homologs of GCN2 (PfeIK1) and TgIF2K-A (PfPK4) have also been described in Plasmodium (malarial) parasites (9, 10).
The importance of eIF2α phosphorylation in translational control in the adaptive processes to stress has been determined in yeast and mammalian systems by allelic gene replacement involving substitution of alanine for the serine-51 phosphorylation site in eIF2α (S51A) (11–14). In this study, we engineered mutant parasites incapable of phosphorylating eIF2α by substituting alanine for the regulatory serine (Ser-71) in TgIF2α. TgIF2α-S71A mutant parasites have decreased viability in vitro and are less virulent in a mouse model of infection. The underlying mechanism for the growth defect does not involve parasite attachment, invasion, replication, egress, or motility but rather is centered on the inability of the TgIF2α-S71A mutant parasites to manage the stress of an extracellular environment. These results provide significant insights into how intracellular parasites survive while they attempt to locate a new host cell.
Results
Generation of Mutant Toxoplasma That Are Incapable of Phosphorylating TgIF2α.
We previously characterized the Toxoplasma eIF2α ortholog (TgIF2α), which possesses the conserved regulatory serine residue (Ser-71) that is phosphorylated during cellular stress (7). To access the impact of TgIF2α phosphorylation in Toxoplasma tachyzoites, we generated a mutant parasite line in which the Ser-71 residue of TgIF2α was substituted to alanine. The TgIF2α-S71A mutation was created by allelic replacement using homologous recombination in RHΔKu80 parasites (15, 16). RHΔKu80 parasites were transfected with a “knock-in” construct that contained a point mutation encoding the S71A substitution along with a minigene encoding chloramphenicol resistance (Fig. 1A). The point mutation generated a unique MscI restriction site in the TgIF2α genomic locus, which was used as a means to select true allelic replacements among the chloramphenicol-resistant clones. The screening was performed by amplifying a fragment of the first exon in the TgIF2α genomic locus and cutting the amplicon with MscI. The MscI-digested PCR product from parental RHΔKu80 parasites [hereafter referred to as “wild-type” (WT)] yields a single DNA fragment of 560 bp; however, two fragments of 340 and 220 bp are produced after allelic replacement (Fig. 1B). We identified two independent clones containing the S71A knockin within the endogenous TgIF2α genomic locus, and both exhibited similar phenotypic properties described below.
Fig. 1.
Generation of parasites containing nonphosphorylatable TgIF2α. (A) Diagram depicting the relevant portion of the TgIF2α genomic locus and the TgIF2α-S71A allelic replacement vector. The dark gray box denotes the beginning of the TgIF2α genomic locus, and the black box represents ≈2.4 kb of upstream sequence. The serine-71 codon (AGC) was mutated to an alanine codon (GCC), which created a unique MscI restriction site within the mutant allele. (B) The first exon of the TgIF2α gene was amplified from WT and TgIF2α-S71A parasites (PCR primers shown in gray in A). The resulting PCR product was digested with MscI and resolved by electrophoresis in 1% agarose gel (inverted image is shown for clarity). In a true allelic replacement, two products (340 and 220 bp) would be visualized instead of one (560 bp). (C) Equal amounts of protein lysates from WT or TgIF2α-S71A tachyzoites were resolved on a 4–12% polyacrylamide gel and transferred for immunoblotting with antibody that specifically recognizes TgIF2α phosphorylated at ser-71 (TgIF2α-P) or total TgIF2α protein.
To confirm that the TgIF2α-S71A clone was unable to be phosphorylated, a Western blot of lysates from parasites treated with the ionophore A23187 was carried out using antisera that specifically recognize TgIF2α phosphorylated at Ser-71 or total TgIF2α protein (7, 8). Ionophore A23187 elicits ER stress and, consistent with our previous study, this ionophore is a potent inducer of TgIF2α phosphorylation in WT parasites (Fig. 1C) (8). By comparison, phosphorylation is absent in the TgIF2α-S71A mutant parasites (Fig. 1C). These results confirm that we created a mutant version of Toxoplasma that cannot phosphorylate TgIF2α.
TgIF2α-S71A Parasites Exhibit Reduced Proliferation in Vitro.
