Abstract
The transcription factor c-myb has emerged as one of the key regulators of vertebrate hematopoiesis. In mice, it is dispensable for primitive stages of blood cell development but essentially required for definitive hematopoiesis. Using a conditional knock-out strategy, recent studies have indicated that c-myb is required for self-renewal of mouse hematopoietic stem cells. Here, we describe and characterize the c-myb mutant in a lower vertebrate, the zebrafish Danio rerio. The recessive loss-of-function allele of c-myb (c-mybt25127) was identified in a collection of N-ethyl-N-nitrosourea (ENU)-induced mutants exhibiting a failure of thymopoiesis. The sequence of the mutant allele predicts a missense mutation (I181N) in the middle of the DNA recognition helix of repeat 3 of the highly conserved DNA binding domain. In keeping with the findings in the mouse, primitive hematopoiesis is not affected in the c-myb mutant fish. By contrast, definitive hematopoiesis fails, resulting in the loss of all blood cells by day 20 of development. Thus, the mutant fish lack lymphocytes and other white and red blood cells; nonetheless, they survive for 2–3 mo but show stunted growth. Because the mutant fish survive into early adulthood, it was possible to directly show that their definitive hematopoiesis is permanently extinguished. Our results, therefore, suggest that the key role of c-myb in definitive hematopoiesis is similar to that in mammals and must have become established early in vertebrate evolution.
Keywords: transcription factor, DNA binding domain, missense mutation, zebrafish
Research on the genetic hierarchy regulating the self-renewal and differentiation of hematopoietic progenitor cells provides critical information for diagnosing and treating a plethora of hematological disorders, both benign and malignant, and serves as a paradigm for stem-cell biology (1).
One of the most important regulators of mammalian hematopoiesis is c-myb (2), an evolutionarily conserved transcription factor (3). c-myb is part of a complex genetic network whose function is to specify and maintain hematopoietic progenitors and to regulate their differentiation (4). Among vertebrates, most genetic studies of c-myb function have been conducted in the mouse model, primarily because the experimental armamentarium is well-developed but also because no c-myb mutations have yet been described in other species.
In zebrafish, primitive hematopoietic activity begins within the first day after fertilization. In the first wave, the cephalic mesoderm gives rise to embryonic macrophages in the so-called rostral blood island (5); shortly thereafter, in a second wave, erythroid precursors localized in the intermediate cell mass give rise to erythrocytes that exit into the circulation at the end of the first day of embryonic development (6). After a brief transition period, which is characterized by the activity of erythromyeloid precursors in the posterior blood island (7), primitive hematopoiesis ends with the formation of the first multipotent precursors that also possess lymphoid potential (8, 9). The latter exhibit self-renewal capacity and are commonly referred to as hematopoietic stem cells (HSCs). Recent work has shown that these cells arise from hemogenic endothelium lining the ventral wall of the dorsal aorta (10, 11), indicating that the cellular processes underlying the generation of these multipotent progenitors are similar in both fish and mammals (12). Studies in the mouse model have suggested that c-myb is required only for definitive hematopoiesis, during which it regulates HSC maintenance and differentiation (13–15). Given the evolutionary conservation of genes regulating functional and temporo-spatial features of primitive hematopoiesis, the question arises as to whether the same applies for definitive hematopoiesis in adult vertebrates. Here, we explore this possibility by studying the phenotypic consequences of an apparent null mutation in the zebrafish gene encoding the c-myb transcription factor.
Results
Identification of a Zebrafish c-myb Mutant.
