Abstract
The combustion of fossil fuels has enriched levels of CO2 in the world’s oceans and decreased ocean pH. Although the continuation of these processes may alter the growth, survival, and diversity of marine organisms that synthesize CaCO3 shells, the effects of ocean acidification since the dawn of the industrial revolution are not clear. Here we present experiments that examined the effects of the ocean’s past, present, and future (21st and 22nd centuries) CO2 concentrations on the growth, survival, and condition of larvae of two species of commercially and ecologically valuable bivalve shellfish (Mercenaria mercenaria and Argopecten irradians). Larvae grown under near preindustrial CO2 concentrations (250 ppm) displayed significantly faster growth and metamorphosis as well as higher survival and lipid accumulation rates compared with individuals reared under modern day CO2 levels. Bivalves grown under near preindustrial CO2 levels displayed thicker, more robust shells than individuals grown at present CO2 concentrations, whereas bivalves exposed to CO2 levels expected later this century had shells that were malformed and eroded. These results suggest that the ocean acidification that has occurred during the past two centuries may be inhibiting the development and survival of larval shellfish and contributing to global declines of some bivalve populations.
Keywords: bivalve larvae, climate change, ocean acidification
More than 8 Pg of carbon dioxide (CO2) is released annually into our planet’s atmosphere via the combustion of fossil fuels (1). About one-third of anthropogenically derived CO2 has entered the world’s oceans during the past two centuries (2) and atmospheric and surface ocean CO2 levels are expected to reach ∼750 ppm by 2100 (3, 4). CO2 entering the ocean decreases the availability of carbonate ions (CO3−2) and reduces ocean pH, a process known as ocean acidification. These changes in ocean chemistry may have dire consequences for ocean animals that produce hard parts made from calcium carbonate (CaCO3). The experimental enrichment of CO2 to levels expected in the coming century has been shown to dramatically alter the growth, survival, and morphology of numerous calcifying organisms including coccolithophores, coral reefs, crustose coralline algae, echinoderms, foraminifera, and pteropods (5–7). Many shellfish also produce calcareous shells, and juvenile and adult clams, mussels, and oysters have been shown to be adversely affected by elevated CO2 (8–12). The earliest life history stages of shellfish, larvae, have been shown to be especially vulnerable to high CO2, displaying large declines in survival and delays in metamorphosis at levels predicted to occur later this century, suggesting recruitment of these populations may be adversely impacted by ocean acidification (12–14).
Although it is clear that calcifying ocean animals such as shellfish are sensitive to the increases in CO2 projected for the future, the extent to which the rise in CO2 that has occurred since the dawn of the industrial revolution has impacted these populations is poorly understood. Here we present experiments that examined the effects of past (250 ppm), present (390 ppm), and future (>400 ppm) CO2 concentrations on larvae of two species of shellfish: the Northern quahog or hard clam, Mercenaria mercenaria, and the bay scallop, Argopecten irradians. These bivalves are ecologically and commercially valuable resources: US mollusk harvests are $750 million annually (15), with ecosystem services far exceeding that value (16, 17). For experiments, CO2 was delivered via a gas proportionator system and CO2 levels in seawater were determined by quantifying dissolved inorganic carbon and pH during experiments using an EGM-4 Environmental Gas Analyzer (PP Systems) and the program CO2SYS (http://cdiac.ornl.gov/ftp/co2sys/). Dissolved inorganic carbon was measured with a methodological precision of ±3.6% and full recovery (102 ± 3%) of Dr. Andrew Dickson’s (Scripps Institution of Oceanography, University of California at San Diego, La Jolla, CA) certified reference material for total inorganic carbon in seawater [Batch 102 = 2,013 μmol dissolved inorganic carbon (DIC) kg seawater−1] was obtained with our analytical procedures. Static delivery of CO2 at rates that turned over experimental vessels several times an hour resulted in constant pH levels during experiments [<0.5% relative standard deviation (RSD) within treatments based on multiple daily measurements]. The rates of larval growth, development, and survivorship and lipid content of larvae were monitored through metamorphosis. Differences in the sizes and shells of larvae and early juvenile stage individuals were documented by cross-sectioning individuals and observing them with an SEM.