Our initial characterization of the TgIF2α-S71A parasites indicated that the mutants took longer to lyse monolayers of host cells compared with WT. To directly assess whether the mutant parasites had decreased fitness relative to WT, we performed “head-to-head” competition assays (17) in which an equal number of WT and TgIF2α-S71A parasites were inoculated into the same culture flask of host cells, human foreskin fibroblasts (HFF) (Fig. 2A). We refined this comparative fitness assay to take advantage of TaqMan probes and real-time PCR as a means to distinguish between the WT (WT-TgIF2α-FAM) or mutant (S71A-TgIF2α-VIC) alleles of the TgIF2α gene (Fig. 2A). Genomic DNA was collected and examined at the time of parasite inoculation of the host cells (day 0) and then sampled again every 3 days. At day 0, the PCR analysis validated that equal numbers of TgIF2α-S71A mutant and WT parasites were present in the mixed culture, but by day 3 the mutant was significantly outgrown by the WT (Fig. 2B). By day 9, the TgIF2α-S71A mutant parasites were no longer detectable in the HFF culture (Fig. 2B).
Fig. 2.
TgIF2α-S71A mutants are outcompeted by WT parasites in vitro. (A) Equal numbers of WT and TgIF2α-S71A parasites (shown as white and black, respectively) were grown in mixed culture in a T25-flask (gray). Every 3 d the parasites were physically removed from the host cells and passed onto a fresh monolayer, and a portion was used for genomic DNA (gDNA) isolation. The gDNA was used as a template for a TaqMan-based fitness assay designed to distinguish WT and mutant parasites. This assay used two probes: WT-TgIF2α-FAM (white) was used to detect the WT allele, whereas S71A-TgIF2α-VIC (black) was used to identify the mutant (S71A) allele. (B) Percentage of WT and S71A mutant allele was determined using SDS software version 1.2.1 (Applied Biosystems) and plotted for each day sampled (white bars, WT; black bars, S71A). Error bars represent the SE from three replicate samples. (C) Parasites (104) were cultured in 12-well plates containing confluent HFF monolayers. Percentage of host cell lysis was evaluated at day 5, 6, and 7 by washing each well with PBS and staining the remaining host cells with Coomassie Brilliant Blue. A digital image of each well was recorded and analyzed using Alpha Innotech software to determine the percentage of the monolayer disrupted by WT vs. TgIF2α-S71A mutants, as represented by white or black bars, respectively. Data shown are from a single representative experiment performed three times with similar results.
We also performed a standard parasite growth assay that measures the disruption of the host cell monolayer over time. WT parasites lysed the host cell monolayer (≈90%) 6 d after inoculation, but an equal number of TgIF2α-S71A parasites only destroyed ≈18% of the host cells at day 6 (Fig. 2C). By day 7, the mutant parasites had destroyed ≈90% of the host cell monolayer, 24 h slower than WT parasites. Together, these results indicate that TgIF2α phosphorylation is important for Toxoplasma tachyzoites to progress normally through host cell cultures.
TgIF2α-S71A Parasites Are Defective in Adapting to the Extracellular Environment.
To determine the cause of the growth retardation of the TgIF2α-S71A mutant, we examined each step in the tachyzoite lytic cycle. Using a standard red/green attachment and invasion assay, we detected no difference in the ability of TgIF2α-S71A mutants to adhere or penetrate host cells (Fig. S1). We also found no defect in gliding motility (Fig. 3A) or the ability of TgIF2α-S71A parasites to exit from host cells upon ionophore-induced egress (Fig. 3B).
Fig. 3.
Toxoplasma motility and egress in TgIF2α-S71A mutants. (A) WT and TgIF2α-S71A mutant parasites were allowed to adhere and glide along a glass coverslip for 30 min. The parasites and surface protein “trails” were detected with mouse anti-Sag1. The percentage of parasites with trails was plotted for both WT parasites (gray) and the TgIF2α-S71A mutant (black). The number of ΔKu80 or TgIF2α-S71A parasites with trails was recorded from a minimum of six independent microscope fields. (B) WT and TgIF2α-S71A parasites were cultured in a monolayer of HFF cells overnight. To induce egress, the infected monolayers were exposed to 2 μM A23187 for 0, 0.5, 1, 2, 3, or 4 min. After each time interval, parasites were fixed with cold methanol, and the percentage of egress was calculated for both WT (black boxes) and TgIF2α-S71A parasites (white circles), recorded from 10 random microscope fields.
