Abstract
Dengue virus (DENV) modifies cellular membranes to establish its sites of replication. Although the 3D architecture of these structures has recently been described, little is known about the cellular pathways required for their formation and expansion. In this report, we examine the host requirements for DENV replication using a focused RNAi analysis combined with validation studies using pharmacological inhibitors. This approach identified three cellular pathways required for DENV replication: autophagy, actin polymerization, and fatty acid biosynthesis. Further characterization of the viral modulation of fatty acid biosynthesis revealed that a key enzyme in this pathway, fatty acid synthase (FASN), is relocalized to sites of DENV replication. DENV nonstructural protein 3 (NS3) is responsible for FASN recruitment, inasmuch as (i) NS3 expressed in the absence of other viral proteins colocalizes with FASN and (ii) NS3 interacts with FASN in a two-hybrid assay. There is an associated increase in the rate of fatty acid biosynthesis in DENV-infected cells, and de novo synthesized lipids preferentially cofractionate with DENV RNA. Finally, purified recombinant NS3 stimulates the activity of FASN in vitro. Taken together, these experiments suggest that DENV co-opts the fatty acid biosynthetic pathway to establish its replication complexes. This study provides mechanistic insight into DENV membrane remodeling and highlights the potential for the development of therapeutics that inhibit DENV replication by targeting the fatty acid biosynthetic pathway.
Keywords: membrane remodeling, RNAi, replication complex
Dengue virus (DENV) is the causative agent of dengue fever, dengue hemorrhagic fever, and toxic shock syndrome (1). These diseases are prevalent in tropical regions around the world, where the mosquito vectors thrive. A total of 50 to 100 million DENV-related infections occur annually worldwide (2). Despite the large burden to human health, basic research into the development of DENV antiviral therapy has been limited.
All positive-strand RNA viruses remodel cytosolic membranes to establish sites of replication (reviewed in ref. 3). These structures play a critical role in the viral life cycle, likely by increasing the local concentration of replicating viral components and by sequestering viral antigens from recognition by host immune surveillance mechanisms. Following the initial translation and processing of DENV proteins, cellular membranes are remodeled to establish cytosolic replication complexes (RCs). DENV nonstructural protein 4A (NS4A) has been proposed to be sufficient for DENV membrane remodeling, perhaps performing a structural role by inducing membrane curvature (4). This function requires a processing event in which the C terminus of NS4A is removed by the viral NS2B-3 protease.
Recently, the 3D structure of DENV RCs has been determined by electron tomography (5). That study clearly demonstrated that viral replication takes place on double-membrane vesicles that are contiguous with the endoplasmic reticulum (ER). Interestingly, there also appears to be physical linkage between sites of replication and assembly. Although we now know much about the structure of RCs and some of the viral factors that contribute to this process (reviewed in ref. 6), we still know very little about the cellular processes that contribute to their formation.
Viruses rely on host machinery to complete their life cycles. Recently, genome-wide RNAi screens have identified host factors required for hepatitis C virus (HCV) (7), HIV (8–10), influenza virus (11), and West Nile virus (WNV) (12) replication. Although a genome-wide screen has been published for DENV in Drosophila melanogaster cells (13), no such study has been done to identify directly the mammalian factors required for DENV infection. Because of the large amount of information generated by these studies, research to determine the biological relevance of the implicated genes has been slow. In contrast, we and other groups have focused on interrogating specific subsets of host genes that may be involved in viral replication (14–18). These targeted studies facilitate an in-depth analysis of the role of specific genes and pathways in viral replication. Recently, we performed an analysis of membrane trafficking genes in HCV replication that implicated phosphatidylinositol signaling and endocytic trafficking as being critical for HCV RC formation (14).
In this study, we performed a parallel analysis to identify cellular cofactors of DENV replication. We reasoned that by using identical siRNAs, transfection techniques, and cell lines between the DENV and HCV studies, we could uncover virus-specific differences in the constellation of host cofactors required for viral replication. The siRNAs we tested are a partial collection of the library used to evaluate cofactors of HCV infection (14), including siRNAs that target host genes involved in pathways that have been reported to be important for the replication of positive-strand RNA viruses. We systematically interrogated this library to determine which siRNAs significantly inhibited DENV replication. Through this analysis, we determined that DENV replication is dependent on at least three major cellular pathways: fatty acid biosynthesis, actin polymerization, and autophagy. We then further characterized the modulation of cellular fatty acid synthesis during DENV infection.
Results
Identification of Host Cofactors of DENV Replication.
Cellular membrane trafficking pathways are likely to be important for all stages of the viral life cycle. To study the role of host genes in DENV replication specifically, and not viral entry or egress, we used a DENV-luciferase replicon in which the structural genes were replaced with a Renilla luciferase gene fused to the first 20 amino acids of the capsid protein, followed by the foot-and-mouth disease virus protease 2A, which self-cleaves to release the luciferase protein from DENV NS1 (Fig. S1A). As proof of principle, siRNAs targeting the DENV replicon produced a significant decrease in replicon luciferase expression (P ≤ 0.05) (Fig. S1B). This reduction was similar to that of a polymerase-defective GDD→GΔΔ DENV replicon (Fig. S1C).