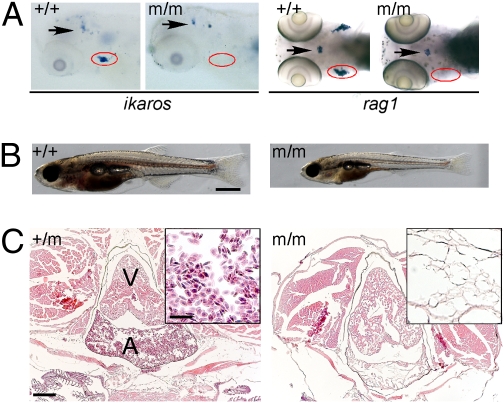
We have previously reported on a collection of mutant zebrafish lines identified in forward genetic screens for abnormalities in thymopoiesis (16). Here, we describe the positional cloning of the gene responsible for the recessive hematopoietic phenotype in line IP109 (t25217). Heterozygous fish are indistinguishable from homozygous wild-type animals. The mutant was originally recognized owing to its complete lack of ikaros- and rag1-expressing hematopoietic cells in the thymus (Fig. 1A). Homozygous mutant fish grow slower than their wild-type and heterozygous littermates, and they are distinguished by their severe anemia (Fig. 1B). Indeed, over the course of time, the number of blood cells diminishes, and by about 20 d postfertilization (dpf), virtually no circulating red blood cells are detectable in the vasculature of mutants (Movies S1, S2, S3, S4, S5, S6, S7, and S8). The absence of erythrocytes and other blood cell types is also apparent in histological sections (Fig. 1C). The bloodless phenotype is accompanied by smaller body size, developmental retardation including incomplete ossification, and lack of sexual maturation (Fig. S1). Although they lack detectable red blood cells from about 3 wk of age onward (Fig. S1), mutant fish survive until 2–3 mo of age. We presume that the mutants achieve residual oxygenation of their tissues through diffusion to an extent that is compatible with life into adulthood.
Fig. 1.
Phenotype of IP109 mutants. (A) Whole-mount RNA in situ hybridization of wild-type (+/+) and homozygous IP109 mutant (−/−) embryos with probes for ikaros (lateral views; Left) and rag1 (dorsal views; Right) at 5 d postfertilization (dpf). Note that ikaros is also expressed in neurons (arrows); this hybridization signal serves as an internal positive control for the hybridization process. In the hybridizations with rag1, a gh probe labels growth hormone-producing cells in the hypophysis (arrows) and serves as control for hybridization. The region of the thymus is encircled. No differences were seen between +/+ and +/m fish. Table S1 has probe details. (B) Macroscopic view of wild-type (Left) and mutant (Right) fish at 20 dpf. Note the smaller size and pale appearance of mutants. (Scale bar: 1 mm.) (C) Histological sections through the regions of the heart (A, atrium; V, ventricle) of heterozygous (Left) and homozygous mutant (Right) fish at 8 wk of age. Note the complete absence of erythrocytes in the mutant fish (Fig. S1). H&E staining. (Scale bars: 400 μm; Inset, 100 μm.)