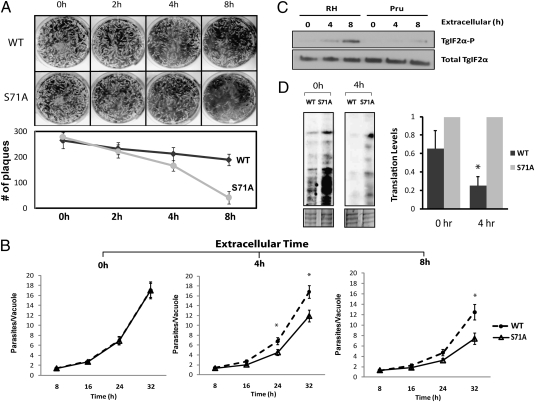
With no measurable defects in entering or exiting host cells, we considered two possibilities that were not mutually exclusive: (i) the TgIF2α-S71A mutants may be defective in asexual replication, and (ii) exposure to the extracellular environment constituted a stress that TgIF2α-S71A mutants are ill-equipped to withstand. To test these possibilities, we performed two independent types of parasite growth measurements: plaque assays and doubling assays. Previously we collected parasites in the HFF culture as they egressed, which could lead to differences in the time the tachyzoites resided outside the host cells in the medium. To obtain a homogenous parasite population that had nominal exposure to the extracellular environment, we physically separated intracellular parasites from their host. The freed tachyzoites were immediately passed onto a fresh HFF monolayer (0 h) or deprived of host cells by incubating in extracellular medium for 2, 4, or 8 h (Fig. 4A). Without appreciable exposure to the extracellular environment consisting of DMEM plus 1% FBS (0 h), WT and TgIF2α-S71A mutants formed a near identical number of plaques, suggesting that the inability to phosphorylate TgIF2α has no appreciable impact on parasite replication. However, TgIF2α-S71A mutants were much more sensitive to being deprived of their host cells. Compared with WT, TgIF2α-S71A mutants showed a significant reduction in plaque numbers after an 8-h exposure to the extracellular environment (Fig. 4A).
Fig. 4.
TgIF2α-S71A mutants less viable than WT after exposure to the extracellular environment. (A) Intracellular WT and TgIF2α-S71A parasites were physically removed from host cells and then incubated without host cells in DMEM plus 1% FBS at 37 °C in 5% CO2 for 0, 2, 4, or 8 h. After each time point, 5 × 102 parasites were passed onto a fresh HFF monolayer in a 12-well plate. Upon plaque formation, each well was washed with PBS and stained with Coomassie Brilliant Blue (Upper). The number of plaques formed by the WT (diamonds) or TgIF2α-S71A mutants (circles) were counted using Alpha Innotech imaging software and plotted on a line graph (Lower). (B) Using the same method as above, WT and TgIF2α-S71A mutants were deprived of host cells for 0, 4, or 8 h and then allowed to infect a fresh HFF monolayer. Toxoplasma growth was quantitated by standard parasite doubling assay. The average number of parasites per vacuole is displayed for each counting period (8, 16, 24, and 32 h after inoculation). Results for WT parasites are shown as circles and dotted line; results for TgIF2α-S71A mutants are shown as triangles and solid line. (C) Protein lysates were generated from RH and Pru parasites deprived of host cells for 0, 4, or 8 h. A Western blot was carried out using equal amounts of protein lysate with antibodies that specifically recognize phosphorylated TgIF2α (TgIF2α-P) or total TgIF2α protein. (D) WT or TgIF2α-S71A tachyzoites were incubated in the presence of [35S] Met/Cys for 1 h at 37 °C with 5% CO2 immediately after removal from host cells (0 h) or after a 4-h extracellular incubation. Radiolabeled proteins were resolved on SDS/PAGE for autoradiography (Upper). The SDS/PAGE was stained with Coomassie Brilliant Blue to confirm equal protein loading (Lower). Translation levels were calculated using densitometry values of radiolabeled protein from WT parasites relative to the TgIF2α-S71A mutant. It is noted that uptake of the radiolabeled amino acids in both the WT and mutant parasites was reduced by 60% after 4 h in the extracellular medium, compared with that measured at 0 h. This explains, in part, the lowered levels of radiolabeled proteins in the TgIF2α-S71A parasites at 4 h vs. 0 h. Consequently, we compared WT protein synthesis with mutant at each time point in the accompanying histogram. *P < 0.05.