We next interrogated a siRNA library targeting genes associated with pathways that have been shown to be critical for replication of other viruses (Table S1). The targeted pathways included lipid biogenesis, actin polymerization, ER stress, autophagy, phosphatidylinositol signaling, and cellular vesicular trafficking (14, 19–24). DENV replicon RNAs and siRNA pools targeting one host gene were simultaneously electroporated into Huh-7.5 cells, which were subsequently harvested at 6 and 48 h and assayed for luciferase expression. DENV replication was defined as luciferase levels at 48 h that were normalized for translation of input RNA at 6 h. A gene was categorized as being critical for DENV replication if the gene showed significant inhibition (P ≤ 0.05) of the replicon compared with irrelevant siRNA-treated cells, without altering cell viability (Table 1 and Fig. S2). We then confirmed that the indicated siRNAs reduced RNA accumulation of their targeted genes using gene-specific quantitative real-time RT-PCR assays (Table S2). siRNAs targeting all tested genes reduced target mRNA levels by greater than 60% (Table 1). Further, to ensure that the inhibitory phenotype could not be associated with siRNA off-target effects, we validated each “hit” with two different individual siRNAs targeting the same gene (Table S1). Genes that are critical for DENV replication clustered into three cellular pathways: actin polymerization (CDC42 and DIAPH1), fatty acid synthesis (ACACA and FASN), and ER stress/autophagy (ATF6, XBP1, and ATG12). The remaining two genes, RAB7L1 and GNB2L1, function in trans-Golgi network to late endosome trafficking and as a scaffolding protein, respectively (Table 1).
Table 1.
Host genes required for DENV RNA replication
| Percent inhibition compared with irrelevant treated cells | |||
| Gene | DENV replicon | Host RNA | Gene function |
| ACACA | 70 ± 4.9% | 71 ± 0.4% | Fatty acid synthesis |
| FASN | 68 ± 9.9% | 90 ± 0.6% | Fatty acid synthesis |
| CDC42 | 60 ± 8.6% | 94 ± 2.3% | Actin remodeling |
| DIAPH1 | 63 ± 0.5% | 74 ± 1.5% | Actin remodeling |
| ATF6 | 66 ± 11.5% | 84 ± 0.8% | ER stress/autophagy |
| XBP1 | 77 ± 5.4% | 95 ± 1.3% | ER stress/autophagy |
| ATG12 | 76 ± 0.7% | 70 ± 1.6% | Autophagy |
| RAB7L1 | 87 ± 2.4% | 89 ± 2.1% | Vesicular trafficking |
| GNB2L1 | 61 ± 8.8% | 84 ± 0.5% | Protein kinase C signaling |
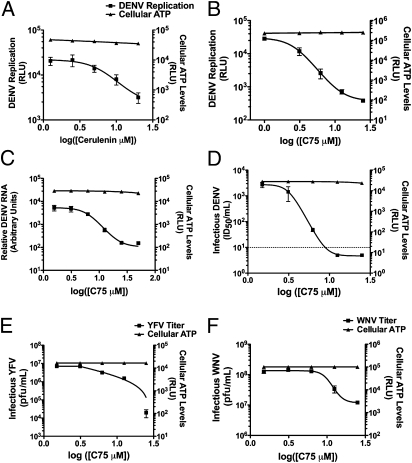
The significance of the identified pathways for DENV replication was then validated with pharmacological inhibitors. 3-Methyl adenine, which inhibits autophagy, has been previously reported to inhibit DENV replication (25, 26). Treatment with Cytochalasin D (Sigma), which inhibits actin polymerization, produced a statistically significant (P < 0.05) dose-dependent inhibition of DENV replication (Fig. S3). The requirement of fatty acid synthesis for DENV replication was tested with two related inhibitors, Cerulenin (MP Biomedicals) and C75 (Cayman Chemical). Both of these inhibitors, which target fatty acid synthase (FASN), showed statistically significant (P < 0.05) dose-dependent inhibition of DENV replication (Fig. 1 A and B). The inhibitory effect of C75 on DENV replication was particularly impressive, reducing DENV replication ∼2 log10 using both replicon (Fig. 1B) and DENV-2 (Fig. 1C). Indeed, infectious DENV was not detected in the presence of higher concentrations of C75 (Fig. 1D). There was no major decrease in cellular viability on addition of the drugs (Fig. 1), indicating that the inhibition of viral replication could be attributed to the inhibition of the targeted cellular process.
Fig 1.
Effects of pharmacological inhibitors on DENV replication. DENV replicon RNAs were introduced into Huh-7.5 cells. At 24 h postelectroporation, the cells were treated with the indicated concentrations of Cerulenin (A) and C75 (B), maintained for 24 h, and assayed for luciferase activity. RLU, relative luciferase units. Huh-7.5 cells were DENV-infected [multiplicity of infection (MOI) = 1] for 4 h and then treated with the indicated C75 concentrations. Twenty-four hours postinfection, viral RNA levels (C) or released virus (D) was quantified along with cellular ATP levels. The dotted line indicates the limit of detection. Baby hamster kidney cells were infected with YFV 17D (E; MOI = 1), or Vero cells were infected with WNV NY99 (F; MOI = 1) and treated as above. Data represent the average ± SEM.