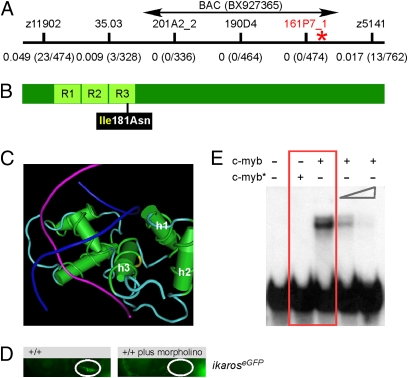
The gene responsible for the phenotype in IP109 mutants was mapped to chromosome 23; the marker closest to the mutation is located on BAC BX927365, with a genetic distance of ≤0.1 cM. Interestingly, this BAC also contains the c-myb locus (Fig. 2A). Because c-myb has been implicated in the regulation of definitive hematopoiesis in mice (13–15), the gene was sequenced in its entirety. This analysis revealed the presence of a thymidine to adenine (T > A) transversion (corresponding to nucleotide position 220627 in accession number BX927365.7 and nucleotide position 652 in accession number NM_131266). This causes a change from isoleucine to asparagine at amino acid residue 181, which is located in the middle of the DNA recognition helix of the R3 repeat of the evolutionarily conserved DNA binding domain (17) (Fig. 2B). Interestingly, a mutation at residue 181 is among the many changes that occur in the avian myeloblastosis virus variant of chicken c-myb (18). The side chain of isoleucine 181 points away from the DNA, and therefore, the replacement of a hydrophobic residue with a polar dicarboxylic amino acid likely affects the overall structure and/or stability of the R3 repeat (Fig. 2C and Fig. S2). The results of three additional experiments suggested that the phenotype in IP109 fish was indeed caused by the mutation in c-myb. First, all 257 mutant fish (identified by the absence of rag1 expression in the thymus) were found to be homozygous for the mutation, indicating close genetic linkage (<0.2 cM). Second, a c-myb–specific antisense morpholino resulted in a phenocopy (as determined by the lack of thymocytes) of the IP109 mutants (Fig. 2D). Third, thymopoietic abnormalities in 9 of 11 IP109 mutants were rescued after injection of a BAC clone (Fig. 2A) encompassing a wild-type c-myb gene [P < 0.000111, Fisher exact probability test (one-tailed)]. The missense mutation in the conserved DNA binding domain is an indication that the function of c-myb as a sequence-specific transcription factor might be compromised. To examine this possibility, wild-type and mutant forms of the DNA binding domains were expressed in Escherichia coli, purified, and used for electrophoretic mobility shift assays with an oligonucleotide containing a canonical c-myb recognition sequence. As expected, the mutant protein lacks DNA binding activity in vitro (Fig. 2E). Although we cannot exclude the possibility that the mutant form of c-myb has residual activity in vivo, our results suggest that it corresponds to a null mutation.
Fig. 2.
A deleterious missense mutation in the DNA binding domain of zebrafish c-myb. (A) Genetic mapping of the IP109 mutation on chromosome 23. The locations of informative markers are indicated; recombination frequencies are given in brackets below their names (details in Table S3). The position of BAC BX927365 encompassing the c-myb gene that was used for complementation analysis is shown. (B) Schematic indicating the location of the isoleucine (Ile) to asparagine (Asn) missense mutation relative to the three repeat domains (R) of the DNA binding domain in the c-myb protein. (C) The side chain of isoleucine 181 in the third helix (h3) of repeat 3 (yellow area on the carbon trace) points away from the DNA double helix (strands in blue and magenta) to the first (h1) and second (h2) helix of repeat 3. This figure was rendered using Cn3D from PDB ID 1MSE (17). (D) Injection of an antisense c-myb morpholino oligonucleotide into wild-type fish transgenic for an ikaros:eGFP reporter recapitulates the thymic homing defect observed in c-myb mutants. The thymic rudiment is encircled. Lateral views, 4 dpf. (E) The mutant version of c-myb lacks in vitro DNA binding activity. The N-terminal one-half (amino acids 1–318) of the mouse c-myb protein, encompassing the highly conserved DNA binding domain, was expressed in E. coli; the I181N mutation was introduced by site-directed mutagenesis. The mutant protein (c-myb*) does not interact with the radioactively labeled DNA probe containing a c-myb consensus binding site (red box); excess unlabeled binding sites compete with the radiolabeled probe (two right-most lanes). Equal amounts of wild-type and mutant proteins were used (Fig. S2).
Abnormal Hematopoiesis in c-myb Mutants.