We next measured the doubling time of WT and TgIF2α-S71A mutant parasites that were passed immediately into fresh host monolayers (0 h extracellular exposure). Both grew at a similar rate during the 32-h time course (Fig. 4B). As we observed in the plaque assay, the inability to phosphorylate TgIF2α compromises parasite recovery from the stress of extracellular exposure. WT parasites deprived of host cells for 8 h have an average number of 12 parasites per vacuole at 32 h after inoculation, whereas TgIF2α-S71A mutants only achieve an average of 7 parasites per vacuole (Fig. 4B).
These studies suggest that TgIF2α becomes phosphorylated after egress to initiate a parasitic stress response that promotes survival until a new host cell can be invaded. Consistent with this idea, we found that phosphorylation of TgIF2α increases during prolonged exposure to the extracellular environment (Fig. 4C). Furthermore, the type I (RH) parasites, which were used in this study, are considered to be a more robust Toxoplasma strain in vitro compared with a type II (Pru) strain. Interestingly, there was significantly greater TgIF2α phosphorylation in type I parasites compared with type II after exposure to the extracellular environment (Fig. 4C). This suggests that there can be differences among Toxoplasma strains in the efficacy of the stress responses, which can account for the viability differences in in vitro analyses.
Because TgIF2α phosphorylation elicits repression of general protein synthesis, we measured translation levels by incubating extracellular WT and TgIF2α-S71A tachyzoites in medium containing [35S] Met/Cys radiolabel immediately after physical removal from host cells or after a 4-h exposure to the extracellular environment. Radiolabeled parasite proteins were then resolved by SDS/PAGE and visualized by autoradiography (Fig. 4D). WT parasites showed a modest reduction in the level of radiolabeled protein compared with mutant TgIF2α-S71A parasites when these parasites were incubated with the [35S] Met/Cys radiolabel immediately after removal from host cells (Fig. 4D). However, a 4-h incubation in the extracellular environment caused a significant reduction in radiolabeled protein in WT parasites relative to the TgIF2α-S71A mutant, consistent with the idea that TgIF2α phosphorylation leads to lowered translation. This translation measurement was carried out in parasites cultured in the extracellular medium in the absence of an added stress agent. When applied immediately after removal from host cells, we noted that there was no difference in 35S uptake between WT and the TgIF2α-S71A parasites. However, there was a 60% reduction in the uptake of radiolabeled amino acids in both WT and mutant parasites after 4 h in the extracellular medium compared with those parasites analyzed immediately after harvesting. This finding suggests that extracellular Toxoplasma tachyzoites experience stress outside the host cells that normally elicits translational control. By not appropriately reducing protein synthesis, the TgIF2α-S71A parasites are unable to implement this core response pathway that is central for stress resistance. One source of this extracellular stress may be impaired transport processes that impair nutrient uptake.
Reduced Virulence of TgIF2α-S71A Parasites in Vivo.
Because Toxoplasma incapable of phosphorylating eIF2α exhibit reduced fitness in vitro, we next addressed whether the TgIF2α-S71A mutant would have decreased virulence in vivo. To test this idea, we injected 100 WT or TgIF2α-S71A mutant parasites into female BALB/c mice immediately after the organisms egressed from human cell hosts or after a 4-h incubation outside the host cells. In this model for acute toxoplasmosis, hypervirulent RH strain Toxoplasma typically produces a moribund mouse in ≈7 d (168 h). The course of infection was very similar for each animal in its designated group, and all animals within that group were moribund at the same time. In our study, mice infected with WT parasites immediately after physical removal from host cells become moribund at 147 h, but mice infected with TgIF2α-S71A parasites showed a 26-h delay, not becoming moribund until 172 h (Table 1). Furthermore, animals injected with WT parasites that were maintained outside host cells for 4 h became moribund at 164 h, whereas animals infected with the TgIF2α-S71A parasites became moribund after 197 h, a delay of 33 h. Additionally, the mean parasite body burden was determined to assess parasite doubling time in vivo. WT parasites had a doubling time of 8.7 h when injected into mice immediately after egress, compared with a 9.9-h doubling time for the TgIF2α-S71A mutant (Table 1). The doubling time of the WT parasites increased by 0.6 h to 9.3 h after the 4 h extracellular incubation. The TgIF2α-S71A mutant doubling time increased significantly (nearly 2 h), from 9.9 h to 11.8 h after a 4-h exposure to the extracellular environment (Table 1). These results indicate that the TgIF2α-S71A parasites also have reduced virulence in an animal model of infection.