We examined whether FASN inhibition affected the replication of other flaviviruses. Cells infected with yellow fever virus (YFV) and WNV were treated with C75, and the release of extracellular virus was quantified. The addition of C75 significantly inhibited the release of infectious viral particles for both viruses (Fig. 1 E and F). Thus, infections by three distinct flaviviruses are inhibited by C75 treatment.
DENV Infection Induces a Relocalization of FASN.
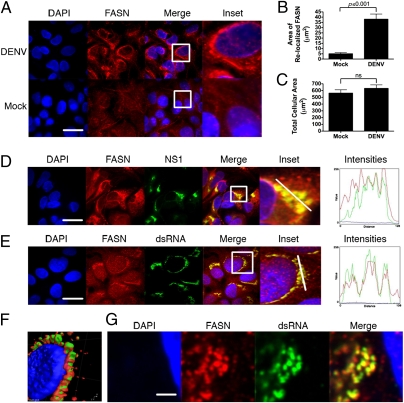
We next investigated whether DENV infection perturbed components of fatty acid biosynthesis. The subcellular localization of FASN, a major enzyme in fatty acid biosynthesis and a hit in our RNAi analysis, was altered dramatically by DENV infection. FASN has a diffuse staining pattern in mock-infected cells, whereas it produces a distinct ring around the nucleus in infected cells (Fig. 2A). Quantification of FASN relocalization using ImageJ (National Institutes of Health) analysis software showed that the difference between infected and uninfected cells is highly significant (P < 0.001; Fig. 2B). There was no significant difference in the size of DENV-infected cells as compared with mock-infected cells, eliminating the possibility that cytosolic shrinkage influenced FASN localization (Fig. 2C).
Fig. 2.
FASN is relocalized to sites of DENV replication. (A) Huh-7.5 cells were mock- or DENV-infected at a multiplicity of infection of 1–5, fixed 36–48 h postinfection, and probed with a monoclonal antibody against FASN. Insets reflect zoomed areas of the boxed regions. (Scale bar: 30 μm.) ImageJ quantitation of the fluorescent intensity of FASN staining in DENV-infected cells (B) compared with mock-infected cell size and cell size in DENV-infected or mock-infected cells (C) (average ± SEM). ns, not significant. Infected cells were probed with antibodies against FASN and the DENV NS1 (D) or dsRNA (E), the DENV replication intermediate. Insets reflect zoomed areas of the boxed regions. (Scale bar: 30 μm.) (F) 3D reconstruction of a z-stack taken through a DENV-infected cell stained with antibodies to dsRNA (green) and FASN (red). (G) Two-photon microscopy of a DENV-infected cell probed for dsRNA and FASN. Intensities plots represent the fluorescent intensity of red, green, and blue pixels along the white line in the inset panels. (Scale bar: 1,500 nm.)
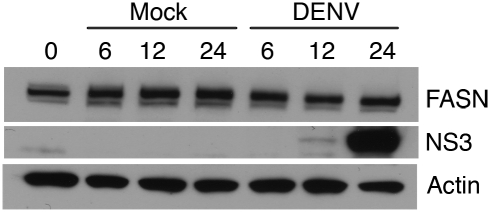
We next examined the colocalization of FASN with markers of DENV replication. Huh-7.5 cells were infected with DENV-2 for 24 h, fixed, and probed with antibodies to FASN and to either DENV NS1, which associates with DENV RCs, or dsRNA, the DENV replication intermediate. FASN colocalized with dsRNA and NS1 (Fig. 2 D and E). A 3D reconstruction of serial images in the Z dimension of an infected cell stained with antibodies to FASN and dsRNA demonstrated that both antibodies label the same perinuclear structures (Fig. 2F). High-resolution two-photon microscopy revealed that the FASN/dsRNA-positive complexes were composed of vesicular clusters reminiscent of published RC tomography structures (Fig. 2G). DENV infection does not produce a perceptible increase in protein abundance (Fig. 3), suggesting that the increased FASN fluorescence primarily reflects alterations in FASN localization and concentration. This contrasts with HCV infection, which stimulates FASN accumulation (27). From these analyses, we conclude that the FASN is relocalized to sites of active DENV replication.
Fig. 3.
FASN levels are not dramatically changed during DENV infection. Huh-7.5 cells were DENV-infected at a multiplicity of infection of 5. At the indicated times postinfection, cells were lysed and protein was extracted. Western blot analysis was performed to determine the relative levels of FASN protein. The blot was stripped and reprobed with actin antibodies as a loading control or with NS3 antibodies to indicate DENV infection.
DENV NS3 Interacts with FASN.