Results and Discussion
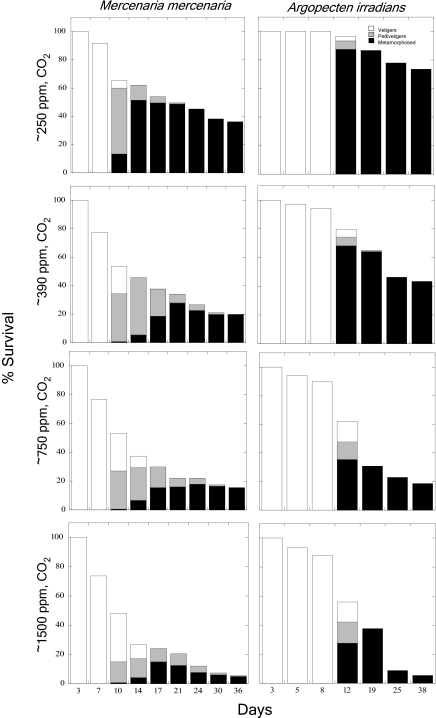
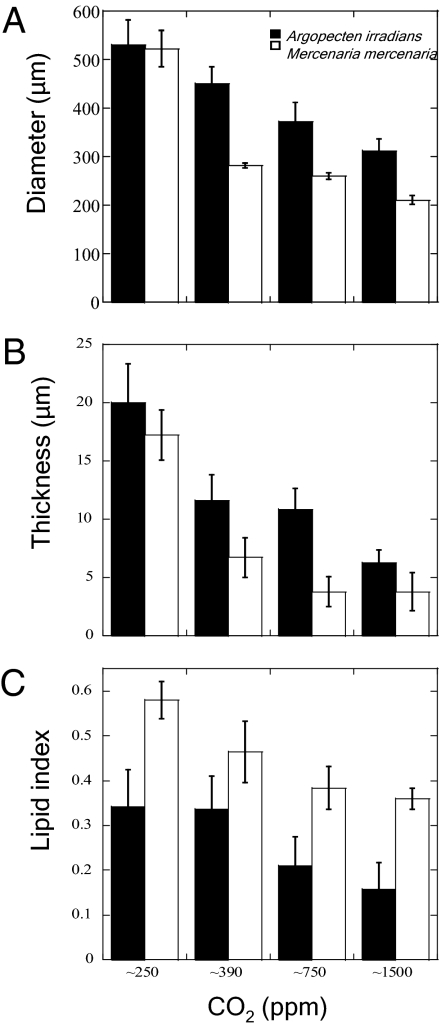
Larvae grown under near preindustrial levels of CO2 (250 ppm) displayed the highest rates of metamorphosis, growth, and survival. After 36 d of development, 40% of M. mercenaria grown under ∼250 ppm CO2 had survived, whereas only 20% survived at modern day CO2 levels (∼390 ppm), and only 6% survived at ∼1,500 ppm CO2 (P < 0.001; Fig. 1). A. irradians displayed similar patterns, with 74% of individuals surviving 38 d under ∼250 ppm CO2, 43% surviving at ∼390 ppm, and only 5.4% remaining at ∼1,500 ppm CO2 (P < 0.001; Fig. 1). Larvae grown under the lowest CO2 levels displayed remarkably faster rates of metamorphosis compared with individuals grown under present day CO2. For example, after 14 d of development, 51% of M. mercenaria larvae had fully metamorphosed at ∼250 ppm CO2, whereas <7% had done so under higher levels of CO2 (P < 0.001; Fig. 1). After 12 d of development, A. irradians larvae displayed a somewhat similar trend because 87% of the larvae had metamorphosed at ∼250 ppm CO2, whereas 68% had done so at ∼390 ppm CO2 (P < 0.001; Fig. 1). The mean diameters attained by both species of larvae also were strongly affected by CO2. M. mercenaria and A. irradians larvae grown under 250 ppm CO2 (523 ± 38 and 531 ± 51 μm) were significantly larger than those grown under present day (282 ± 5 and 449 ± 35 μm) and higher (210 ± 9 and 311 ± 26 μm at ∼1,500 ppm) levels of CO2 (P < 0.001; Fig. 2) These trends in the size of individuals were obvious during the examination of individuals under SEM (Figs. 3 and 4).
Fig. 1.
Development and survival of M. mercenaria and A. irradians larvae. Percent survival and developmental stage (veliger, pediveliger, and metamorphosed) of larvae grown under four levels of CO2, ∼250, 390, 750, and 1,500 ppm (Table 1). The relative SD of larval survival among replicated vessels per treatment for all times points and experiments was 4% (n = 4 per treatment).
Fig. 2.
Diameters, shell thickness, and lipid index of bivalve larvae grown under a range of CO2 concentrations. Data are from four levels of CO2, ∼250, 390, 750, and 1,500 ppm. (A) Diameters of M. mercenaria (day 24) and A. irradians (day 20). (B) Thickness of M. mercenaria (day 36) and A. irradians (day 52) shells at midpoint between the hinge and valve edge of the upper and lower shell of cross sectioned individuals. (C) Lipid index (lipid area/total area) for M. mercenaria (day 24) and A. irradians (day 20). Error bars represent SD of replicated vessels per treatment (n = 4 per treatment).
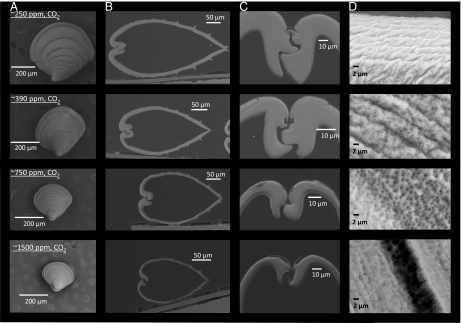
Fig. 3.