Table 1.
Virulence of WT vs. TgIF2α-S71A mutants in vivo
| Group | Time to moribund state (h) | Parasite body burden | Doubling time (h) |
| 0-h extracellular incubation | |||
| WT | 147 | 1.14 ± 0.2 e7 | 8.7 |
| TgIF2α-S71A | 172 | 1.68 ± 0.4 e7 | 9.9 |
| 4-h extracellular incubation | |||
| WT | 164 | 2.0 ± 0.6 e7 | 9.3 |
| TgIF2α-S71A | 197 | 1.13 ± 0.2 e7 | 11.8 |
BALB/c mice were injected with 100 WT or TgIF2α-S71A parasites immediately after harvesting or after a 4-h incubation in extracellular medium. The time to moribund state and parasite body burden at the moribund state was recorded for each infected animal.
Discussion
Eukaryotic cells have evolved mechanisms to tolerate stresses encountered in their environments. A well characterized stress response pathway conserved from yeast to humans centers on the phosphorylation of eIF2α, which reduces translation initiation and provides the cell with time to reprogram its genome to alleviate damage resulting from an insult. We and others have found this stress response to be conserved in early-branching protozoa, including parasitic species such as the Apicomplexa and kinetoplastids (7, 10, 18).
Here we describe a unique function for eIF2α phosphorylation in the obligate intracellular protozoan, Toxoplasma, which centers on the ability of the parasite to survive without the resources and shelter supplied by its host cell. This study shows that without eIF2α phosphorylation, tachyzoites have a diminished capacity to remain virulent the longer the parasites are deprived of host cells. The decrease in virulence as a result of not being able to phosphorylate eIF2α is also seen when the mutant parasites are used in the mouse model of acute toxoplasmosis. The data indicate that intracellular parasites are stressed when outside of a host cell, and a key part of managing this extracellular stress involves translation control mediated by the phosphorylation of eIF2α. Consistent with this idea, we found that type I RH strain parasites exhibit more robust TgIF2α phosphorylation compared with type II parasites exposed to the extracellular environment (Fig. 4C). It has recently been shown that the laboratory RH strain used here survives outside of host cells longer than type II strains (3). The ability of RH strain to elicit a faster and stronger eIF2 kinase stress response upon egress may help explain why RH strain is hardier in vitro and in vivo relative to type II strain.
There are four eIF2 kinases encoded in the Toxoplasma genome, designated TgIF2K-A through -D. TgIF2K-A possesses a transmembrane domain and was found to be localized to the parasite ER (8). Upon treatment with ER stress agents, TgIF2K-A is released from its association with BiP, suggesting shared activation mechanisms with those described in yeast and mammals (14). TgIF2K-B is a cytosolic eIF2 kinase lacking homology to previously characterized eIF2 kinases (8). TgIF2K-C and -D are less well characterized but resemble GCN2, an eIF2 kinase that is documented to respond to nutrient deficiency. Which of the TgIF2Ks is involved in phosphorylating TgIF2α when parasites are exposed to extracellular stress encountered by Toxoplasma is currently not clear. The nature of the extracellular stress could be the absence of the host cell protecting or buffering the parasite from deficiencies in nutrients or metabolites, or it could be a range of damaging environmental insults. Consistent with this idea, we found that uptake of the radiolabeled amino acids was reduced after 4-h exposure to extracellular stress. Reduced translation by phosphorylation of TgIF2α may allow Toxoplasma to conserve resources and give the parasite time to reconfigure gene expression to circumvent the extracellular stress. We note that Toxoplasma does not possess basic leucine zipper transcription factors, such as ATF4, which are preferentially translated in response to eIF2α phosphorylation in mammalian cells (19, 20). Presently, the only transcription factors identified in Apicomplexa resemble the AP2 domain family described in plants (21, 22). It is inviting to speculate that alternative regulators, such as the AP2 factors, may be the targets for preferential translation in these parasites.