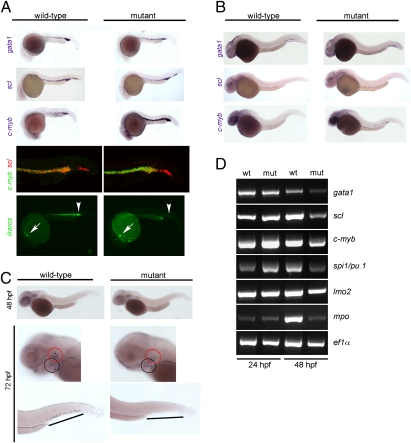
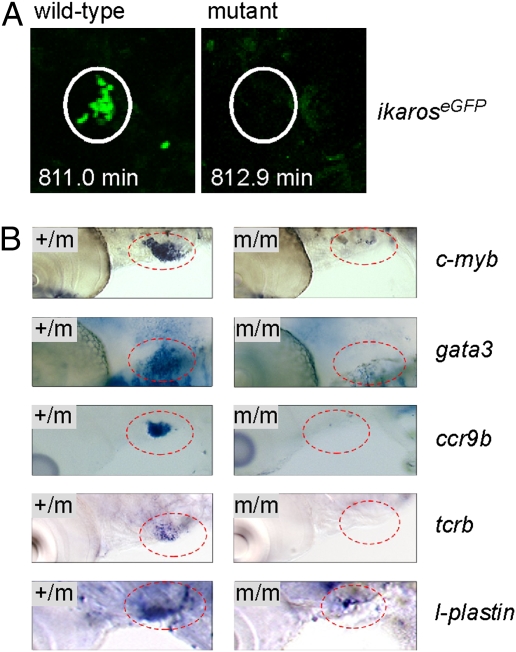
To examine the early stages of hematopoiesis, whole-mount RNA in situ hybridizations with several gene-specific probes were performed. In the zebrafish, the first hematopoietic precursors with multilineage differentiation capacity, the so-called erythromyeloid precursors (EMPs) are found in the posterior blood island (PBI) and can be conveniently detected by hybridization with a gata1 probe. At 24 h postfertilization (hpf), the number of EMPs seems to be slightly reduced in c-myb mutants, whereas the number of hematopoietic progenitor cells expressing scl is similar in wild-type and mutant fish (Fig. 3A). However, in cells coexpressing scl and c-myb, the expression levels of the latter are much higher in the mutants, which are clearly indicated by double-fluorescent in situ hybridization (Fig. 3A and Fig. S3). Furthermore, although the expression levels of ikaros are not affected in embryonic macrophages of mutants, ikaros expression is greatly diminished in the intermediate cell mass (ICM) and PBI regions, indicating a reduction in the number of lymphoid progenitors (Fig. 3A). Collectively, these data indicate that even the early stages of definitive hematopoiesis in c-myb mutants are abnormal. Differences between wild-type and mutant fish are also detectable at 36 hpf (Fig. 3B) and later stages (Fig. 3C). The severe impairment of definitive hematopoiesis is also evident in expression analyses using RT-PCR; the results confirm the transient up-regulation of c-myb, which is also evident for spi1/pu.1, and show that, at 48 hpf, the expression levels of all hematopoietic genes are much lower in mutants compared with controls (Fig. 3D). Thus, even in the absence of c-myb function, primitive hematopoiesis nevertheless gives rise to cells of the myeloid and erythroid lineages. However, in keeping with the progressive overall reduction of hematopoietic progenitor cells and the specific reduction of lymphocyte progenitors (Fig. 3A), the thymus is not colonized in mutant embryos (Fig. 4A and Fig. S4), although the thymic anlage as such is not affected (Fig. S4). As a consequence, thymopoiesis is completely absent in c-myb mutants, compatible with findings in the mouse (19). For instance, in addition to the lack of ikaros and rag1 expression (Fig. 1A), no expression of c-myb, gata3, ccr9b, and tcrb genes is observed in the thymic anlage (Fig. 4B). Moreover, no functional tcrb transcripts could be found in mutant embryos at 5 dpf (0/7 embryos), whereas they could be readily detected in heterozygous fish [3/3; P < 0.0084, Fisher exact probability test (one-tailed)]. Time-lapse video recordings of fluorescent cells in transgenic ikaros:eGFP reporter fish indicated that, although tissue macrophage-like cells display their characteristic migratory behavior, lymphocyte progenitors fail to develop and hence, do not appear in the thymic anlage of c-myb mutants (Movies S9 and S10). Collectively, these experiments suggest that T cell development is aborted at an early prethymic stage in c-myb mutants.