SEM images of M. mercenaria larvae grown under a range of CO2 concentrations. SEM images of 36-d-old M. mercenaria grown under different levels of CO2, ∼250, 390, 750, and 1,500 ppm (Table 1). (A) Images of individual larvae under each CO2 level. (B) Hinge to valve cross sections of individuals under each CO2 level. (C) The hinge of individuals under each CO2 level. (D) A magnification of the outermost shell of individuals under each CO2 level.
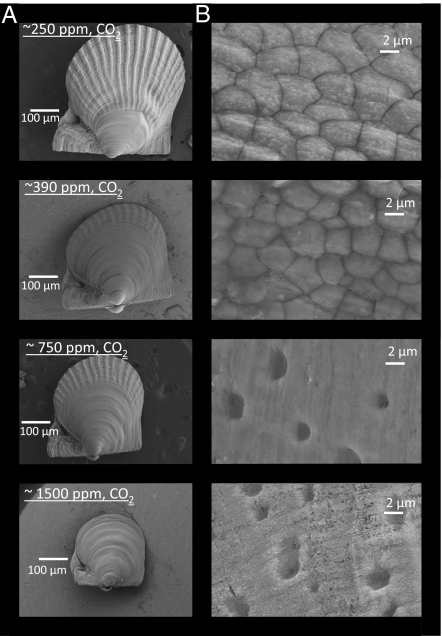
Fig. 4.
SEM images of 52-d-old A. irradians grown under different levels of CO2:, ∼250, 390, 750, and 1,500 ppm, (Table 1). (A) Image of a full individual larvae under each CO2 level. (B) A magnification of the outermost shell of individuals under each CO2 level.
Levels of CO2 strongly influenced the early formation of M. mercenaria and A. irradians shells. For example, after 17 d of development M. mercenaria shells were 17 ± 2 μm thick under ∼250 ppm CO2, 6.7 ± 2 μm at ∼390 ppm CO2, and 3.8 ± 1 μm at ∼750 ppm and ∼1,500 ppm CO2 (P < 0.001; Figs. 2 and 3). A. irradians shells also decreased in thickness with increasing CO2 being 20 ± 3, 12 ± 1, 11 ± 1, and 6.3 ± 1 μm thick under ∼250, 390, 750, and 1,500 ppm CO2 (P < 0.001, Fig. 2), respectively. Beyond impacting shell thickness, elevated levels of CO2 severely altered the development of the hinge structure of early stage bivalves. As CO2 levels increased from ∼250 to ∼1,500 ppm, there were dramatic declines in the size, integrity, and connectedness of the hinge (Fig. 3). Although the M. mercenaria hinge displayed a “tongue and groove” pattern under low CO2 (250 and 390 ppm), under higher CO2 concentrations the hinge and associated hinge teeth became increasingly separated and detached. Given that the bivalve hinge facilitates opening and closing of shells, allowing for intake of food and the excretion of waste (18), the compromised hinges observed under elevated CO2 may hinder the ability of individuals to obtain and process suspended particles for nutrition. This hypothesis is consistent with changes in lipid stores of larval shellfish exposed to differing CO2 concentrations. For both species, with each increasing level of CO2, the lipid content (as estimated by an index) decreased significantly (P < 0.001; Fig. 2). Increasing CO2 concentrations also caused marked changes in the morphology of the outer edge of juvenile shells (Figs. 3 and 4). With increasing levels of CO2, this region of the shell became increasingly riddled with holes, pockmarks, and crevices, observations consistent with other juvenile and larval shellfish reared under high CO2 (10, 14), suggesting CaCO3 shells were malforming and/or dissolving under more acidic conditions. Altered shell morphology was also obvious in juvenile scallops that had distinct ridges, characteristic of later stages of development, under preindustrial CO2, whereas individuals reared under higher CO2 conditions lacked ridges, a sign of slower development (Fig. 4 and 19).
Shell integrity is one of the most important lines of defense for larval and juvenile bivalve shellfish, because shells provide physical support for soft and delicate internal organs (20) and protection from benthic and pelagic predators and suspended particles (21, 22). As such, the thinner, frailer shells displayed by early life history bivalves reared under modern day and elevated CO2 would likely make individuals more vulnerable to predation and/or other environmental stressors. Similarly, within an ecosystem setting, larvae that accumulate fewer lipids (Fig. 2) are generally slower to metamorphose (19) and are more likely to perish once settled (23). Finally, individuals with extended metamorphosis times (Fig. 1) and that are smaller (Fig. 2) would be susceptible to greater rates of predation and natural mortality (23, 24). Hence, within an ecosystem setting, mortality rates of early life history bivalves that develop under modern day and higher CO2 levels would be expected to be even greater than the rates observed during our experiments. Given that bivalves in coastal areas naturally experience extremely high mortality rates in the transition from larvae to benthic juveniles (9), increases in mortality due to elevated CO2 could have profound effects on estuarine bivalve populations (5).