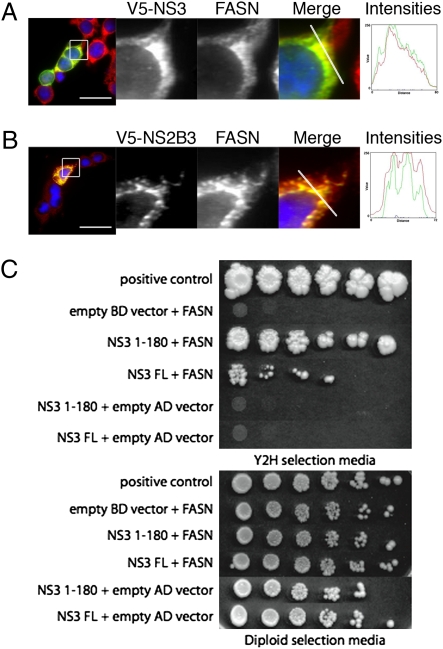
To determine the mechanism of FASN relocalization during DENV infection, we transfected V5-tagged expression constructs containing each of the individual DENV nonstructural proteins (Table S3) into Huh-7.5 cells and examined the localization of FASN and the respective DENV protein (Fig. S4). We observed significant colocalization of FASN with DENV NS3 but not with any of the other nonstructural proteins (Fig. S4). To ensure that this interaction was not cell type-specific, we repeated these experiments in HEK 293T cells. We were again able to see significant colocalization of FASN with NS3 expressed alone (Fig. 4A). Because NS3 normally associates with membranes via interaction with its NS2B cofactor, we expressed NS2B-3 and verified that NS3 staining was more associated with the ER and again colocalized with FASN (Fig. 4B).
Fig. 4.
DENV NS3 interacts with FASN. HEK 293T cells were transfected with a V5-tagged NS3 (A) or V5-tagged NS2B-3 (B) expression construct. Seventy-two hours posttransfection, cells were fixed and stained with antibodies to detect the tagged NS3 (green) and FASN (red). The intensity plots represent the fluorescent intensities along the line indicated in the merge panel. (Scale bar: 30 μm.) (C) Ten-fold serial dilutions of diploid yeast transformed with AD-FASN and BD-NS3 constructs (or their respective controls) were plated on yeast two-hybrid selection media or diploid selection media. Growth on the yeast two-hybrid selection media indicates an interaction between the proteins.
In parallel, two-hybrid system screening of a human cDNA library for NS3 interactions identified FASN, as described in Materials and Methods. The cDNA encoding the interacting portion of FASN was recloned and tested for interaction with NS3. We observed a specific interaction between FASN and NS3, whereas all control plasmid combinations yielded the appropriate results (Fig. 4C). Thus, NS3 can form a complex with FASN in the absence of other viral proteins. The N-terminal 180 amino acids of NS3 interact strongly with FASN, indicating that the NS3 protease domain is involved in FASN complex formation (Fig. 4C).
Induction of de Novo Lipid Biosynthesis and Incorporation of Newly Synthesized Lipids in DENV Replication Fractions.
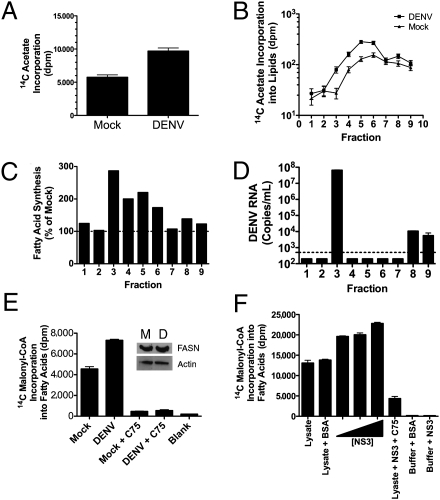
Given that FASN is required for viral replication and localizes with DENV RCs, we hypothesized that DENV infection may stimulate de novo fatty acid biosynthesis at sites of viral replication. To test this hypothesis, a confluent monolayer of human embryonic lung (HEL) cells was pulsed with 14C-acetate [a metabolite that can be used by FASN after conversion into acetyl-CoA to synthesize fatty acids (28, 29)] before viral infection. HEL cells were used in this assay because they are contact-inhibited, which helped to reduce basal lipid biosynthesis associated with cell growth. We first verified that HEL cells were appropriate for this study by reproducing the FASN phenotypes that were observed in DENV-infected Huh-7.5 cells (Fig. S5) and then proceeded with the FASN assay. Thirty-six hours after DENV infection, lipids were extracted from a postnuclear fraction and analyzed for radiolabel incorporation. A 170% increase in total labeled lipids accumulated in the postnuclear fraction of DENV-infected cells as compared with mock-infected cells (Fig. 5A).
Fig. 5.
DENV infection stimulates fatty acid biosynthesis, and de novo synthesized lipids are incorporated into sites of DENV replication. HEL cells were pulsed with 14C-acetate for 4 h, rinsed, DENV- or mock-infected for ∼36 h, and lysed, and the postnuclear fraction of the cells was recovered. The lysate was lipid-extracted and quantified (A) or applied to an OptiPrep gradient (B–E). (B) Lipid extraction of the fractions to determine the amount of radiolabel incorporated into lipids. (C) Percent increase in radiolabel in each fraction from DENV-infected cells compared with mock infection. (D) DENV RNA in each fraction as determined by RT-PCR. The dotted line at 500 copies represents the detection limit of the assay. (E) Cell lysates from DENV- or mock-infected cells were incubated with 14C-malonyl–CoA as a direct measure of fatty acid biosynthesis. The FASN inhibitor C75 was applied to verify that the radiolabel incorporation was attributable to FASN activity. Protein was extracted from the DENV and mock reactions and blotted for the presence of FASN and actin. (F) Huh-7.5 cell lysates were incubated with 14C-malonyl–CoA and increasing concentrations of purified recombinant NS3 (2, 4, or 8 μg) or a corresponding BSA control. C75 (1 mM) was added in parallel to a sample containing NS3. Lipids were extracted, and the radiolabel incorporation was quantified via scintillation counting. The buffer-only control indicates the background of the assay.