In addition to the rapid proliferation of tachyzoites, pathogenesis of Toxoplasma also involves the ability of the tachyzoites to differentiate into bradyzoites. Bradyzoites form a tissue cyst that can remain in the host organism for life. During immunosuppressive conditions, the bradyzoites can reemerge as an acute tachyzoite infection. Consistent with their quiescent nature, latent bradyzoites maintain TgIF2α in its phosphorylated state, and it was suggested that this stress response may also function to lower protein synthesis and conserve resources in the dormant parasitic cyst (8). The TgIF2α-S71A mutant was created in the hypervirulent RH type I strain of Toxoplasma; although well suited to study the proliferate stage, the strain has largely lost its ability to develop into bradyzoites in vitro and in vivo (3). To further examine the role of TgIF2α phosphorylation in bradyzoites, it is important to generate the TgIF2α-S71A mutant in type II strain Toxoplasma. Such a mutant is likely to prove challenging to generate because type II strains grow more slowly and are less amenable to genetic manipulation than type I strains.
In addition to our previous observation that phosphorylated TgIF2α accumulates in latent bradyzoites, this study shows that TgIF2α phosphorylation is also important during the tachyzoite lytic cycle, specifically during the critical time when the parasite is without a host cell. Therefore, pharmacological targeting of the TgIF2α stress response pathway promises to have multiple benefits in fighting both acute and chronic forms of toxoplasmosis. It would be of great interest to assess whether eIF2 phosphorylation and translation control also contribute to the survival of other intracellular pathogens during the times in their life cycle when they are outside of host cells.
Methods
Allelic Replacement Vector for TgIF2α-S71A.
To generate the allelic replacement construct, two fragments (−26,00 bp to −1,411 bp and −1,238 bp to +440 bp) were amplified and inserted on opposing ends of the CAT minigene cassette within the pminCAT/HXGPRT+ vector, as illustrated in Fig. 1A (23). The ≈1.2-kb 5′ TgIF2α flanking sequence (−2,600 bp to −1,411 bp) was inserted between the NotI and BamHI sites using the oligonucleotides “5′S71A for + NotI” and “5′S71A rev + BamHI.” Sequences for primers used for this study are in Table S1. The 3′ TgIF2α flanking fragment (−1,238 bp to +440 bp), which includes the entire first exon of TgIF2α as well as ≈1.2 kb of upstream sequence, was inserted into the BclI site of the construct described above. A point substitution was generated to change Ser71 to Ala71 using the QuikChange XL mutagenesis kit (Stratagene) and the oligonucleotides “TgIF2α S71A quikchange 1F” and “TgIF2α S71A quikchange 1R.”
Generation of TgIF2α-S71A Mutant Parasites.
Twenty-five micrograms of the TgIF2α-S71A allelic replacement vector was linearized with NotI and transfected into RH strain ΔKu80 parasites (15, 16) as previously described (23). Parasites were cultivated in confluent monolayers of HFF under standard conditions (DMEM plus 1% FBS in a humidified incubator at 37 °C with 5% CO2). Transgenic parasites were selected in 20 μM chloramphenicol and cloned by limiting dilution. Parasite clones were screened by immunoblot analysis of the phosphorylation status of TgIF2α phosphorylation after stress with 5 μM A23187 for 1 h (8). Positive clones were confirmed independently using a PCR-based approach: replacement of the Ser71-encoding nucleotides with those that encode Ala71 creates a unique MscI restriction site. The designated portion of the TgIF2α locus was amplified using “S71A screen for” and “S71A screen rev” and digested with MscI (Fig. 1A). Clones with the S71A substitution yield two bands of 340 and 220 bp, whereas the parental strain yields a single 560-bp band.
TgIF2α Phosphorylation Detection and Protein Radiolabeling.
Phosphorylation of TgIF2α was monitored by Western blotting performed as described by Narasimhan et al. (8). Intracellular parasites were released from HFF host cells by physical disruption (scraping and/or syringe passage) and purified by filtration through 3-μm polycarbonate filters (23). The purified extracellular parasites were incubated in DMEM containing 1% FBS at 37 °C under 5% CO2 for the designated length of time. Fifty micrograms of protein lysates were separated by electrophoresis using a 10% Bis-Tris acrylamide gel (Invitrogen). Proteins were transferred to nitrocellulose membranes and probed with either rabbit anti-TgIF2α antibody (diluted 1:10,000) or phospho-specific (Ser71) TgIF2α antibody (diluted 1:500) followed by an anti-rabbit IgG–horseradish peroxidase conjugate (GE Healthcare) (7, 8). Total and phospho-TgIF2α was visualized using an ECL Western blotting substrate (Pierce).