Fig. 3.
Characterization of primitive hematopoiesis. (A) Whole-mount RNA in situ hybridizations (WISH) were performed at 24 hpf with probes specific for scl (merge composed of three pictures), gata1, and c-myb. Hybridizations using single probes are shown in the top three panels. A double-fluorescent in situ hybridization for c-myb (green fluorescence) and scl (red fluorescence) is shown in the penultimate panel (merge of 22 optical slices at 5-μm thickness for wild-type and 20 slices for mutant embryos; Fig. S3 has additional data). The expression of ikaros was visualized using an ikaros:eGFP transgenic reporter (bottom panel). Note the normal number and fluorescence intensity of embryonic macrophages located in the anterior part of the embryos (arrows) and the diminished signals in the caudal hematopoietic tissue (CHT) and intermediate cell mass (ICM) of mutants (arrowheads); the embryonic macrophages are highly motile cells (Movies S9 and S10). (B) WISH analyses at 36 hpf using the indicated probes. All photographs are composites of three pictures, with the exception of the gata1 hybridization of mutant embryo, which is composed of four pictures. (C) WISH analyses for c-myb expression at the indicated time points. The photographs for the 48-hpf time-point are merged from three pictures. In the images taken at 72 hpf, the pharyngeal arches are marked with a black circle, and the thymic rudiment is marked with a red circle (Middle); the hematopoietic tissue in the tail regions (Bottom) is indicated by lines. (D) Gene-expression analysis in c-myb mutants. RT-PCR was performed at the indicated time points for the indicated genes; elongation factor 1 α (ef1α) serves as a control for cDNA integrity and a standard.
Fig. 4.
Failure of thymopoiesis in c-myb mutants. (A) Lack of thymus colonization in c-myb mutants transgenic for ikaros:eGFP. Still photographs were taken from Movies S9 and S10 at the indicated time points of the observation period (t0 = 55 hpf), equivalent to ∼68 hpf. Note the cluster of green cells in the thymus (encircled), whereas the thymus of mutants lacks such cells. A progenitor cell approaching the thymus is seen at the bottom right corner of the image of wild-type fish. (B) No evidence for thymopoiesis in early larvae. WISH was done with probes indicated at 5 dpf; the thymic area (encircled) is shown. Note the presence of small numbers of cells expressing l-plastin in the c-myb mutants, a marker associated with the myelo-monocytic lineage. This most likely represents embryonic macrophages situated in the thymus (Movies S9 and S10).
Failure of Definitive Hematopoiesis in c-myb Mutants.