We next examined whether the enhanced FASN activity occurred in subcellular fractions that contain DENV RCs. HEL cells were labeled with 14C-acetate and DENV- or mock-infected as before. After harvest of the cell lysate, the postnuclear fraction was applied to an OptiPrep (Invitrogen) gradient and centrifuged. Fractions were collected from the gradient, lipid-extracted, and analyzed for radiolabel incorporation (Fig. 5B). Fractions 3 through 6 from the DENV-infected cells contained significant increases in radiolabeled lipid incorporation compared with mock-infected cells. Interestingly, the overall pattern of radiolabeled lipid incorporation into the fractions was different between DENV- and mock-infected cells (Fig. 5B). This suggests that fatty acid synthesis in DENV-infected cells may be enhanced in specific subcellular compartments. To determine if the radiolabel was increased in fractions in which DENV RNA was present, we extracted viral RNA from the fractions and quantified it by real-time RT-PCR. The vast majority of the DENV RNA was in fraction 3 (Fig. 5D). This corresponds to the fraction with the largest percent increase (>280%) in lipid-associated radiolabel in DENV-infected cells as compared with mock-infected cells (Fig. 5C).
Although 14C-acetate incorporation is a standard FASN assay, the substrate can also be incorporated into other classes of lipids, such as cholesterol. To test specifically whether FASN activity is altered in DENV-infected cells, we performed in vitro fatty acid synthesis assays with 14C-malonyl–CoA, which is a dedicated substrate for fatty acid synthesis. DENV- or mock-infected HEL cell lysates, which contained equivalent amounts of FASN as determined by Western blot analysis, were then tested for FASN activity (Fig. 5E). We observed a significant increase in newly synthesized labeled fatty acids from DENV-infected lysates as compared with mock-infected lysates (P < 0.05) (Fig. 5E). 14C-malonyl–CoA incorporation into lipids could be reduced to background levels by the addition of the FASN inhibitor C75, confirming the requirement of FASN activity for radiolabel incorporation into fatty acids (Fig. 5E).
Since FASN activity increased in DENV-infected cells without major changes in protein levels, we hypothesized that NS3 may directly stimulate FASN activity. Recombinant NS3 was purified, and increasing concentrations of NS3 (or a BSA control) were incubated with uninfected cellular lysates and analyzed by fatty acid synthesis via 14C-malonyl–CoA incorporation. We observed a dose-dependent increase in fatty acid synthesis as NS3 concentrations were increased, which can be inhibited by C75 (Fig. 5F). From these data, we conclude that DENV NS3 recruits FASN to sites of replication and stimulates fatty acid synthesis.
Discussion
This study is part of a parallel RNAi analysis of cellular pathways involved in HCV and DENV replication. The HCV analysis identified requirements of actin dynamics, endocytic trafficking, and phospholipid kinases for viral replication (14). Surprisingly, DENV replication does not share a need for the majority of these cellular cofactors. In particular, silencing phosphatidylinositol 4-kinase IIIα (PI4K-IIIα), which inhibits HCV replication by 99%, has no effect on DENV replication. Instead, data from RNAi, pharmacological inhibitors, microscopy, and biochemical assays implicate three cellular pathways in DENV replication: (i) ER stress/autophagy, (ii) actin polymerization, and (iii) fatty acid biosynthesis. This indicates that members of the Flaviviridae, although sharing the property of reorganizing cellular membranes to establish sites of replication, may have evolved different mechanisms of cellular remodeling.
The requirement of ER stress and autophagy for DENV replication is supported by a number of earlier studies (25, 26, 30–33). These earlier studies proposed that DENV RCs occur on autophagosomes, similar to what was initially proposed for some picornaviruses (19, 32, reviewed in ref. 33). However, electron tomography studies clearly demonstrate that DENV RCs reside in membrane structures that are contiguous with the ER rather than distinct autophagosomes (5). Thus, although there is a requirement for autophagy during DENV infection, its role is likely indirect.
A role for actin dynamics in DENV replication was indicated by the identification of CDC42 and DIAPH1 as cofactors of DENV replication. The role of actin polymerization in DENV replication may involve the trafficking of viral and/or cellular factors to sites of replication. Interestingly, CDC42 is also required for HCV replication (7, 14), indicating that there may be a conserved role for actin-based trafficking between the two viruses during replication.
DENV replication requires the expression of ACACA and FASN, whose products catalyze the first two dedicated steps in the fatty acid biosynthetic pathway: conversion of acetyl-CoA to malonyl-CoA (34) and the generation of palmitoyl-CoA (35), respectively. The significance of this pathway in DENV replication was confirmed using the pharmacological inhibitors Cerulenin and C75, which covalently bind to the active site cysteine of the β-keto-acyl synthase moiety and prevent fatty acid biogenesis (28). The inhibition of DENV infection with C75 is consistent with the findings of a recent report (36). The previously undescribed effect of C75 on DENV replicons demonstrated in this study defines a specific role for FASN in DENV replication. However, it is a distinct possibility that there may be additional roles for fatty acid biogenesis during DENV infection, including posttranslational protein modifications or a role in generating excess lipids for virion envelopment.