In experiments in which parasites were radiolabeled, equal numbers of extracellular tachyzoites were resuspended in labeling media, DMEM without l-methionine, l-cysteine, l-glutamine, or sodium pyruvate (Invitrogen #21013-024) supplemented with 5% FBS, 1 mM l-glutamine, and 0.5 mM sodium pyruvate. Express Protein Label Mix (0.145 mCi) containing [35S]methionine and [35S]cysteine (PerkinElmer Life Sciences) was added to the sample and incubated for 1 h. Samples were washed twice in PBS, and a portion was counted to determine the uptake of the radiolabeled amino acids, as previously described (8). Although there was no difference in 35S uptake between WT and the TgIF2α-S71A parasites, there was a 60% reduction in the uptake of radiolabeled amino acids after 4 h in the extracellular medium compared with those parasites analyzed immediately after harvesting. Parasites were resuspended in lysis buffer and sonicated. Equal amounts of total protein from each lysate preparation were separated by SDS/PAGE, and radiolabeled proteins were visualized by autoradiography. Additionally, the SDS/PAGE gel was stained with Coomassie Brilliant Blue to confirm equal protein loading. Translation levels were calculated using densitometry values of radiolabeled protein from WT parasites relative to the TgIF2α-S71A mutant. Results are presented as means SE that were derived from three independent experiments. Student's t test was used to determine statistical significance.
Competitive Parasite Fitness Assay Using TaqMan Probes.
The comparative fitness assay was based on a protocol outlined in ref. 17. After filter purification, 5 × 105 parental ΔKu80 (WT) and TgIF2α-S71A mutant parasites were mixed in 10 mL DMEM + 1% FBS and added to a T-25 cm2 flask containing a monolayer of HFF host cells. A sample of the mixed parasites was collected every 72 h for a total of 9 d. At each time point, 105 parasites were used to infect a fresh HFF monolayer. Genomic DNA was isolated from each parasite sample using the DNeasy kit (Qiagen). TaqMan-based allelic discrimination assay was performed using forward and reverse oligonucleotides “TgIF2α TaqMan for” and “TgIF2α TaqMan rev” and a combination of probes used to identify the WT or mutant allele (“FAM-WT TgIF2α” and “VIC-TgIF2α-S71A,” respectively). PCR reactions were performed in triplicate using the 7500 Real-Time PCR System and analyzed with relative quantification software (SDS software, version 1.2.1; Applied Biosystems).
Parasite Growth Assays.
Toxoplasma doubling assays were performed as previously described (24). Intracellular ΔKu80 and TgIF2α-S71A parasites were physically removed from host cells by syringe passage. A total of 105 of each were immediately applied to a fresh monolayer of HFFs grown on coverslips (0 h), or incubated in culture media at 37 °C with 5% CO2 in absence of host cells for 4 or 8 h before infection. The number of parasites per vacuole was visualized every 8 h by immunofluorescence assay using the DNA intercalator DAPI. Experiments were carried out in triplicate using separate biological samples. Toxoplasma growth in culture was also evaluated by a standard plaque assay (23). Five hundred ΔKu80 or TgIF2α-S71A mutant parasites were allowed to infect an HFF monolayer; the degree of host cell lysis was evaluated on day 5–7 using Coomassie Brilliant Blue staining. In some cases, the number of plaques was counted. Alternatively, the area of clearing representing the degree of monolayer disruption was determined using an Alpha Innotech Imaging system. Experiments were carried out in triplicate using separate biological replicates. Results from a single representative experiment are shown.
Lytic Cycle Assays.
The red/green adhesion and invasion assay was carried out as previously described (25). To evaluate Toxoplasma motility, 105 ΔKu80 or TgIF2α-S71A parasites were allowed to glide along a poly-l-lysine–coated coverslip for 30 min. Adhered parasites and surface protein trails were detected with a mouse anti-Sag1 immune serum as previously described (26). The number of ΔKu80 or TgIF2α-S71A parasites with trails was recorded from a minimum of six independent microscope fields. Parasite egress assays were performed as previously described (27).
Animal Studies.