Our findings regarding the effects of future CO2 levels on larval shellfish are consistent with recent investigations of ocean acidification demonstrating that calcifying organisms will experience declines in survival and growth, as well as malformed CaCO3 shells and hard parts (25). However, our examination of the development of larval shellfish at levels of CO2 present before the industrialization of the planet provides important insight regarding the potential effects ocean acidification has had on calcifying organisms during the past two hundred years. Consistent with our findings, larval oysters (Crassostrea virginica) have displayed slightly larger shell area when grown under preindustrial CO2 levels compared with modern levels (26).
During the ∼24 million years before the industrial revolution, atmospheric CO2 levels are estimated to have been relatively static, likely fluctuating in a narrow range significantly below the concentrations present today (27, 28). Moreover, periods of higher CO2 before this era may not have been accompanied by lower pH and carbonate ion concentrations because the oceans may have buffered the more gradual changes in CO2 that have occurred through geological history (3, 29). The evolution of calcification in ocean animals is unknown, and the multiple forms of CaCO3 synthesized by modern day calcifiers (calcite, aragonite, amorphous CaCO3, and high magnesium CaCO3) differ widely in their vulnerabilities to dissolution under lower pH (30). Although the precise evolutionary tracks of modern bivalves remain somewhat uncertain (31), fossil evidence suggests that 906 of the 958 living genera of bivalve mollusks, including the species presented here, have a record that began in the mid- to late Cenozoic with the greatest continuous increase in genera between ∼15 and ∼25 Mya (32), a period of estimated lower CO2 levels compared with today (27, 28). Together with our results, this suggests that ocean acidification since the industrial revolution may have applied selection pressure on modern marine bivalves and may continue to do so in the future.
The shallow marine environments that many marine bivalves occupy can harbor dynamic levels of pH and CO2 (33, 34) and the precise degree of phenotypic plasticity of survival among bivalve larvae in the face of higher CO2 has not been established. Adaptation and evolution could promote the proliferation of bivalve strains that are more resistant to the increases in ocean CO2 expected in the coming century and some calcifying organisms may even benefit from higher CO2 levels (25, 35). Importantly, however, the current rates of increase in atmospheric CO2 are significantly faster than any recorded in tens of millions of years (27, 28), suggesting this evolutionary challenge may be without precedent for extant calcifying species.
A comparison of our two study species may provide insight into future evolutionary pressure of ocean acidification on marine calcifiers. Globally, M. mercenaria has a larger, more diverse geographic distribution (36) than A. irradians (37), an attribute that generally provides resistance to evolutionary pressures (38) such as increasing CO2 levels. In addition, predicted extinction rates are higher for the marine mollusk family Pectinidae, which includes A. irradians, than the Veneridae family, which includes M. mercenaria (39). This information, combined with the more dramatic declines in survival displayed by A. irradians under higher CO2 levels compared with M. mercenaria (Fig. 1), suggests A. irradians may face a greater evolutionary challenge in adapting to future increases in CO2 concentrations.
Precipitous declines in wild populations of bivalves during the 20th century have been attributed to overfishing, loss of habitat, hypoxia, and harmful algal blooms (40, 41). Our results suggest that ocean acidification is another process that may have contributed to the declines of these populations in the recent past and could further impact bivalve population densities and diversity in the future. Looking forward, marine organisms will be threatened by aspects of climate change beyond elevated CO2, including higher temperatures. Given that the rise in ocean temperatures projected for the coming century (4) is within a range that could also hinder the growth and survival of bivalve larvae (19, 42), future studies should consider the impact of higher CO2 in conjunction with temperature changes in line with such projections.
Materials and Methods
CO2 Treatments and Measurements.