We observed a profound redistribution of FASN following DENV infection to sites of DENV replication. The mechanism of FASN relocalization is via interaction with DENV NS3, which specifically colocalizes with FASN in NS3 and NS2B-3–transfected cells. The N-terminal 180 amino acids of NS3, which contain the protease domain, are sufficient for FASN interaction in yeast two-hybrid analysis. DENV infection increases fatty acid biosynthesis, and the de novo synthesized lipids cofractionate, at least partially, with DENV RNA. These data suggest that DENV infection modulates fatty acid biosynthesis to increase lipid biogenesis to establish or expand its RCs. Interestingly, FASN activity (but not protein levels) is increased in DENV-infected cells. Purified NS3 enhances the specific activity of FASN in vitro. It is possible that NS3 directly binds and modifies FASN activity or, alternatively, that the interaction is indirect through another cellular protein. The mechanism by which NS3 stimulates FASN-specific activity is currently under investigation.
We propose the following model of DENV RC formation based on these data and previous publications. DENV alters ER membranes in a two-step process. NS4A-2K forms the structural component of the membrane remodeling, likely by inducing membrane curvature. Thus, NS4A-2K expression alone can produce structures with similar morphology as are observed in DENV-infected cells (4). However, extensive membrane curvature would drastically reduce the effective surface area and functionality of the ER. Therefore, DENV NS2B-3 recruits FASN to sites of RC formation for fatty acid generation. These fatty acids could then be modified and incorporated into the ER membrane, leading to membrane expansion and increased membrane fluidity conducive to the generation of membrane curvature. This model is consistent with that proposed for Brome Mosaic Virus, which requires enzymes in the fatty acid metabolic pathway for increased membrane fluidity and membrane remodeling (37, 38). Fatty acid biosynthesis may also act in concert with cholesterol synthesis to alter the membrane composition of DENV RCs. This is based on the enhanced cholesterol accumulation at sites of viral replication observed in cells infected with the related flavivirus, Kunjin, and the requirement of some components of cholesterol biosynthesis for DENV replication (17, 39).
This mechanism is fundamentally different from what has been proposed for another member of the Flaviviridae, HCV. A number of groups identified PI4K-IIIα as a requirement for HCV replication (7, 14, 16, 40, 41), and it was shown that it localizes with and is required for HCV RCs (14). An analysis of these studies proposed a model in which the generation of phosphatidylinositol-4-phosphate, the end product of PI4K-IIIα, serves to nucleate cellular vesicles and the ER with HCV replicase proteins, resulting in HCV RC formation (42). This model has recently been extended to enteroviruses, which rely on the related PI4K-IIIβ (43). Flaviviruses, on the other hand, appear to stimulate de novo synthesis of fatty acids and cholesterol to generate membranes for remodeling.
This report and others have identified fatty acid synthesis as a requirement for the replication of at least eight different viruses. Human cytomegalovirus and influenza A virus infections may require fatty acid synthesis to modulate membrane composition for viral budding or protein modifications (44). It is also required for productive HCV (27, 45) and EBV (46) infection by increasing the surface display of cellular receptors and the transcription of certain viral genes, respectively. The fact that so many diverse viruses depend on this process raises the intriguing possibility of the development of a broad-spectrum antiviral therapy targeting fatty acid biosynthesis. Further work defining the exact requirements for fatty acid biosynthesis during infection, as well as the mechanism of viral manipulation of the pathway, is an important next step in developing possible therapies.
Materials and Methods
Cells, Viruses, and Inhibitors.
Huh-7.5 cells, a subline derived from the hepatocyte Huh7 cell line (47); HEL cells; HEK 293T cells (American Type Culture Collection); baby hamster kidney cells; and Vero cells were used. Cells were maintained in DMEM-high glucose supplemented with 0.1 mM nonessential amino acids, 5% (vol/vol) FBS, and penicillin-streptomycin (Invitrogen). DENV-2 16681, YFV 17D, and WNV NY99 were used. Pharmacological inhibitors used were Cytochalasin D, Cerulenin, and C75. Reagents for individual DENV protein expression and purification are described in SI Materials and Materials.
RNAi Analysis.
siRNA assays were performed as previously described (15) with slight modifications, as described in SI Materials and Materials.
Immunofluorescence Analysis.
Cells were plated on glass coverslips, infected, fixed in methanol, and blocked, and antibody was added in 1× PBS + 0.1% saponin. Antibodies used include FASN polyclonal (Abcam) or monoclonal (BD Biosciences), dsRNA (J2 monoclonal antibody; English and Scientific Consulting Bt.), NS1 monoclonal antibody (Abcam), and V5 epitope polyclonal antibody (Sigma). Secondary antibodies were species-appropriate Alexa-Fluor 488 or 594 secondary antibody (Molecular Probes). Stained coverslips were mounted with Prolong Gold with DAPI (Invitrogen) and imaged using an Olympus DSU spinning disk confocal microscope or a Leica SP5 tandem scanner two-photon spectral confocal system, using 100× oil objectives. Digital images were taken using Slidebook 4.1 software (Olympus) and processed using ImageJ and Adobe Photoshop.