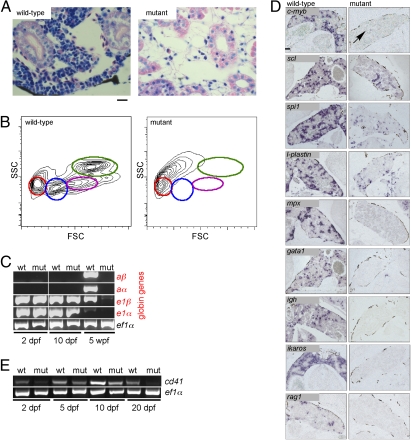
In contrast to mice with a null mutation in c-myb (14), a large fraction of c-myb mutant fish reaches adolescence. Hence, it was possible to directly examine the state of definitive hematopoiesis in these fish; our results indicate that it is severely compromised in the absence of c-myb. Mutants lack hematopoietic tissue in the head kidney, as shown in histological sections (Fig. 5A) and by flow cytometry (Fig. 5B); in addition, expression of adult globin genes is not detectable (Fig. 5C). In keeping with these observations, an extensive survey of the expression of relevant genes by RNA in situ hybridization indicated the complete absence of markers for immature cells of erythroid (gata1) and lymphoid (ikaros, rag1) lineages (Fig. 5D), compatible with the absence of red blood cells in these fish (Fig. 1C and Fig. S1) and the lack of thymocytes (Fig. 4, Fig. S4, and Movies S9 and S10) and B cells (Fig. 5D). A few cells expressing markers associated with the myelo-monocytic lineage (spi1 and l-plastin) were detectable, whereas cells expressing the granulocytic marker mpx were completely absent (Fig. 5B), compatible with the notion that short-lived progeny are no longer present after cessation of productive hematopoiesis. The former might represent descendants of long-lived embryonic macrophages, because they could also be observed in many tissues, including the thymus (Fig. 4B). In wild-type fish, the expression of scl, a marker associated with hematopoietic progenitor cells, was detected close to the nephric ducts and the intertubular space; some ventral renal tubules also express this gene. By contrast, scl expression is lacking in the intertubular space in c-myb mutant fish, pointing to a dramatic reduction of hematopoietic progenitor cells. Likewise, a large number of c-myb–expressing cells are present in wild-type fish, whereas only very few c-myb–expressing cells are found in the mutants. Using expression of cd41 as a marker for hematopoietic stem cells, it seems that such cells are greatly diminished, if not absent, by 20 dpf (Fig. 5E); because cd41 is also expressed in the thrombocyte lineage, its steadily declining levels also argue against a transient increase of such cells, contrary to what was observed in hypomorphic mouse c-myb mutants (20, 21). If hematopoietic progenitor cells survive until 2 mo of age in the mutant, their subsequent differentiation must be severely comprised. Collectively, the data show that the c-mybI181N mutation results in a state of anemia and immunodeficiency.
Fig. 5.
Failure of adult hematopoiesis in c-myb mutants. (A) Histological sections of the head kidney at 7 wk of age (Giemsa staining). Note the lack of hematopoietic cells in the mutant tissue. (Scale bar: 10 μm.) (B) Flow cytometric analysis of whole kidney marrow (6 wk of age) according to side scatter (SSC) and forward scatter (FSC) characteristics. Circles denote the positions of various cell types detectable in wild-type fish: red, erythrocytes; blue, lymphocytes and thrombocytes; magenta, precursors; green, myelomonocytes. The residual cells obtained from disintegrated mutant kidney tissue lack these characteristic features. (C) Lack of adult hemoglobin gene expression in c-myb mutants. RT-PCR was performed at the indicated time points for adult (prefix a) and embryonic (prefix e) globin genes; ef1α serves as a control for cDNA integrity and as a standard. (D) RNA in situ hybridization of head kidney sections was performed with the indicated probes. The signals seen with spi1 and l-plastin presumably originate from long-lived embryonic macrophages; a single c-myb positive cell is indicated (arrow). In mutant tissue, no signals are observed for mpx, gata1, ikaros, rag1, and igh. (Scale bar: 100 μm.) (E) Diminishing expression of cd41 in mutant embryos and larvae. RT-PCR was performed at the indicated time points; ef1α serves as a control for cDNA integrity and as a standard.