A gas proportionator system (Cole Parmer Flowmeter system, multitube frame) was used to deliver CO2 gas to seawater treatments at multiple rates. The gas proportionator mixed appropriate flow rates of 5% carbon dioxide gas, low carbon dioxide gas, and pressurized air (∼390 ppm CO2) to yield the concentrations of carbon dioxide desired for experiments at a net flow rate (350 ± 5 mL min−1) that turned over the volume of plexiglass covered experimental beakers >400 times daily. Experiments were repeated with tanked gas premixed at each specific CO2 level and nearly identical seawater chemistry and larval responses were obtained. For experiments, the CO2 gas mixtures from the proportionator system were continuously delivered to the bottom of four replicated, polypropylene 1-L beakers containing 0.2 μm filtered seawater from eastern Shinnecock Bay, NY. With continuous bubbling, all treatment beakers remained saturated with respect to oxygen (∼8 mg L−1). To quantify precise CO2 levels attained in experimental beakers, seawater in beakers was bubbled for 24 h and analyzed at the start (immediately before the addition of larvae and phytoplankton) and at the end (larvae removed, phytoplankton present) of each experiment using an EGM-4 Environmental Gas Analyzer (PP Systems) system that quantifies total dissolved inorganic carbon levels after separating the gas phase from seawater using a Liqui-Cel Membrane (Membrana). This instrument provided a methodological precision ±3.6% for replicated measurements of total dissolved inorganic carbon and provided full recovery (102 ± 3%) of Dr. Andrew Dickson’s (Scripps Institution of Oceanography, University of California at San Diego, La Jolla, CA) certified reference material for total inorganic carbon in seawater (batch 102 = 2,013 μmol DIC kg seawater−1). Levels of CO2 were subsequently calculated based on measured levels of total inorganic carbon, pH (total scale; mol kg seawater−1), temperature (∼24 °C), salinity (∼28 ppt), and first and second dissociation constants of carbonic acid in seawater according to Roy et al. (43) using the program CO2SYS (http://cdiac.ornl.gov/ftp/co2sys/). Multiple daily measurements of pH (calibrated prior each use with NIST traceable standards, ± 0.002, Orion Star Series Benchtop pH meter; Thermo Scientific) indicated experiment beakers maintained a constant pH level throughout all experiments (<0.5% RSD within treatments).
Experimental Design.
The drafted recommendations of the “best practices” for small microcosm experiments set forth by European Project on Ocean Acidification (EPOCA) were followed for this project. For example, aeration of seawater was used to reach a target pCO2 level, the ideal mechanism to manipulate seawater carbon chemistry (43). Experiments were conducted using two species of bivalves: M. mercenaria and A. irradians. For each experiment, four levels of carbon dioxide were administered: a high level (∼1,500 ppm CO2), predicted for the year 2250; a moderate level (∼750 ppm CO2), predicted for the year 2100 (3, 44); ambient air (∼390 ppm CO2); and a near preindustrial level (∼250 ppm CO2; 25, 26 and Table 1). A. irradians and M. mercenaria larvae were obtained from locally obtained broodstock spawned at the East Hampton Shellfish Hatchery.
Table 1.
Temperature, pH, carbonate chemistry, alkalinity, and salinity (±SD) during the four-level CO2 experiments with M. mercenaria, and A. irradians larvae
| Parameter | Near preindustrial CO2 | Ambient, present day CO2 | Year 2100 CO2 | Year 2200 CO2 |
| M. mercenaria | ||||
| Temperature (°C) | 24 ± 0.52 | 24 ± 0.52 | 24 ± 0.52 | 24 ± 0.52 |
| pH | 8.171 ± 0.022 | 8.052 ± 0.036 | 7.801 ± 0.004 | 7.532 ± 0.021 |
| pCO2 (ppm) | 247.1 ± 6.231 | 380.0 ± 33.02 | 742.3 ± 9.111 | 1516 ± 31.21 |
| Ωcalcite | 5.31 ± 0.47 | 4.53 ± 0.41 | 2.82 ± 0.05 | 1.67 ± 0.05 |
| Ωaragonite | 3.42 ± 0.30 | 2.92 ± 0.26 | 1.82 ± 0.03 | 1.08 ± 0.03 |
| Total DIC (μmol L1) | 1646 ± 94.21 | 1831 ± 52.34 | 1947 ± 21.33 | 2108 ± 18.06 |
| CO32− (μmol L−1) | 208.0 ± 20.22 | 178.0 ± 16.03 | 111.0 ± 1.806 | 66.0 ± 1.904 |
| Alkalinity (TA) | 1938 ± 117.3 | 2070 ± 66.42 | 2080 ± 22.63 | 2127 ± 49.71 |
| Salinity | 28.0 ± 1.0 | 28.0 ± 1.0 | 28.0 ± 1.0 | 28.0 ± 1.0 |
| A. irradians | ||||
| Temperature (°C) | 24 ± 0.51 | 24 ± 0.52 | 24 ± 0.52 | 24 ± 0.52 |
| pH | 8.170 ± 0.026 | 8.041 ± 0.044 | 7.801 ± 0.005 | 7.530 ± 0.011 |
| pCO2 (ppm) | 244.1 ± 4.006 | 386.5 ± 40.04 | 738.9 ± 9.941 | 1529 ± 35.05 |
| Ωcalcite | 5.18 ± 0.06 | 4.55 ± 0.47 | 2.81 ± 0.06 | 1.66 ± 0.05 |
| Ωaragonite | 3.34 ± 0.35 | 2.94 ± 0.30 | 1.81 ± 0.04 | 1.07 ± 0.03 |
| Total DIC (umol L−1) | 1613 ± 53.54 | 1850 ± 30.98 | 1941 ± 25.54 | 2101 ± 9.221 |
| CO32− (μmol L1) | 202.0 ± 23.42 | 180.0 ± 18.44 | 111.0 ± 2.341 | 66.02 ± 1.911 |
| Alkalinity (TA) | 1899 ± 35.24 | 2090 ± 50.01 | 2075 ± 26.84 | 2146 ± 11.21 |
| Salinity | 28.0 ± 1.0 | 28.0 ± 1.0 | 28.0 ± 1.0 | 28.0 ± 1.0 |