Yeast Two-Hybrid Assay.
Yeast strains and standard two-hybrid assay protocols are described in SI Materials and Methods. DENV NS3 fused to the Gal4 DNA-binding domain was screened against a human cDNA library cloned into the yeast two-hybrid vector pOAD.103 (48). Activation domain inserts from positive yeast two-hybrid colonies were PCR-amplified and sequenced. To confirm the yeast two-hybrid interaction between NS3 and FASN, the FASN fragment (nucleotides 2680–3108 of NM_004104.4) identified in the original yeast two-hybrid screen was amplified from human cDNA; cloned into pOAD.103 by homologous recombination in BK100; and mated with R2HMet yeast containing empty pOBD2 vector, pOBD2-NS3-FL, or pOBD2-NS3-1-180. Diploid yeast was selected on synthetic dropout (SD) medium lacking tryptophan and leucine. Liquid cultures of midlog-phase yeast were diluted to an OD600 of 1.0; subjected to fivefold serial dilutions in dH2O; spotted on solid SD medium lacking tryptophan, leucine, uracil, and histidine; and supplemented with 1 mM 3-amino-1,2,4-triazole. Plates were incubated at 30 °C until colonies appeared and were imaged on an Epson 1240U flatbed scanner.
Fatty Acid Synthesis Assay.
A confluent 10-cm dish of HEL cells was pulsed with 15 μCi of 14C-acetate in 9 mL of DMEM supplemented with 2% (vol/vol) FBS for 4 h, rinsed, and then infected at a multiplicity of infection of 1 for ∼36 h. Cells were washed with PBS; scraped into 2 mL of MEPS buffer [5 mM MgSO4, 5 mM EGTA, 35 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 0.25 M sucrose] supplemented with complete protease inhibitor (Roche), DTT, and PMSF; and mechanically disrupted by passage through a needle. The resulting lysate was centrifuged at 2,000 × g at 4 °C for 2 min. The postnuclear supernatant fraction was applied to a discontinuous OptiPrep gradient composed of equal volumes of 80%, 50%, 30%, and 10% OptiPrep in 1× PBS and spun overnight at 4 °C at 30,000 rpm in a SW41 rotor (Beckman Coulter). One-milliliter fractions were collected and analyzed for the presence of the radiolabel via scintillation counting or RNA via quantitative RT-PCR. Lipids were methanol/chloroform-extracted according to the Folch procedure (49). RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's instructions.
In Vitro Fatty Acid Synthesis Assay.
Confluent HEL cells grown in 10-cm dishes were DENV- or mock-infected for 36 h, rinsed, scraped into assay buffer [20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM DTT, 100 mM potassium phosphate], lysed via dounce homogenization, and then spun to remove nuclei. The postnuclear supernatant was combined with 170 μM acetyl-CoA, 500 μM NADPH, and 0.15 μCi of 14C-malonyl–CoA in glass tubes; incubated at 37 °C for 2 h; and stopped with 2:1 methanol/chloroform. Lipids were extracted as above and quantified via scintillation counting. Huh-7.5 cell lysates were used for the purified NS3 FASN assay.
Supplementary Material
Acknowledgments
We thank Charles Rice (The Rockefeller University) for providing Huh-7.5 cells, Bernard Roizman (University of Chicago) for providing the HEL cells, Claire Huang and Rich Kinney (Centers for Disease Control and Prevention, Fort Collins, CO) for providing the DENV-2 16681 infectious clone, Rich Kinney for providing WNV NY99, and Jim Strauss (Caltech) for providing YFV 17D. We acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence (National Institutes of Health Award 1-U54-AI-057153). N.S.H. is funded by National Institutes of Health Training Grant T32 AI065382-01. R.J.K. acknowledges support from the National Institute of Allergy and Infectious Disease (Grant AI45976). D.J.L. acknowledges support from the Ralph W. and Grace M. Showalter Research Trust.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010811107/-/DCSupplemental.