Discussion
For about two decades, the potential role of c-myb in definitive hematopoiesis has been intensely scrutinized. Its pivotal function in hematopoiesis was originally discovered in mice; nullizygous mice are severely anemic and die in midgestation, because definitive hematopoiesis does not occur (14). This phenotype is recapitulated in the mutant zebrafish. The hematopoietic phenotypes observed in animals carrying hypomorphic c-myb alleles (20, 21), chimeric animals established from mixtures of wild-type and c-myb–deficient cells (15, 19), and mice with lineage-specific inactivation of c-myb (13) suggested that this transcription factor is a pleiotropic regulator of hematopoiesis with nonredundant roles in progenitor cells as well as in their differentiating progeny. Here, we show that, in general, the role of c-myb in the regulation of self-renewal of hematopoietic progenitor cells and multilineage differentiation as originally defined in mice is evolutionarily conserved. As expected, not all aspects of the phenotypes observed in mice with hypomorphic c-myb alleles are seen in the fish mutant. For instance, thrombocytosis that occurs in some hypomorphs of mouse c-myb (21, 22) is not observed in the mutant zebrafish. Whether this reflects functional differences of the c-myb transcription factor between mouse and zebrafish or is because of the particular kind of missense mutation remains to be investigated. While this manuscript was under review, a report appeared describing a c-myb mutation in the teleost Oryzias latipes (23). Unlike the situation in mouse and zebrafish, the loss of c-myb in medaka already affects primitive hematopoiesis, supporting the notion of species-specific differences in the requirement for c-myb in early and later stages of hematopoiesis.
The unique biology of the fish has allowed us to study the function of c-myb in adult animals, obviating the need for conditional lineage-specific gene inactivation or other experimental manipulations. Anemic zebrafish are able to survive for several weeks (24), presumably owing to their ability to oxygenate tissues through diffusion and to the minimal but nevertheless protective function of long-lived innate immune cells established during embryonic hematopoiesis. By contrast, fish with complete failure of erythropoiesis, for instance, because of mutations in gata1 (25), are incapable of surviving into adulthood. This suggests that, after fish have developed beyond a critical threshold, they can tolerate lack of oxygen much better than at earlier stages. It seems that the critical transition between primitive and definitive hematopoiesis occurs between 15 dpf (when gata1 deficiency takes its toll) and 20 dpf (when the function of primitive hematopoiesis has lapsed).
In conclusion, we have identified the first c-myb null allele in lower vertebrates and have shown that the function of c-myb in definitive hematopoiesis is evolutionarily conserved, contributing to the growing evidence that the genetic regulation of general hematopoietic functions has deep evolutionary roots (26). Because c-myb mutants survive into adulthood while lacking most of their hematopoietic cells, they could serve as a model to assess, through cell transplantation, the functional capacity of different hematopoietic cell types without incurring damage to the hematopoietic stromal compartments before cell transfer (27).
Materials and Methods
Animals.
Details of the forward genetic screens to identify genes regulating thymopoiesis have been described (16); IP109 mutants belong to the Tübingen arm of our screen. The ikaroseGFP line was described previously (28). The animal experiments reported here were approved by the regional government.
In Situ Hybridization Analysis.
Procedures for RNA in situ hybridization were described previously (16). Double in situ hybridization was carried our according to procedures described in ref. 29. Probes are listed in Table S1.
Gene-Expression Analysis.
The primers for RT-PCR analysis of adult and embryonic gene expression have been described (30, 31); the primers for cd41 were described in ref. 7. Other primers are listed in Tables S2 and S3.
Biochemistry.
Details describing the expression, purification, and functional analysis of wild-type and mutant forms of the c-myb DNA binding domains can be found in SI Materials and Methods.
Live Microscopy of Fish.
The procedures for live imaging were described previously (28).
Morpholino and BAC Injections.
Injection of a c-myb–specific antisense morpholino was used to phenocopy the c-myb mutation; phenotypic rescue was attempted by injection of a BAC clone encompassing a wild-type c-myb gene (details in SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank the members of the Tübingen and Freiburg screening group for help during the identification of the IP109 mutant, Mike Bialecki, Dagmar Diekhoff, Fernando Mateos, Tanna Franz, and Monika Held for help during various stages of the project, and the Deutsche Forschungsgemeinschaft and the Max-Planck Society for financial support.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 17067.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004640107/-/DCSupplemental.