A culture of Isochrysis galbana (Tahitian strain, T-Iso) was maintained in exponential phase growth using standard culture conditions and added at a density of 2 × 107 cells daily to each experimental beaker (2 × 104 mL−1) as a food source. This algal species administered at this density and at this rate is known to produce high growth rates and survivorship of shellfish larvae through metamorphosis (19, 42, 45). To promote the high survivorship, containers that were in contact with larvae were never exposed to chemicals or detergents (45). To discourage the growth of bacteria during experiments, an antibiotic solution (5,000 units of penicillin, 5 mg of streptomycin, and 10 mg of neomycin per mL of solution, No.4083; Sigma-Aldrich) was added to each beaker at 1% its original concentration at the beginning of each experiment and during each water change (approximately two times weekly). This antibiotic mixture at this concentration has been shown to have no negative effects on the growth and survivorship of shellfish larvae (45). For each experiment, ∼200 larvae were distributed to each experimental beaker, achieving an environmentally realistic abundance of larvae (42). Each treatment began with ∼900 mL to allow enough beaker volume for the algal culture to be added daily as a food source. Twice weekly during experiments, larvae were gently poured onto a 64-μm mesh, and the condition (live or dead) and developmental stage of each larvae (veligers, pediveligers, and metamorphosed) was determined visually under a dissecting microscope; every individual larvae was counted at every water change. Larvae from each beaker (n = 4, per treatment) were removed, counted, observed, and transferred into a new beaker with new filtered seawater, food, and antibiotics within a 15 min period. Throughout experiments, all beakers were submerged in a water bath maintained at 24 °C via the use of commercially available heaters and chillers. This temperature generally yields high growth rates for A. irradians and M. mercenaria larvae (19, 42). Percent survivorship of all larvae was determined at each of the biweekly water changes when the numbers of larvae in each stage of veligers, pediveligers, and metamorphosed juveniles were quantified. Experiments were terminated after all surviving larvae in all treatments had metamorphosed. To statistically evaluate the effect of CO2 treatments on larval survival, goodness of fit tests (G Tests) were performed (46).
SEM.
To document differences in the size and structure of larval and early juvenile shellfish exposed to differing levels of CO2, randomly chosen individuals (n = 4 per treatment) were mounted for SEM in two distinct ways. Firstly, to image the outside of shells, individuals were attached at 45° relative to a level surface to a conductive substrate using carbon, double-sided tape and were subsequently coated with ∼12 nm of gold using an Edwards 150B rotary pump. To image the thickness and internal dimensions, cross-sections of shellfish were prepared. Individuals were mounted on glass microscope slides using UV-curing adhesive coating (Locite 4304) and were impregnated with low-viscosity epoxy (Stuers’ Specifix-20) under vacuum outgassing, a step that did not alter the original shape or size of individuals. After curing, the epoxy mount was progressively ground and polished to the centerline (hinge to shell edge) of the shellfish using silicon carbide sandpapers, followed by successively finer diamond polishing grits (15, 6, and 3 μm), 0.05 μm aluminum oxide suspension, and finally with colloidal silica. All individuals were cross-sectioned at the same location (hinge to shell edge) across the shell. This mount was then attached to a conductive substrate using carbon double-sided tape and coated with ∼4 nm of gold. SEM images were collected on both types of samples with a Leo (Zeiss) Model #1550 electron microscope using a high voltage of 20 KV and a Robinson backscatter detector. All components of individual bivalve shells displayed in Figs. 3 and 4 were probed using advanced EDAX/EDA microanalysis in the LEO (Zeiss) Model #1550 electron microscope and were confirmed to contain almost exclusively C, O, and Ca.
Size and Lipid Analysis.
To estimate the relative lipid content of larvae, Nile Red stain was used to bind to neutral lipids and fluoresce under an FITC filter on an epifluorescent microscope (23, 47). A Nile Red stock solution was made of 1.25 mg of Nile Red crystals in 100 mL of acetone. Randomly selected larvae (n = 15) from each treatment were stained with a 1:9 dilution of the stock solution and 0.2 μm filtered seawater. Larvae were exposed to the stain for ∼1.5 h, rinsed with filtered seawater, and digitally photographed with a Roper Scientific Photometrics CoolSNAP ES camera under an epifluorescent microscope. Digital images of each larva were analyzed for the area of lipid accumulation and the diameter and the area of individuals using ImageJ. A lipid index was estimated by dividing the area of the larvae containing the fluorescing lipids by the total larval area, thereby allowing for direct comparisons among treatments. One-way ANOVAs and posthoc Tukey multiple comparison tests were performed to examine the differences among larval lipid indexes, shell length, and thickness, at each CO2 level.