References
- 1.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.Miller S, Krijnse-Locker J. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol. 2008;6:363–374. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller S, Kastner S, Krijnse-Locker J, Bühler S, Bartenschlager R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J Biol Chem. 2007;282:8873–8882. doi: 10.1074/jbc.M609919200. [DOI] [PubMed] [Google Scholar]
- 5.Welsch S, et al. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A. Pathogenesis of flavivirus infections: Using and abusing the host cell. Cell Host Microbe. 2009;5:318–328. doi: 10.1016/j.chom.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Tai AW, et al. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008;4:495–504. doi: 10.1016/j.chom.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Brass AL, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 10.König R, et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008;135:49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao L, et al. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454:890–893. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan MN, et al. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455:242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sessions OM, et al. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger KL, et al. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci USA. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randall G, et al. Cellular cofactors affecting hepatitis C virus infection and replication. Proc Natl Acad Sci USA. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaillancourt FH, et al. Identification of a lipid kinase as a host factor involved in hepatitis C virus RNA replication. Virology. 2009;387:5–10. doi: 10.1016/j.virol.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell C, et al. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389:8–19. doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Ng TI, et al. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology. 2007;45:1413–1421. doi: 10.1002/hep.21608. [DOI] [PubMed] [Google Scholar]
- 19.Jackson WT, et al. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherry S, et al. COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLoS Pathog. 2006;2:e102. doi: 10.1371/journal.ppat.0020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belov GA, Fogg MH, Ehrenfeld E. Poliovirus proteins induce membrane association of GTPase ADP-ribosylation factor. J Virol. 2005;79:7207–7216. doi: 10.1128/JVI.79.11.7207-7216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Street A, Macdonald A, McCormick C, Harris M. Hepatitis C virus NS5A-mediated activation of phosphoinositide 3-kinase results in stabilization of cellular beta-catenin and stimulation of beta-catenin-responsive transcription. J Virol. 2005;79:5006–5016. doi: 10.1128/JVI.79.8.5006-5016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bost AG, Venable D, Liu L, Heinz BA. Cytoskeletal requirements for hepatitis C virus (HCV) RNA synthesis in the HCV replicon cell culture system. J Virol. 2003;77:4401–4408. doi: 10.1128/JVI.77.7.4401-4408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackenzie JM, Jones MK, Westaway EG. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J Virol. 1999;73:9555–9567. doi: 10.1128/jvi.73.11.9555-9567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YR, et al. Autophagic machinery activated by dengue virus enhances virus replication. Virology. 2008;374:240–248. doi: 10.1016/j.virol.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panyasrivanit M, Khakpoor A, Wikan N, Smith DR. Co-localization of constituents of the dengue virus translation and replication machinery with amphisomes. J Gen Virol. 2009;90:448–456. doi: 10.1099/vir.0.005355-0. [DOI] [PubMed] [Google Scholar]
- 27.Yang W, et al. Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology. 2008;48:1396–1403. doi: 10.1002/hep.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58:4611–4615. [PubMed] [Google Scholar]
- 29.Pizer ES, Wood FD, Pasternack GR, Kuhajda FP. Fatty acid synthase (FAS): A target for cytotoxic antimetabolites in HL60 promyelocytic leukemia cells. Cancer Res. 1996;56:745–751. [PubMed] [Google Scholar]
- 30.Khakpoor A, Panyasrivanit M, Wikan N, Smith DR. A role for autophagolysosomes in dengue virus 3 production in HepG2 cells. J Gen Virol. 2009;90:1093–1103. doi: 10.1099/vir.0.007914-0. [DOI] [PubMed] [Google Scholar]
- 31.Umareddy I, et al. Dengue virus serotype infection specifies the activation of the unfolded protein response. Virol J. 2007;4:91. doi: 10.1186/1743-422X-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor MP, Kirkegaard K. Modification of cellular autophagy protein LC3 by poliovirus. J Virol. 2007;81:12543–12553. doi: 10.1128/JVI.00755-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor MP, Kirkegaard K. Potential subversion of autophagosomal pathway by picornaviruses. Autophagy. 2008;4:286–289. doi: 10.4161/auto.5377. [DOI] [PubMed] [Google Scholar]
- 34.Tong L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell Mol Life Sci. 2005;62:1784–1803. doi: 10.1007/s00018-005-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith S. The animal fatty acid synthase: One gene, one polypeptide, seven enzymes. FASEB J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- 36.Samsa MM, et al. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee WM, Ahlquist P. Membrane synthesis, specific lipid requirements, and localized lipid composition changes associated with a positive-strand RNA virus RNA replication protein. J Virol. 2003;77:12819–12828. doi: 10.1128/JVI.77.23.12819-12828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee WM, Ishikawa M, Ahlquist P. Mutation of host delta9 fatty acid desaturase inhibits brome mosaic virus RNA replication between template recognition and RNA synthesis. J Virol. 2001;75:2097–2106. doi: 10.1128/JVI.75.5.2097-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackenzie JM, Khromykh AA, Parton RG. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe. 2007;2:229–239. doi: 10.1016/j.chom.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Trotard M, et al. Kinases required in hepatitis C virus entry and replication highlighted by small interference RNA screening. FASEB J. 2009;23:3780–3789. doi: 10.1096/fj.09-131920. [DOI] [PubMed] [Google Scholar]
- 41.Borawski J, et al. Class III phosphatidylinositol 4-kinase alpha and beta are novel host factor regulators of hepatitis C virus replication. J Virol. 2009;83:10058–10074. doi: 10.1128/JVI.02418-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger KL, Randall G. Potential roles for cellular cofactors in hepatitis C virus replication complex formation. Commun Integr Biol. 2009;2:471–473. doi: 10.4161/cib.2.6.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu NY, et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munger J, et al. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackel-Cram C, Babiuk LA, Liu Q. Up-regulation of fatty acid synthase promoter by hepatitis C virus core protein: Genotype-3a core has a stronger effect than genotype-1b core. J Hepatol. 2007;46:999–1008. doi: 10.1016/j.jhep.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Webster-Cyriaque J, Tomlinson CC, Yohe M, Kenney S. Fatty acid synthase expression is induced by the Epstein-Barr virus immediate-early protein BRLF1 and is required for lytic viral gene expression. J Virol. 2004;78:4197–4206. doi: 10.1128/JVI.78.8.4197-4206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vignali M, et al. Interaction of an atypical Plasmodium falciparum ETRAMP with human apolipoproteins. Malar J. 2008;7:211. doi: 10.1186/1475-2875-7-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.