References
- 1.Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greig KT, Carotta S, Nutt SL. Critical roles for c-Myb in hematopoietic progenitor cells. Semin Immunol. 2008;20:247–256. doi: 10.1016/j.smim.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Davidson CJ, Tirouvanziam R, Herzenberg LA, Lipsick JS. Functional evolution of the vertebrate Myb gene family: B-Myb, but neither A-Myb nor c-Myb, complements Drosophila Myb in hemocytes. Genetics. 2005;169:215–229. doi: 10.1534/genetics.104.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns CE, et al. A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood. 2009;113:5776–5782. doi: 10.1182/blood-2008-12-193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 6.Detrich HW, 3rd, et al. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci USA. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertrand JY, et al. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand JY, Kim AD, Teng S, Traver D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development. 2008;135:1853–1862. doi: 10.1242/dev.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissa K, et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111:1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 12.Boisset J-C, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 13.Lieu YK, Reddy EP. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc Natl Acad Sci USA. 2009;106:21689–21694. doi: 10.1073/pnas.0907623106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mucenski ML, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 15.Sumner R, Crawford A, Mucenski M, Frampton J. Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene. 2000;19:3335–3342. doi: 10.1038/sj.onc.1203660. [DOI] [PubMed] [Google Scholar]
- 16.Schorpp M, et al. Conserved functions of Ikaros in vertebrate lymphocyte development: Genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J Immunol. 2006;177:2463–2476. doi: 10.4049/jimmunol.177.4.2463. [DOI] [PubMed] [Google Scholar]
- 17.Ogata K, et al. Solution structure of a specific DNA complex of the Myb DNA-binding domain with cooperative recognition helices. Cell. 1994;79:639–648. doi: 10.1016/0092-8674(94)90549-5. [DOI] [PubMed] [Google Scholar]
- 18.Klempnauer KH, Gonda TJ, Bishop JM. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: The architecture of a transduced oncogene. Cell. 1982;31:453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- 19.Allen RD, 3rd, Bender TP, Siu G. c-Myb is essential for early T cell development. Genes Dev. 1999;13:1073–1078. doi: 10.1101/gad.13.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandberg ML, et al. c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev Cell. 2005;8:153–166. doi: 10.1016/j.devcel.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Metcalf D, et al. Anomalous megakaryocytopoiesis in mice with mutations in the c-Myb gene. Blood. 2005;105:3480–3487. doi: 10.1182/blood-2004-12-4806. [DOI] [PubMed] [Google Scholar]
- 22.Malaterre J, et al. c-Myb is required for progenitor cell homeostasis in colonic crypts. Proc Natl Acad Sci USA. 2007;104:3829–3834. doi: 10.1073/pnas.0610055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriyama A, Inohaya K, Maruyama K, Kudo A. Bef medaka mutant reveals the essential role of c-myb in both primitive and definitive hematopoiesis. Dev Biol. 2010;345:133–143. doi: 10.1016/j.ydbio.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Brownlie A, et al. Positional cloning of the zebrafish sauternes gene: A model for congenital sideroblastic anaemia. Nat Genet. 1998;20:244–250. doi: 10.1038/3049. [DOI] [PubMed] [Google Scholar]
- 25.Belele CL, et al. Differential requirement for Gata1 DNA binding and transactivation between primitive and definitive stages of hematopoiesis in zebrafish. Blood. 2009;114:5162–5172. doi: 10.1182/blood-2009-05-224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene. 2004;23:7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- 27.Traver D, et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 28.Bajoghli B, et al. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Jowett T. Double in situ hybridization techniques in zebrafish. Methods. 2001;23:345–358. doi: 10.1006/meth.2000.1147. [DOI] [PubMed] [Google Scholar]
- 30.Brownlie A, et al. Characterization of embryonic globin genes of the zebrafish. Dev Biol. 2003;255:48–61. doi: 10.1016/s0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 31.Chan F-Y, et al. Characterization of adult alpha- and beta-globin genes in the zebrafish. Blood. 1997;89:688–700. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.