Acknowledgments
We are grateful for our supply of larvae from the East Hampton Shellfish Hatchery. We thank Jim Quinn for SEM assistance and James Waldvogel for cross sectioning assistance during this project. Constructive reviews came from two anonymous reviewers. This research was supported by the New Tamarind Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Le Quere C, et al. Trends in the sources and sinks of carbon dioxide. Nat Geosci. 2009;2:831–836. [Google Scholar]
- 2.Sabine CL, et al. The oceanic sink for anthropogenic CO2. Science. 2004;305:367–371. doi: 10.1126/science.1097403. [DOI] [PubMed] [Google Scholar]
- 3.Caldeira K, Wickett ME. Oceanography: Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- 4.I.P.C.C. Intergovernmental Panel on Climate Change. Summary for Policymakers. In: Solomon, et al., editors. The Physical Sciences Basis. Working Group I Contribution to the Fourth Assessment Report of the IPCC. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 5.Guinotte JM, Fabry VJ. Ocean acidification and its potential effects on marine ecosystems. Ann N Y Acad Sci. 2008;1134:320–342. doi: 10.1196/annals.1439.013. [DOI] [PubMed] [Google Scholar]
- 6.Kleypas JA, et al. Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A Guide for Future Research, Report of a Workshop Held 18–20 April 2005, St. Petersburg, Florida, Sponsored by NSF, NOAA, and U.S. Geological Survey. Seattle: NOAA / Pacific Marine Environmental Laboratory; 2006. Impacts of ocean acidification on coral reefs and other marine calcifiers. Contribution 2897. [Google Scholar]
- 7.Riebesell U, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- 8.Gazeau F, et al. Impact of elevated CO2 on shellfish calcification. Geophys Res Lett. 2007;34 L07603:1–5. [Google Scholar]
- 9.Green MA, Jones ME, Boudreau CL, Moore RL, Westman BA. Dissolution mortality of juvenile bivalves in coastal marine deposits. Limnol Oceanogr. 2004;49:727–734. [Google Scholar]
- 10.Green MA, Waldbusser GG, Reilly SL, Emerson K, O’Donnell S. Death by dissolution: Sediment saturation state as a mortality factor for juvenile bivalves. Limnol Oceanogr. 2009;54:1037–1047. [Google Scholar]
- 11.Kurihara H, Asai T, Kato S, Ishimatsu A. Effects of elevated pCO2 on early development in the mussel Mytilus galloprovincialis. Aquat Biol. 2008;4:225–233. [Google Scholar]
- 12.Kurihara H, Kato S, Ishimatsu A. Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas. Aquat Biol. 2007;1:91–98. [Google Scholar]
- 13.Talmage SC, Gobler CJ. The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica) Limnol Oceanogr. 2009;54:2072–2080. [Google Scholar]
- 14.Watson SA, Southgate PC, Tyler PA, Peck LS. Early larval development of the sydney rock oyster Saccostrea glomerata under near-future predictions of CO2 driven ocean acidification. J Shellfish Res. 2009;28:431–437. [Google Scholar]
- 15.Cooley SR, Doney SC. Anticipating ocean acidification’s economic consequences for commercial fisheries. Environ Res Lett. 2009;4:1–8. [Google Scholar]
- 16.Costanza R, et al. The value of the world’s ecosystem services and natural capital. Nature. 1997;387:253–260. [Google Scholar]
- 17.Cooley SR, Kite-Powell HL, Doney SC. Ocean acidification’s potential to alter global marine ecosystem services. Oceanography (Wash DC) 2009;22:172–180. [Google Scholar]
- 18.Eble AE. In: Anatomy and Histology of Mercenaria mercenaria. Biology of the Hard Clam. Kraeuter JN, Castagna M, editors. Amsterdam: Elsevier; 2001. pp. 117–216. [Google Scholar]
- 19.Cragg SM. Development, Physiology, Behaviour, and Ecology of Scallop Larvae. In: Shumway SE, Parsons GJ, editors. Scallops: Biology, Ecology, and Aquaculture. Amsterdam: Elsevier; 2006. pp. 45–122. [Google Scholar]
- 20.Carriker MR. Influence of suspended particles on biology of oyster larvae in estuaries. Am Malacol Bull Special Ed. 1986;3:41–49. [Google Scholar]
- 21.Carriker MR. The shell and ligament. In: Kennedy VS, Newwell RIE, Eble AE, editors. The Eastern Oyster: Crassostrea virginica. College Park, MD: Maryland Sea Grant College, University of Maryland System; 1996. pp. 75–168. [Google Scholar]
- 22.Purcell JE, Cresswell FP, Cargo DG, Kennedy VS. Differential ingestion and digestion of bivalve larvae by the scyphozoan Chrysaora quinquecirrha and the ctenophore Mnemiopsis leidyi. Biol Bull. 1991;180:103–111. doi: 10.2307/1542433. [DOI] [PubMed] [Google Scholar]