Female BALB/c mice (18–20 g) were injected i.p. with 100 ΔKu80 or TgIF2α-S71A parasites (at least eight mice per group) harvested immediately after egress from HFF cells or after a 4-h incubation in culture media. Animals were monitored twice daily until significant illness was observed, at which time the moribund animals were killed. Time to moribund state was recorded for each infected group of mice. To assess parasite burden at harvest, peritoneal fluid was removed and organism content was determined by direct counting (28). Knowing the original inoculum and the total number of organisms recovered per mouse, the time of infection was used to calculate a doubling time based on an exponential model of growth.
Supplementary Material
Acknowledgments
We thank Dr. Vern Carruthers (University of Michigan Medical School, Ann Arbor, MI) for supplying the RHΔKu80 parasites and his protocol for the red/green invasion assay; Dr. John Boothroyd (Stanford University, Palo Alto, CA) for supplying rabbit anti-Sag1; and Pam Torkelson (Indiana University School of Medicine) for technical assistance. Support for this research was provided through American Heart Association Grant 0920034G (to B.R.J.) and Awards R21AI084031 (to R.C.W. and W.J.S.) and R01GM49164 (to R.C.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007610107/-/DCSupplemental.
References
- 1.Hill DE, Chirukandoth S, Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- 2.Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–623. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan A, Behnke MS, Dunay IR, White MW, Sibley LD. Phenotypic and gene expression changes among clonal type I strains of Toxoplasma gondii. Eukaryot Cell. 2009;8:1828–1836. doi: 10.1128/EC.00150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 5.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal. 2007;9:2357–2371. doi: 10.1089/ars.2007.1764. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan WJ, Jr., Narasimhan J, Bhatti MM, Wek RC. Parasite-specific eIF2 (eukaryotic initiation factor-2) kinase required for stress-induced translation control. Biochem J. 2004;380:523–531. doi: 10.1042/BJ20040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narasimhan J, et al. Translation regulation by eukaryotic initiation factor-2 kinases in the development of latent cysts in Toxoplasma gondii. J Biol Chem. 2008;283:16591–16601. doi: 10.1074/jbc.M800681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fennell C, et al. PfeIK1, a eukaryotic initiation factor 2alpha kinase of the human malaria parasite Plasmodium falciparum, regulates stress-response to amino-acid starvation. Malar J. 2009;8:99. doi: 10.1186/1475-2875-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Möhrle JJ, Zhao Y, Wernli B, Franklin RM, Kappes B. Molecular cloning, characterization and localization of PfPK4, an eIF-2alpha kinase-related enzyme from the malarial parasite Plasmodium falciparum. Biochem J. 1997;328:677–687. doi: 10.1042/bj3280677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dever TE, et al. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 12.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 13.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 14.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 15.Fox BA, Ristuccia JG, Gigley JP, Bzik DJ. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot Cell. 2009;8:520–529. doi: 10.1128/EC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh MH, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8:530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fohl LM, Roos DS. Fitness effects of DHFR-TS mutations associated with pyrimethamine resistance in apicomplexan parasites. Mol Microbiol. 2003;50:1319–1327. doi: 10.1046/j.1365-2958.2003.03756.x. [DOI] [PubMed] [Google Scholar]
- 18.Moraes MC, et al. Novel membrane-bound eIF2alpha kinase in the flagellar pocket of Trypanosoma brucei. Eukaryot Cell. 2007;6:1979–1991. doi: 10.1128/EC.00249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 21.Iyer LM, Anantharaman V, Wolf MY, Aravind L. Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int J Parasitol. 2008;38:1–31. doi: 10.1016/j.ijpara.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 22.De Silva EK, et al. Specific DNA-binding by apicomplexan AP2 transcription factors. Proc Natl Acad Sci USA. 2008;105:8393–8398. doi: 10.1073/pnas.0801993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roos DS, Donald RG, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 24.Fichera ME, Bhopale MK, Roos DS. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob Agents Chemother. 1995;39:1530–1537. doi: 10.1128/aac.39.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh MH, et al. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 2003;22:2082–2090. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 27.Black MW, Arrizabalaga G, Boothroyd JC. Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol Cell Biol. 2000;20:9399–9408. doi: 10.1128/mcb.20.24.9399-9408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai G, Radzanowski T, Villegas EN, Kastelein R, Hunter CA. Identification of STAT4-dependent and independent mechanisms of resistance to Toxoplasma gondii. J Immunol. 2000;165:2619–2627. doi: 10.4049/jimmunol.165.5.2619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.