- 23.Phillips NE. Effects of nutrition-mediated larval condition on juvenile performance in a marine mussel. Ecology. 2002;83:2562–2574. [Google Scholar]
- 24.Tamburri MN, Zimmer-Faust RK. Suspension feeding: Basic mechanisms controlling recognition and ingestion of larvae. Limnol Oceanogr. 1996;41:1188–1197. [Google Scholar]
- 25.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Annu Rev Mater Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 26.Miller AW, Reynolds AC, Sobrino C, Riedel GF. Shellfish face uncertain future in high CO2 world: Influence of acidification on oyster larvae and growth in estuaries. Plos ONE. 2009;4:e5661. doi: 10.1371/journal.pone.0005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagani M, Zachos JC, Freeman KH, Tipple B, Bohaty S. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science. 2005;309:600–603. doi: 10.1126/science.1110063. [DOI] [PubMed] [Google Scholar]
- 28.Pearson PN, Palmer MR. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature. 2000;406:695–699. doi: 10.1038/35021000. [DOI] [PubMed] [Google Scholar]
- 29.Caldeira K, Berner R, Sundquist ET, Pearson PN, Palmer MR. Seawater pH and atmospheric carbon dioxide. Science. 1999;286:2043a. doi: 10.1126/science.284.5421.1824. [DOI] [PubMed] [Google Scholar]
- 30.Mann S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry. New York: Oxford University Press; 2001. [Google Scholar]
- 31.Harper EM, Taylor JD. In: The Evolutionary Biology of the Bivalvia. JAC, editor. 2000. (Geological Society, London, Special Publications,) [Google Scholar]
- 32.Jablonski D, Roy K, Valentine JW, Price RM, Anderson PS. The impact of the pull of the recent on the history of marine diversity. Science. 2003;300:1133–1135. doi: 10.1126/science.1083246. [DOI] [PubMed] [Google Scholar]
- 33.Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B. Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science. 2008;320:1490–1492. doi: 10.1126/science.1155676. [DOI] [PubMed] [Google Scholar]
- 34.Salisbury J, Green M, Hunt C, Campbell J. Coastal acidification by rivers: A new threat to shellfish? Eos Trans AGU. 2008;89:513. [Google Scholar]
- 35.Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2 induced ocean acidification. Geology. 2009;37:1131–1134. [Google Scholar]
- 36.Harte ME. Systematics and Taxonomy. In: Kraeuter JN, Castagna M, editors. Biology of the Hard Clam. Amsterdam: Elsevier; 2001. pp. 3–43. [Google Scholar]
- 37.Brand AR. Scallop ecology: Distributions and behaviour. In: Shumway SE, Parsons GJ, editors. Scallops: Biology, Ecology, and Aquaculture. Amsterdam: Elsevier; 2006. pp. 651–713. [Google Scholar]
- 38.Jablonski D. Heritability at the species level: Analysis of geographic ranges of cretaceous mollusks. Science. 1987;238:360–363. doi: 10.1126/science.238.4825.360. [DOI] [PubMed] [Google Scholar]
- 39.Roy K, Hunt G, Jablonski D. Phylogenetic conservatism of extinctions in marine bivalves. Science. 2009;325:733–737. doi: 10.1126/science.1173073. [DOI] [PubMed] [Google Scholar]
- 40.Gobler CJ, Lonsdale DJ, Boyer GL. A review of the causes, effects, and potential management of harmful brown tide blooms caused by Aureococcus anophagefferens (Hargraves et Sieburth) Estuaries. 2005;28:726–749. [Google Scholar]
- 41.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 42.Carriker MR. Embryogenesis and Organogenesis of Veligers and Early Juveniles. In: Kraeuter JN, Castagna M, editors. Biology of the Hard Clam. Amsterdam: Elsevier; 2001. pp. 77–115. [Google Scholar]
- 43.Roy RN, et al. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45 °C. Mar Chem. 1993;44:249–267. [Google Scholar]
- 44.Riebesell U, Fabry VJ, Hansson L, Gattuso JP. Guide to Best Practices for Ocean Acidification Research and Data Reporting. Luxembourg: Publications Office of the European Union; 2010. [Google Scholar]
- 45.Zeebe RE, Zachos JC, Caldeira K, Tyrrell T. Oceans. Carbon emissions and acidification. Science. 2008;321:51–52. doi: 10.1126/science.1159124. [DOI] [PubMed] [Google Scholar]
- 46.Padilla DK, Doall MH, Gobler CJ, Hartson A, O’Boyle K. Brown tide alga, Aureococcus anophagefferens, can affect growth but not survivorship of Mercenaria mercenaria larvae. Harmful Algae. 2006;5:736–748. [Google Scholar]
- 47.Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd Ed. New York: W.H. Freeman and Company; 1995. [Google Scholar]
- 48.Castell LL, Mann R. Optimal staining of lipids in bivalve larvae with Nile Red. Aquaculture. 1994;119:89–100. [Google Scholar]